Abstract
Objective
Albumin-to-alkaline phosphatase ratio (AAPR), a newly developed blood biomarker, has been reported to have prognostic value in several types of cancer. This study aimed to investigate the predictive value of AAPR in patients with early-stage hepatocellular carcinoma (HCC) undergoing radiofrequency ablation (RFA) as initial therapy.
Methods
This retrospective study analyzed 445 patients with newly diagnosed HCC undergoing RFA as initial therapy. A series of survival analyses were performed to evaluate the prognostic value of AAPR. Univariate and multivariate analyses were performed to identify independent prognostic factors. An AAPR-based nomogram was constructed, and its predictive performance was validated.
Results
Patients with a low AAPR had a significantly reduced recurrence-free survival (RFS) and overall survival (OS) compared with those with a high AAPR. AAPR was found to be an independent prognostic indicator and showed superior discrimination efficacy than other liver function indices. The AAPR-based nomogram had a concordance index value of 0.72 (95% confidence interval [CI]: 0.65–0.79) in the training cohort and 0.72 (95% CI: 0.63–0.81) in the validation cohort, which significantly outperformed other existing staging systems.
Conclusions
AAPR serves as a promising indicator of prognosis in patients with early-stage HCC undergoing RFA. The AAPR-based nomogram might contribute to individualized prognosis prediction and clinical decision making.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death and the sixth most common cancer worldwide [Citation1,Citation2]. The treatment of HCC patients remains a multidimensional issue, which requires consideration of tumor characteristics, liver function, and physical status. For patients with early-stage HCC, surgical treatments including hepatic resection (HR) and liver transplantation are preferred curative options [Citation3]. However, liver transplantation is not a feasible treatment option for the majority of patients due to the shortage of donor organs, whereas HR is not often suitable for patients with severe cirrhosis and poor liver function. Radiofrequency ablation (RFA) yields clinical outcomes comparable to those of HR in early-stage HCC [Citation4,Citation5], and thus, RFA has been recognized as a minimally invasive therapy for patients with small HCCs and compromised liver function.
In recent studies, several clinical and biochemical factors have been proposed as prognostic indicators for survival after RFA, such as serum alfa-fetoprotein (AFP), γ-glutamyl transpeptidase (γ-GT), albumin (ALB), alkaline phosphatase (AKP), tumor size, and tumor number [Citation6,Citation7]. However, none of these factors have satisfactory prediction power. Hence, an accurate and reliable biomarker must be determined to identify RFA patients with a higher risk for tumor recurrence and unfavorable prognosis.
Albumin-to-alkaline phosphatase ratio (AARP), which is calculated by dividing the serum ALB level by the serum AKP level, is considered a novel prognostic index in several malignancies including HCC [Citation8–10], cholangiocarcinoma [Citation11], pancreatic ductal adenocarcinoma [Citation12], and urothelial carcinoma [Citation13]. As major indices of liver function, ALB reflects nutritional status and liver synthetic function, while AKP is an indicator of liver tissue damage. Investigators have revealed that AAPR not only reflects liver function reserve but is also correlated with tumor cell proliferation and inflammation reactions [Citation14]. However, no study has explored the predictive value of AAPR in patients with early-stage HCC undergoing RFA.
In the present study, we aimed to investigate the prognostic value of pretreatment AAPR in patients with early-stage HCC undergoing RFA as initial therapy and to develop an AAPR-based nomogram to identify patients with an unfavorable prognosis.
Methods
Patients
We retrospectively reviewed 445 patients with newly diagnosed HCC undergoing RFA as initial therapy in the Liver Cancer Institute at Zhongshan Hospital between March 2007 and June 2016. The inclusion and exclusion criteria are listed in . Patients were randomly divided into the training cohort (n = 297) and validation cohort (n = 148) in a 2:1 ratio.
Table 1. Criteria for patient selection.
Patients’ demographic and clinicopathological data were obtained: age, sex, etiology, presence of liver cirrhosis, pretreatment laboratory assessments, and tumor-related characteristics. Haematological and biochemical parameters were examined 3 days before RFA was performed, including alanine aminotransferase (ALT, U/L), ALB (g/L), total bilirubin (TB, μmol/L), AKP (U/L), γ-GT (U/L), and AFP (ng/mL), and routine blood tests were conducted. AARP was calculated by the dividing the serum ALB (g/L) level by the serum AKP level (U/L). This study was a retrospective study and approved by the institutional review board. Informed consent was obtained from each patient for using their data in the study.
