Abstract
Objective
To define an optimal cutoff time to distinguish early and late recurrence in hepatocellular carcinoma (HCC) patients after radiofrequency ablation (RFA), and to determine the risk factors and patterns of early recurrence.
Materials and methods
This retrospective study included HCC patients who developed recurrence after RFA as the primary therapy at three Chinese hospitals from January 2011 to December 2016. The best cutoff time to define early and late recurrence was determined based on differences in post recurrence survival (PRS). The clinical variables were assessed by univariate and multivariate logistic regression analyses.
Results
A total of 279 eligible patients were included. The optimal cutoff time interval after RFA to differentiate early and late recurrence was identified as 12 months (p = 0.029). The independent risk factors of early recurrence were multiple tumors, alpha fetoprotein (AFP) levels, gamma-glutamyl transferase (γ-GT), and serum albumin (ALB) levels. A well-discriminated nomogram was constructed to predict risk of early recurrence. The incidence of intrahepatic distant recurrence (IDR) alone and IDR + extrahepatic recurrence (ER) in early recurrence group was significantly higher than those in late recurrence group (80.73% vs. 66.47%, p = 0.009).
Conclusion
Twelve months was determined as the optimal cutoff time for differentiating early and late recurrence after RFA for HCC patients. The factors affecting early recurrence after RFA were multiple tumors, AFP levels, ALB level, and γ-GT level. Patients in early recurrence cohort were more likely to develop IDR alone or IDR + ER.
Introduction
Liver cancer is the sixth most common cancer and the fourth leading cause of cancer-related death worldwide [Citation1]. Hepatocellular carcinoma (HCC) accounts for 75%∼85% of all liver cancers [Citation1]. Of note, China had 369,000 new cases of HCC and 366,000 HCC-related deaths in 2018, accounting for about 50% of global HCC cases [Citation1,Citation2]. The therapeutic options for HCC depends on stage of the disease, the underlying liver condition and performance status of the patient [Citation3,Citation4]. Liver transplantation is the first-line curative therapy for early-stage HCC (single or up to 3 tumors ≤3 cm, no extrahepatic metastasis or major vessel invasion), which is always limited by organ scarcity and high cost [Citation3,Citation4]. Meanwhile, surgical resection is not feasible for patients with multiple tumors, cirrhosis, poor hepatic reserve and portal hypertension [Citation3,Citation4].
For patients with very early-stage HCC (single tumor ≤2 cm), radiofrequency ablation (RFA) has been accepted as the first-line therapy [Citation3]. For patients with early-stage HCC who are not suitable for surgical resection or transplantation, RFA has been accepted as an alternative curative therapy [Citation3]. However, the long-term prognosis of HCC is still limited because of the high tumor recurrence rate up to 60% within 5 years after radical therapies [Citation5].
HCC recurrence after radical therapies has been defined as early and late recurrence, based on the temporal distribution, meanwhile, risk factors for the development of early and late recurrence also differ [Citation6,Citation7]. Although the terms ‘early recurrence’ and ‘late recurrence’ of HCC are often mentioned in both the academic research and clinical practice, a scientific definition of early recurrence is still lacking [Citation6]. Different cutoff values reported by previous studies ranged from 8 months to 24 months after radical therapies for HCC [Citation8–12]. Some researchers have defined early and late recurrence by overall survival (OS) or disease free survival (DFS) [Citation13,Citation14]. Post recurrence survival (PRS) has also been used as an indicator to distinguish early and late recurrence after surgical resection for HCC or pancreatic cancer, because it can objectively reflect the prognosis of tumor recurrence compared with OS [Citation8,Citation9,Citation15]. However, the optimal cutoff time to differentiate early and late recurrence of HCC after RFA has not been studied.
Previous studies have shown that patients who develop early recurrence have poorer prognosis than those who develop late recurrence [Citation8,Citation9]. Many researchers have considered that early recurrence develops from intrahepatic micro-metastasis, while late recurrence appears to be due to de novo carcinogenesis [Citation11,Citation12,Citation16]. Previous studies reported early recurrence after hepatectomy to be associated with aggressive oncological features, such as large tumor size, high alpha-fetoprotein (AFP) level, high albumin-bilirubin (ALBI) ratio, poor differentiation, and microvascular invasion (MVI) [Citation8,Citation9,Citation12,Citation17]. However, the risk factors associated with scientific definition of ‘early recurrence’ after RFA for HCC have not been studied.
Determining the optimal cutoff time of time recurrence and understanding the patterns and risk factors of early recurrence will help interventional oncologists to predict the prognosis of HCC patients, plan surveillance strategies, and formulate adjuvant therapeutic and preventive strategies. Therefore, this multicenter retrospective study was conducted to identify an optimal cutoff time for defining early recurrence after RFA in HCC and to determine the risk factors and patterns of early recurrence. Meanwhile, a nomogram was developed to predict risk of early recurrence.
Materials and methods
Study cohort
This study was approved by the Institutional Review Boards of the National Cancer Center, First Hospital of Shanxi Medical University and Beijing Hospital of Traditional Chinese Medicine. Written informed consent for the treatment was obtained from each patient. The need for written informed consent to publish the data was waived by the Ethics Committees, since the personal details of these patients were kept confidential.
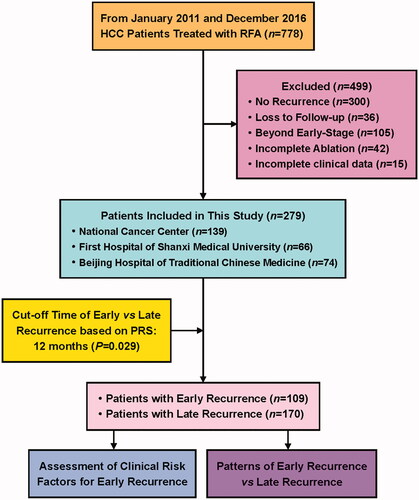
The present study is a retrospective review of prospectively maintained database of adult patients (≤80 years old) having HCC treated by RFA with a curative intent at the National Cancer Center, First Hospital of Shanxi Medical University and Beijing Hospital of Traditional Chinese Medicine from January 2011 to December 2016. Patients with HCC who refused to undergo surgery, or those who were not feasible for surgery because of cirrhosis, poor hepatic reserve and portal hypertension received RFA after the diagnosis. The present study focused on HCC recurrence, and excluded patients who did not developed recurrence until the end of follow-up or death, similar to the previous studies on postsurgical early recurrence [Citation8,Citation9]. Inclusion criteria were: (1) HCC confirmed by histology or noninvasive diagnostic criteria according to the European Association for the Study of Liver (EASL) [Citation18]; (2) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (3) patients with very early-stage (single tumor ≤2 cm, BCLC 0 stage) or early-stage HCC (up to 3 tumors ≤3 cm, BCLC A stage); (4) absence of extrahepatic metastasis or major vessel invasion; (5) patients who underwent RFA with complete tumor ablation. Exclusion criteria were: (1) diagnosis of primary HCC or recurrence without using contrast-enhanced (CE) magnetic resonance imaging (MRI) [Citation19]; (2) patients without recurrence until the end of follow-up or death; (3) patients who were lost to follow up; (4) patients who first underwent RFA at other centers; (5) patients who underwent surgery as the primary treatment and RFA for recurrence. The flowchart of patient selection and study design is shown in .
We included all clinically relevant variables prior to RFA based on the previous studies [Citation20,Citation21]. These variables included age, gender, maximum tumor size, number of tumors, tumor location, number of ablation sessions, etiology of HCC, cirrhosis, Child-Pugh class, ALBI, serum AFP levels, liver function tests [serum alkaline phosphatase (AKP), serum albumin (ALB), aspartic transaminase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), serum γ-glutamyl transpeptidase (γ-GT) levels, platelet (PLT) count, and international normalized ratio (INR).