Treatment and follow-up
Details of the treatment procedure have been described in a previous study [Citation15]. Generally, RFA is performed using the Cool-tip system (Valleylab, Boulder, CO, USA) or RITA system (RITA Medical Systems Inc., Mountain View, CA). The procedures were performed percutaneously with the use of real-time ultrasound under local anesthesia. Repeat ablations were performed to cover the entire tumor to obtain a sufficiently safe margin of 0.5–1 cm.
Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) were performed 4 weeks after RFA to evaluate the effectiveness of ablation therapy. The treatment was considered successful if imaging showed the absence of contrast enhancement or abnormal wash-out within or around the ablation zone [Citation16].
All patients underwent follow-up in the outpatient clinic 1 month after the initial RFA and thereafter, underwent follow-up every 2–3 months. Physical examinations, routine blood tests, liver function tests, tumor maker tests, and abdominal ultrasound were performed. Contrast-enhanced CT and/or MRI were performed in patients whose test results suggested tumor recurrence.
Statistical analysis
Quantitative variables were expressed as means ± standard deviations and compared using the Student’s t test and Mann–Whitney U test, while categorical variables were expressed as counts with percentages and compared using the χ2 and Fisher’s exact test. Recurrence-free survival (RFS) was defined as the duration from the date of the initial RFA to the first recording of recurrence or death. Overall survival (OS) was defined as the duration from the date of the initial RFA until death or the last follow-up.
The optimal cutoff value of AAPR was determined using X-tile version 3.6.1 (Yale University, New Haven, CT, USA) [Citation17] in relation to OS. Consequently, the patients were dichotomized into either the low-AAPR group or high-AAPR group. Survival curves were estimated using the Kaplan–Meier survival function and compared using a log-rank test. Univariate analysis was performed to identify the significant factors associated with HCC prognosis. All variables that had a p value of <0.1 in the univariate analysis were further fitted into the multivariate Cox proportional hazards regression model. The concordance index (C-index) values, receiver operating characteristic (ROC) curves, and likelihood ratio test χ2 (LAT χ2) were used to assess the abilities of different liver function indices in predicting survival outcomes. A nomogram was developed using β coefficients from the Cox regression model to determine the proportional prognostic effects of the enrolled variables. Calibration plots with 1,000 sets of bootstrap resamples were drawn to estimate the predictive efficacy of the model. In addition, C-index values, areas under the ROC curve (AUCs), Akaike information criteria (AIC), and Bayesian information criteria (BIC) were employed to compare the discrimination efficacy of the current nomogram with those of the existing staging systems, including the 8th edition of the American Joint Committee on Cancer TNM staging system (AJCC TNM-8) [Citation18], the Cancer of the Liver Italian Program (CLIP) score [Citation19], the Okuda staging system [Citation20], the Japan Integrated Staging (JIS) score [Citation21], the Italian Liver Cancer tumor staging and integrated prognostic staging system (the ITA.LI.CA staging system) [Citation22], and the Chinese University Prognostic Index (CUPI) [Citation23].
All statistical analyses were performed under the supervision of a senior statistician (Li-Hong Huang) using Stata version 15.1 (StataCorp, College station, TX) and R version 3.5.1. Differences were considered significant when the two-tailed p value was <0.05.
Results
Patient’s characteristics
The baseline characteristics of the training and validation cohorts are presented in . The training cohort consisted of 221 men (74.4%) and 76 women (25.6%), with a mean age of 58.7 ± 10.9 years. In the validation cohort, 233 patients (78.5%) had hepatitis B virus (HBV) infection, while 12 (4.0%) had hepatitis C virus (HCV) infection. Liver cirrhosis was detected in 184 patients (62.0%). Only 2 patients (0.7%) exhibited Child-Pugh (CP) grade B, and 72 (24.2%), 199 (67.0%), and 26 (8.8%) patients had an albumin-bilirubin (ALBI) grade of 1, 2, and 3, respectively. The mean levels of ALB and AKP were 38.8 ± 5.1 g/dL and 98.2 ± 52.3 U/L, respectively. Regarding tumor characteristics, 65 patients (21.9%) presented with tumors larger than 3 cm, while 47 (15.8%) presented with multiple tumors (less than 3 nodules). A total of 120 (40.4%), 119 (40.1%), and 58 (19.5%) patients had AJCC TNM stage Ia, Ib, and II disease, respectively. All baseline characteristics were comparable between the two cohorts (p > 0.05, ).