Definitions
Cirrhosis was diagnosed by medical imaging (e.g., ultrasonography, CT, MRI elastography), liver function tests (e.g., AST, ALT), and etiology (e.g., HBV, HCV) according to liver cirrhosis guidelines of EASL and Chinese Society of Hepatology (CSH) [Citation22,Citation23]. The major vessels included the main hepatic artery, the first and second branch of the portal vein, and inferior vena cava [Citation24]. Unfavorable locations were defined as sites where the tumor margin was <0.5 cm from the important structures, including the major vessels, primary and secondary intrahepatic bile duct, gallbladder, diaphragm, pericardium, liver capsule and gastrointestinal tract.
RFA procedure
All RFA procedures were performed under local anesthesia using ultrasound or computed tomography (CT) by interventional oncologists with at least 5 years of RFA experience. The radiofrequency generator system (S-1500, MedSphere International Inc, Fremont, CA, USA) was used for the procedure. The type of electrode used (monopolar or expandable multi-tined electrode; MedSphere International Inc, Fremont, CA, USA), the power of the generator, and the ablation strategy were all determined by the operator based on tumor burden, tumor shape, tumor location, manufacturer’s recommendations, and so on. Expanding or overlapping ablation was performed to ensure that all the tumors were ablated at least 0.5 cm beyond the tumor margin when possible [Citation25,Citation26]. Artificial ascites were created before RFA which acted as an insulation layer between the tumor(s) and the adjacent vital structures such as gallbladder, diaphragm, pericardium, liver capsule and gastrointestinal tract [Citation24,Citation27,Citation28]. After the lesions were ablated, the ablation path was cauterized to avoid tumor seeding and hemorrhage.
Follow-up
Post-operative CE imaging such as MRI, CT, or ultrasound were performed to confirm complete ablation. If residual tumor tissue was still present, additional RFA procedures were immediately performed to achieve complete ablation in one procedure [Citation20]. A minimal ablative margin > 0.5 cm beyond the tumor in all directions or/and absence of arterial contrast enhancement and portal venous washout within the ablation zone suggestive of residual tumor one month after ablation was/were considered as complete ablation [Citation25,Citation26,Citation29,Citation30]. Patients were followed-up every 3 months in the first year after RFA and every six months in the subsequent years until tumor recurrence or death. The last follow-up date for this study was September 30th, 2019. Each follow-up visit consisted of clinical history, physical examination, serum AFP levels and CE MRI. Patients with chronic hepatitis B virus (HBV) infection and HBV DNA level >1000 copies/mL were treated with lamivudine, adefovir dipivoxil or entecavir daily [Citation12]. Patients with chronic hepatitis C virus (HCV) infection were treated with interferon and ribavirin [Citation31]. HCC recurrence was treated by the multidisciplinary approach, which included surgical resection, repeat RFA, transarterial chemoembolization (TACE), or systemic therapy (i.e., sorafenib or FOLFOX4 regimen chemotherapy).
Oncological outcomes
The primary outcomes of this study were PRS, RFS and OS. PRS was defined as the time interval from the first recurrence to death. RFS was defined as the time interval from first RFA treatment to first recurrence. OS was defined as the time interval from first RFA treatment to death or the last follow-up. Patients who were still alive at the last follow-up examination should be regarded as censored data.
The recurrence was classified based on the time and location as time recurrence and spatial recurrence, respectively. All recurrences were diagnosed by using contrast-enhanced MRI [Citation19]. Spatial recurrence was classified as local tumor progression (LTP), intrahepatic distant recurrence (IDR) or extrahepatic recurrence (ER). LTP was defined as the appearance of tumor foci at the edge of the ablation zones after RFA [Citation6,Citation26,Citation32]. IDR was defined as appearance new intrahepatic tumor in different liver subsegments from the ablation zones or the same liver subsegment that was not adjacent to the ablation zones after RFA [Citation33–35]. ER was defined as appearance of new extrahepatic metastasis after RFA [Citation6,Citation26,Citation36].
Statistical analysis
Qualitative variables were expressed as frequency (percentages), and compared using Fisher’s exact test or Chi-square test, as appropriate. Quantitative variables were expressed as mean ± standard deviation and compared using the Mann–Whitney U-test or t-test. The Kaplan–Meier method was used to calculate RFS, PRS and OS. The minimum p value of Log-rank test method for cumulative PRS rates difference between the two cohorts was used to evaluate the best cutoff time for distinguishing early and late recurrence cohorts [Citation8,Citation9]. The log-rank test was used to compare the differences between the survival outcomes of early and late recurrence. Univariable and multivariable logistic regression analyses were performed to identify the risk factors for early recurrence. Parameters with a p value < 0.10 on the univariate analysis were included in the multivariate analysis via a forward stepwise model. A two-tailed p value < 0.05 was considered statistically significant. The nomogram was subjected to 500 bootstrap resamples for the internal validation of the analyzed database. A receiver operating characteristic (ROC) curve analysis was performed, and the area under the ROC (AUC) was calculated to evaluate the predictive power of the model. All analyses were conducted using the R software version 3.6.2 (http://www.r-project.org/).
Results
Study cohort
Out of 778 HCC patients who received RFA at three medical centers, 300 patients had no recurrence at the end of follow-up, 36 patients lost to follow up, 105 patients were beyond early-stage, 42 patients received incomplete ablation, and 16 patients had incomplete clinical data. Finally, 279 patients with 590 lesions were included in the present study (139, 66 and 74 HCC patients from the National Cancer Center, the First Hospital of Shanxi Medical University, and Beijing Hospital of Traditional Chinese Medicine, respectively).
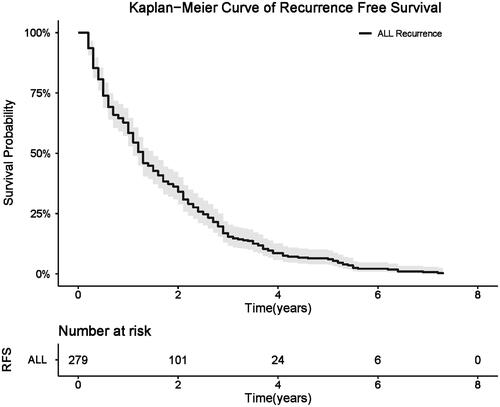
Among all 279 patients, 236 patients were men and 43 patients were women; the mean age was 55.3 ± 11.5 years (range, 34.0–80.0 years). A total of 75 patients (26.9%) had one tumor, 116 patients (41.6%) had two tumors and 88 patients (31.5%) had three tumors, and the median tumor diameter was 2.2 cm (range, 0.9–3.0 cm; mean ± SD, 2.2 ± 0.6 cm). Technical success rate was 95.08% (561/590), and technical effectiveness rate was 100% (590/590). The median follow-up period of the included patients was 46.7 months (range: 2.9–96.0). The median RFS for the study patients was 15.2 months (range: 2.1–87.9), and the 1-, 2-, 3-, and 5-year RFS rates were 60.57%, 35.13%, 15.41%, and 6.09%, respectively ().
Defining early and late recurrence
The median OS and PRS for the whole cohort were 83.0 months (95% CI: 80.1–NA) and 63.5 months (95% CI: 58.6–80.2), respectively. The optimal cutoff time for defining early and late recurrence was determined by assessing PRS at 2-month intervals after RFA () [Citation8,Citation9]. The largest difference in cumulative PRS rates was 12 months after RFA. Therefore, the optimal cutoff time after RFA was taken as 12 months. HCC recurrence within 12 months after first curative RFA was defined as early recurrence and that after 12 months was defined as late recurrence (p = 0.029, ).
Table 1. Comparison of PRS to define the cutoff time for early and late HCC recurrence.
The 1-, 2-, 3-, and 5-year PRS rates of early recurrence group were 82.57%, 69.24%, 62.40%, and 53.03%, respectively. The 1-, 2-, 3-, and 5-year PRS rates of late recurrence group were 91.10%, 82.21%, 73.98%, and 58.40%, respectively. Patients who developed early recurrence had significantly poorer cumulative PRS rates compared with patients who developed late recurrence (QU-PRS: 14.0 vs. 35.7 months; p = 0.029, ).