Table 2. Baseline characteristics of patients in the training and validation cohorts.
Associations between pretreatment AAPR and clinicopathological characteristics
In the training cohort, an AAPR of 0.40 was assigned as the optimal cutoff value using the X-tile statistical software (Supplementary Figure 1). Following this, 180 patients (60.6%) were included in the high-AAPR group (AAPR > 0.40), while the remaining 117 (39.4%) were included in the low-AAPR group (AAPR ≤ 0.40). The association between AAPR and other clinicopathological characteristics are presented in . No differences were observed between the two groups in age, sex, etiology, ALT levels, TB levels, the CP grade, AFP levels, tumor size, tumor number, and TNM stage. Those in the low-AAPR group had significantly higher γ-GT levels and ALBI grades (both p < 0.001) and a lower incidence of liver cirrhosis (p = 0.001) than patients in the high-AAPR group.
Table 3. Associations between AAPR and other characteristics.
Survival
The median follow-up duration was 28.5 months (range: 1–144). At the last follow-up, 90 patients (20.2%) had died while 315 (70.8%) had developed tumor recurrence. In the training cohort, the median RFS was 25 months (95% CI: 21–29.5); the 1-, 3-, and 5-year RFS rates were 68.6%, 36.2%, and 20.4%, respectively. The median OS was 132 months (95% CI: 90–NA); the 1-, 3-, and 5-year OS rates were 96.2%, 79.5, and 69.0%, respectively. No significant survival differences were found between the training and validation cohorts (p = 0.482 for RFS and p = 0.487 for OS).
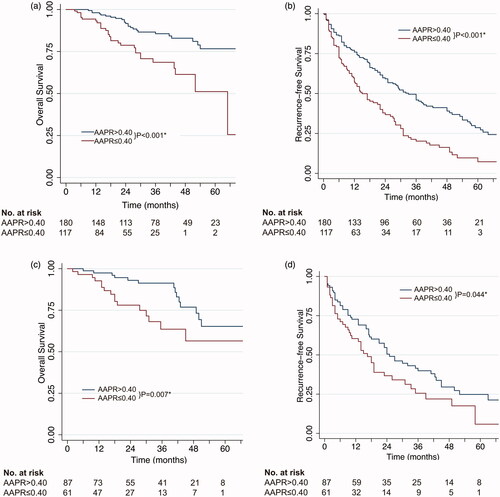
The survival curves of patients classified by AARP values are presented in ; the low-AARP group had a significantly shorter RFS and OS than the high-AARP group. In the training cohort, the 1-, 3-, and 5-year RFS rates of patients with a low AAPR were 57.2%, 20.2%, and 7.3%, while those of patients with a high AAPR were 76.0%, 46.0%, and 28.6%, respectively. In addition, the 1-, 3-, and 5-year OS rates of patients with a low AAPR were 93.2%, 68.6%, and 51.1%, while those of patients with a high AAPR were 98.1%, 85.6%, and 76.7%, respectively (). These findings were reproduced in the validation cohort ().
Figure 1. Kaplan–Meier survival estimates between subgroups classified by AAPR. (a) Overall survival according to AAPR values in the training cohort; (b) recurrence-free survival according to AAPR values in the training cohort; (c) overall survival according to AAPR values in the validation cohort; (d) recurrence-free survival according to AAPR values in the validation cohort. *Statistically significant.
Univariate and multivariate analyses
In the training cohort, the univariate analysis suggested the age, ALT levels, AAPR, the CP grade, the ALBI grade, γ-GT levels, and tumor size were associated with OS. Further multivariate analyses confirmed that age, AARP, γ-GT levels, and tumor size were independent prognostic indicators (). In the validation cohort, the univariate and multivariate analyses demonstrated that AARP and γ-GT levels were strong indicators of OS ().
Table 4. Univariate and multivariate analyses of overall survival in the training and validation cohorts.