Figure 3. Comparison of post recurrence survival (A) and overall survival (B) among HCC patients with early and late recurrence.
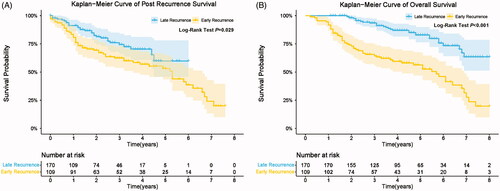
The 1-, 2-, 3-, and 5-year OS rates of early recurrence group were 92.66%, 72.96%, 64.33%, and 56.14%, respectively. The 1-, 2-, 3-, and 5-year OS rates of late recurrence group were 100.00%, 98.13%, 93.45%, and 82.56%, respectively. Patients who developed early recurrence had significantly poorer cumulative OS rates compared with patients who developed late recurrence (p < 0.001, ).
Comparison of baseline characteristics between early and late recurrence groups
The baseline characteristics of the patients in early and late recurrence cohorts are shown in . The number of lesions, Child-Pugh class, AFP levels, ALB levels, and γ-GT levels were different between these two groups (p < 0.05).
Table 2. Comparison of HCC patient demographics with early and late recurrence.
Risk factors for early recurrence
The univariate and multivariate logistic regression analysis found that multiple tumors (HR = 3.058, 95% CI: 1.321–7.080, p = 0.009), AFP level > 400 ng/ml (HR = 2.790, 95% CI: 1.367–5.693, p = 0.005), ALB ≤ 35 g/L (HR = 0.394, 95% CI: 0.158–0.982, p = 0.046), and γ-GT >60 U/L (HR = 1.950, 95% CI: 1.047–3.632, p = 0.035) were independent risk factors of early recurrence ().
Table 3. Multivariable logistic regression analysis of risk factors for early recurrence of HCC.
Constructing the nomogram for early recurrence
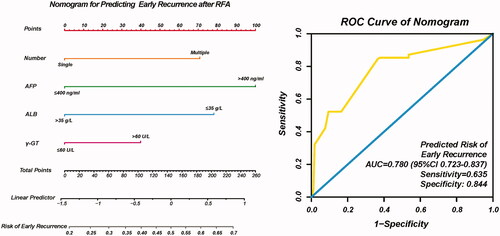
Based on the independent risk factors (multiple tumors, AFP, ALB and γ-GT) identified by the multivariate analysis, a nomogram was developed to predict risk of early recurrence after RFA, as shown in . Each risk factor was allocated a predicting score, and the sum of four scores were located on the total points axis, suggesting the risk of early recurrence after RFA. The AUC of nomogram for predicting risk of early recurrence was 0.780 (95%CI: 0.724–0.837, sensitivity: 635, specificity: 0.844, ).
Complications and side effects
No major complications (Society of Interventional Radiology Classification System C–F) occurred in patients receiving RFA treatment [Citation26,Citation37], 6.41% sessions (20/312) occurred post-ablation syndrome, 8.97% sessions (28/312) occurred 1–2 grade pain, 1.92% sessions (6/312) occurred asymptomatic pleural effusions and 2.56% sessions (8/312) occurred asymptomatic perihepatic fluid or blood.
Recurrence patterns and therapies after recurrence
The recurrence patterns, their characteristics, and therapies after recurrence are shown in and .
Figure 5. Recurrence patterns of HCC after RFA (A); Distribution of various types of recurrence over the follow-up period (B).
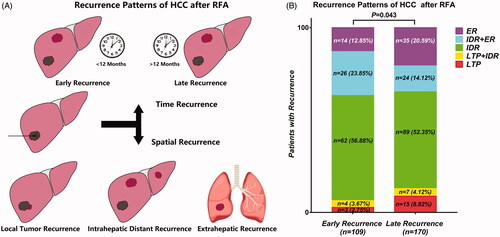
Table 4. Recurrence patterns and therapies after recurrence of HCC patients with early and late recurrence.