Discriminatory powers of liver function-related indices
The discriminatory capacities of AAPR, the ALBI grade, and the CP grade were compared using the C-index, 3- and 5-year AUCs, and LAT χ2 values (, ). In the training cohort, the C-index values of AAPR, the ALBI grade, and the CP grade were 0.64 (95% CI: 0.57–0.71), 0.58 (95% CI: 0.52–0.64), and 0.51 (95% CI: 0.49–0.53), respectively, whereas in the validation cohort, the corresponding C-index values were 0.65 (95% CI: 0.56–0.74), 0.62 (95% CI: 0.56–0.70), and 0.50 (95% CI: 0.49–0.51), respectively. Moreover, the 3- and 5-year AUCs and LAT χ2 values of different criteria also indicated that AAPR may have a better discrimination efficacy than the ALBI grade and CP grade in early-stage HCC undergoing RFA as initial therapy ().
Figure 2. ROC curves showing the discriminatory power of AAPR, the ALBI grade, and the CP grade. (a–b) ROC curves for predicting the 3- and 5-year OS in the training cohort; (c–d) ROC curves for predicting the 3- and 5-year OS in the validation cohort.
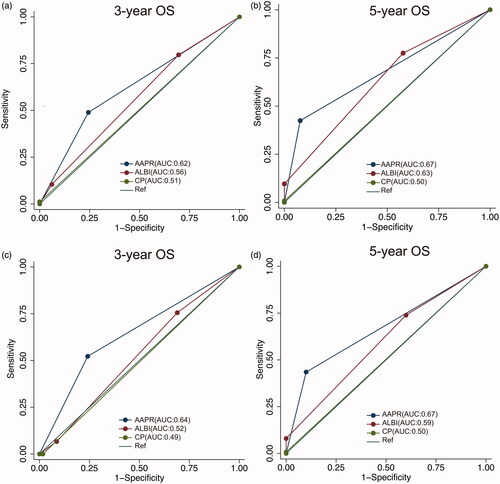
Table 5. Comparison of predictive efficacy among different liver function-related indices.
Construction and validation of the AAPR-based nomogram
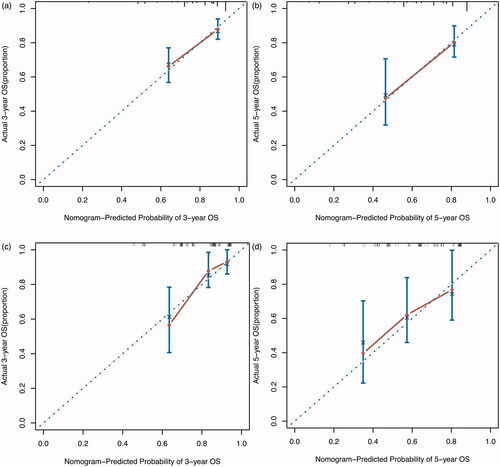
A nomogram was built based on AAPR and other significant prognostic factors identified by the multivariate Cox proportional hazard model. Each enrolled variable in the nomogram was assigned a score (top scale). The projections from the total points (range 0–400) are shown on the bottom scale in and indicated the estimated survival probabilities at 3 and 5 years. The calibration curves of the newly developed nomogram were well-matched with the idealized 45° lines in both the training and validation cohorts ().
Figure 3. The AAPR-based nomogram predicting the 3- and 5-year survival probabilities of early-stage HCC after radiofrequency ablation.
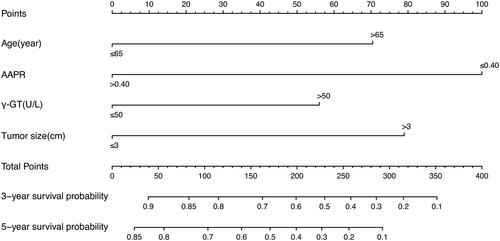
Figure 4. Calibration curves for predicting the 3- and 5-year OS of early-stage HCC patients after radiofrequency ablation. (a,b) Calibration curves for predicting the 3- and 5-year OS in the training cohort; (c,d) calibration curves for predicting the 3- and 5-year OS in the validation cohort.