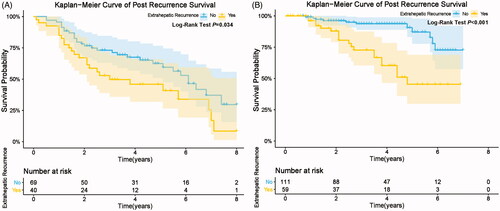
Among the 279 patients with recurrence after RFA, 18 patients (6.5%) had LTP alone, 11 patients (3.9%) had LTP + IDR, 151 patients (54.1%) had IDR alone, 54 patients (19.4%) had IDR + ER, and 45 patients (16.1%) had ER (). The recurrence patterns were statistical different between early and late recurrence cohorts (p = 0.043, ). IDR alone was the most common recurrence pattern in both two recurrence cohorts. The incidence of IDR alone in early recurrence group was higher than that in late recurrence group, but the difference was not significant (62 of 109 [56.7%] vs. 89 of 170 [52.4%], p = 0.459). The incidence rate of IDR + ER in early recurrence group was significantly higher than that in late recurrence group (26 of 109 [23.9%] vs. 24 of 170 [14.1%], p = 0.038). Moreover, the incidence rate of IDR alone and IDR + ER in early recurrence group was significantly higher than those in late recurrence group (88 of 109 [80.7%] vs. 113 of 170 [66.5%], p = 0.009). Subgroup analyses showed that patients with ER (ER, IDR + ER) had significant poorer cumulative PRS rates compared to patients without ER (LTP, IDR, LTP + IDR) (early recurrence: p = 0.034 ; late recurrence: p < 0.001, ).
Figure 6. Comparison of post recurrence survival among HCC patients with and without extrahepatic recurrence in early recurrence group (A) and late recurrence group (B).
The mean size of recurrent tumors in early recurrence cohort was significantly larger than those in late recurrence cohort (2.8 ± 1.4 cm vs. 2.3 ± 1.1 cm, p < 0.001). The following treatments were administered following recurrence: hepatectomy (2.9%), repeat ablation (42.3%), ablation combined with TACE (14.3%), TACE alone (19.7%), TACE combined with sorafenib (7.5%), and FOLFOX4 regimen chemotherapy (13.3%). More patients in early recurrence cohort received potential radical therapies (hepatectomy, second ablation, and ablation combined with TACE) compared to those in late recurrence cohort (64.2% vs. 56.5%).
Discussion
Recurrence of HCC after RFA adversely affects long-term survival. Recurrence has been classified as early and late recurrence in most of the studies [Citation6]. However, there has been no consensus definition of early and late recurrence after RFA in HCC patients. This multicenter retrospective study showed that the optimal cutoff time for identifying early and late recurrence based on postoperative survival to be 12 months. Moreover, multivariate analyses identified that multiple tumors, AFP levels, ALB levels, and γ-GT levels were independent risk factors for early recurrence.
PRS is closely associated with the prognosis of postoperative HCC. This is the first study to define the optimal cutoff time for differentiating early and late recurrence after RFA by using PRS rather than OS, because PRS could avoid the inherent bias associated with OS. Also, PRS more objectively reflects the prognosis of HCC patients after the development of recurrence. PRS has been used by previous studies to determine the optimal cutoff time of early and late recurrence in patients with HCC or pancreatic cancer after surgical resection [Citation8,Citation9,Citation15]. In present study, we concluded that the optimal time for defining early recurrence was 12 months based on the significant difference in PRS between early and late recurrence cohorts. Patients who developed early recurrence had significantly poorer cumulative PRS rates compared with patients who developed late recurrence (p = 0.029). Moreover, patients who developed early recurrence had significantly poorer cumulative OS rates compared with patients who developed late recurrence (p < 0.001).
In previous studies, researchers have defined the optimal cutoff time for early recurrence of HCC after liver resection in the range of 8 to 24 months [Citation8–12]. The reasons for the variability in the cutoff time for early recurrence included use of different procedures for the treatment of primary HCC and recurrent cancer, difference in the etiologies of HCC in the study patients, racial difference, and the different endpoints (OS and PRS) for estimating the best cutoff time. Moreover, distinguishing early recurrence from late recurrence is important for prognostication of the disease, treatment planning and surveillance strategy.
The pathophysiological mechanism and risk factors for early and late recurrence of HCC are different [Citation6,Citation7]. The present study suggested that multiple tumors, AFP level (>400 ng/mL), ALB level (≤35 g/L) and γ-GT level (>60 U/L) before RFA were independent risk factors for early recurrence of HCC after RFA, which are similar to those reported by previous studies [Citation38–40]. These results indicated that aggressive tumor biology and high tumor burden is associated with high-risk early recurrence. It further supports the hypothesis that early recurrence may be due to micro-metastasis of the primary tumor [Citation41,Citation42]. In future studies we intend to explore the histopathological and genetic changes associated with early and late recurrence of HCC. Additionally, determining the risk factors of early recurrence could help to individualize the follow-up and provide early intervention to improve the prognosis. Nomogram has been widely used as a visualization tool for predicting the prognosis of patients with various types of cancers. To the best of our knowledge, the present study is the first to construct a nomogram for predicting risk of early recurrence after RFA based on the risk factors of scientific ‘early recurrence’. The AUC demonstrated that our nomogram was a reliable prognostic prediction model.
In this study, recurrence patterns were statistical different between early and late recurrence cohorts (p = 0.043). The incidence rate of IDR alone in early recurrence group was higher than in late recurrence group, but the difference was not significant (56.9% vs. 52.4%, p = 0.459). We think that the difference was due to more frequent postoperative surveillance in the first year than in subsequent years. The incidence rate of IDR + ER in early recurrence group was significantly higher than that in late recurrence group (23.9% vs. 14.1%, p = 0.038). In our opinion, IDR + ER is more likely to be the next-step metastasis process of IDR. The incidence rate of IDR alone and IDR + ER in early recurrence group was significantly higher than those in late recurrence group (80.7% vs. 66.5%, p = 0.009), which further supports the hypothesis that early recurrence may be due to micro-metastasis of the primary tumor. Specially, patients with ER (ER, IDR + ER) had significant poorer cumulative PRS rates compared to patients without ER (LTP, IDR, LTP + IDR) (early recurrence: p = 0.034; late recurrence: p < 0.001). Different spatial recurrence patterns have different oncological outcomes. Therefore, the results of this study could help improve the management of HCC patients after RFA (adjuvant therapy, monitoring strategy and post recurrence therapy). These results were similar with a previous multi-institutional study conducted by Wei et. al [Citation9], 35.5% of patients in early recurrence cohort had intrahepatic and extrahepatic recurrence compared to 19.8% of patients in late recurrence cohort. Additionally, these investigators found that patients with early recurrence had better OS after curative re-treatment compared to those who received palliative treatment for early recurrence (median OS, 69.0 vs. 21.0 months, p < 0.001) [Citation9]. These results suggest that patients at high risk for early postoperative recurrence should receive more frequent postoperative surveillance strategies, and adjuvant therapy such as sorafenib, lenvatinib, FOLFOX4 regimen chemotherapy, and hepatic artery infusion chemotherapy (HAIC) [Citation3,Citation4,Citation43].
There are some limitations of the present study. First, the study was retrospective in nature with limited sample size and inherent bias. Second, outcomes could not be compared across various treatment methods for recurrence due to the limited data. Third, most of the study patients had HBV-related HCC, hence, the results cannot be extrapolated to other populations, such as Western countries where alcohol and nonalcoholic steatohepatitis are the most common causes of HCC. Fourth, medical imaging features and technology factors of RFA were not collected historically.
In conclusion, based on the PRS analysis, we recommend that recurrence within 12 months should be defined as early recurrence in HCC patients after RFA. Patients in early recurrence cohort were more likely to develop IDR alone or IDR + ER. Multiple tumors, AFP levels, ALB levels, and γ-GT levels were associated with early recurrence after RFA. HCC patients with these risk factors should be provided frequent strict surveillance for early detection of the recurrence. Early detection of recurrence will be helpful in providing timely treatment and improve prognosis.
Disclosure statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462.
- Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758.
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68:783–797.
- Fujiwara N, Friedman SL, Goossens N, et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–549.
- Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2020;22:677–689.
- Wei T, Zhang XF, Bagante F, et al. Early versus late recurrence of hepatocellular carcinoma after surgical resection based on post-recurrence survival: an international multi-institutional analysis. J Gastrointest Surg. 2020.
- Tsilimigras DI, Bagante F, Moris D, et al. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the barcelona clinic liver cancer criteria. Ann Surg Oncol. 2020;27:2321–2331.
- Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235.
- Xu X-F, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154:209–217.
- Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17:422–427.
- Zhou L, Rui JA, Wang SB, et al. Early recurrence in large hepatocellular carcinoma after curative hepatic resection: prognostic significance and risk factors. Hepatogastroenterology. 2014;61:2035–2041.
- Groot VP, Gemenetzis G, Blair AB, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. 2019;269:1154–1162.
- Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16:792–794.
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207.
- Liver EAFTSOT. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Chen Y, Zhao C, Yang Y, et al. Using the Controlling Nutritional Status (CONUT) score for evaluating patients with early-stage hepatocellular carcinoma after radiofrequency ablation: a two-center retrospective study. Cardiovasc Intervent Radiol. 2020;43:1294–1304.
- Yang H, Yang Y, Dou J, et al. Cholecystectomy is associated with higher risk of recurrence after microwave ablation of hepatocellular carcinoma: a propensity score matching analysis. Cancer Biol Med. 2020;17:478–491.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460.
- Chinese Society of Hepatology CMA. Chinese guidelines on the management of liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2019;27:846–865.
- Xu Y, Shen Q, Liu P, et al. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol. 2017;27:3877–3887.
- Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30:2463–2472.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Lin CC, Cheng YT, Chen MW, et al. The effectiveness of multiple electrode radiofrequency ablation in patients with hepatocellular carcinoma with lesions more than 3 cm in size and barcelona clinic liver cancer stage A to B2. Liver Cancer. 2016;5:8–20.
- Ju JX, Zeng QJ, Xu EJ, et al. Intraprocedural contrast-enhanced ultrasound-CT/MR fusion imaging assessment in HCC thermal ablation to reduce local tumor progression: compared with routine contrast-enhanced ultrasound. Int J Hyperthermia. 2019;36:785–793.
- Xie L, Cao F, Qi H, et al. Efficacy and safety of CT-guided percutaneous thermal ablation for hepatocellular carcinoma adjacent to the second porta hepatis. Int J Hyperthermia. 2019;36:1122–1128.
- Zhang L, Ge NL, Chen Y, et al. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol. 2015;32:77.
- Ma YJ, Du LY, Yan LB, et al. Long-term follow-up of HCV patients with sustained virological response after treatment with pegylated interferon plus ribavirin. HBPD Int. 2020.
- Nishikawa H, Inuzuka T, Takeda H, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: a proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol. 2011;46:1418–1426.
- Okuwaki Y, Nakazawa T, Kokubu S, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2747–2753.
- Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43:71–78.
- Maruyama H, Takahashi M, Shimada T, et al. Pretreatment microbubble-induced enhancement in hepatocellular carcinoma predicts intrahepatic distant recurrence after radiofrequency ablation. AJR Am J Roentgenol. 2013;200:570–577.
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202.
- Hayashi M, Shimizu T, Hirokawa F, et al. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am Surg. 2011;77:572–578.
- Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283.
- Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507.
- Pecchi A, Besutti G, De Santis M, et al. Post-transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. WJH. 20145;77:276–284.
- Schmidt C, Marsh JW. Molecular signature for HCC: role in predicting outcomes after liver transplant and selection for potential adjuvant treatment. Curr Opin Organ Transplant. 2010;15:277–282.
- Yang Y, Si T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Biol Med. 2018;15:299–310.