Furthermore, the discriminatory capacity of the AAPR-based nomogram was assessed in comparison with those of existing staging systems including the AJCC TNM-8 system, the CLIP score, the Okuda staging system, the JIS score, the ITA.LI.CA staging system, and the CUPI system (). The C-index values of the AAPR-based nomogram were 0.72 (95% CI: 0.65–0.79) in the training cohort and 0.73 (95% CI: 0.63–0.83) in the validation cohort, respectively. Thus, this nomogram significantly outperformed the existing staging systems. Moreover, the newly developed nomogram also had the largest AUCs and the lowest AIC and BIC values in both the training and validation cohorts. All these results suggest that the AAPR-based nomogram may serve as an effective tool for individualized prognosis prediction in HCC patients undergoing RFA as initial therapy.
Table 6. Comparison of discrimination efficacy between the newly developed nomogram and other staging systems.
Discussion
In the present study, we investigated the prognostic significance of AAPR in HCC patients undergoing RFA. The results indicated that AAPR is an independent prognostic predictor for HCC. More importantly, the AAPR-based nomogram displayed a discrimination power superior to that of the widely used AJCC TNM-8 system in predicting the prognosis of HCC patients undergoing RFA; thus, this nomogram may help in individualized clinical decision making.
Clinically, liver function reserve is a critical concern in predicting the prognosis of HCC [Citation24]. Risk stratification of HCC patients before locoregional treatment is paramount owing to the varying severity of underlying liver disease. In the last decade, the CP grade has been a commonly used measurement of liver function reserve in HCC patients [Citation24]. However, several studies have indicated that the discriminatory ability of the CP grade in early-stage HCC patients was unsatisfactory [Citation25,Citation26]. Indeed, the majority of our HCC patients presented with compensated liver cirrhosis, and only 3 patients (0.7%) exhibited CP grade B disease. Thereby, the CP grade failed to offer satisfactory discrimination in our cohort. In contrast, AAPR exhibited superior discrimination efficacy than the CP grade, indicating its prognostic prediction value in early-stage HCC following RFA.
AAPR is calculated based on two liver function parameters: AKP and ALB; both of these have been found to be correlated with malignant biological behaviors in HCC [Citation27–30]. Investigators have observed that in liver cancer cell lines such as Hep-G2, high levels of AKPase reaction products in the nucleolus are related to cancer cell proliferation [Citation27]. Moreover, AKP is also found to be correlated with epithelial mesenchymal transition phenotypes in HCC; epithelial mesenchymal transition is considered to be a major step in tumor invasion or metastasis [Citation28,Citation29]. Furthermore, ALB is conventionally considered as an indicator of nutritional status, and low levels of ALB indicate a dysfunction of protein synthesis in the liver [Citation31], which may eventually impair immunity. In addition, emerging evidence has revealed that ALB can stabilize cell growth, exert antioxidant effects against carcinogens, and suppress tumor cell proliferation by reducing the phosphorylation of Rb proteins [Citation30]. Based on these data, we speculated that patients with a low AAPR might harbor more aggressive HCC tumors and thus, have an increased risk of tumor relapse and a poor prognosis.
In our series, a low AAPR was significantly correlated with higher levels of γ-GT. In addition, γ-GT was identified as an important prognostic determinant in the newly developed nomogram. Mechanistically, γ-GT is a transferase mainly distributed in the cytoplasm of hepatocytes and the intrahepatic bile duct epithelium of the liver. Increasing evidence has demonstrated that γ-GT is not only a basic parameter indicating liver function but also an important tumor biomarker linked to tumor formation, tumor cell proliferation, and apoptosis [Citation14]. Therefore, the correlation between AAPR and γ-GT levels supports our finding that AAPR is a composite indicator of both liver function reserve and malignant biological phenotype in HCC.
The low-AAPR group showed high ALBI grades but had a low prevalence of liver cirrhosis. In HCC, the ALBI grade is superior to the CP grade in terms of assessing liver function reserve [Citation26]. In addition, recent studies have demonstrated that the ALBI grade is associated with higher levels of AFP and more advanced tumor stage, which is correlated with malignant biological behavior and poor prognosis [Citation32], similar to the association of AAPR in HCC. Moreover, HCC patients without cirrhosis, in whom HCC is presumably attributed to the direct carcinogenic effect of HBV infection, tend to harbor larger-sized or poorly differentiated tumors, contributing to the unfavorable prognosis of early-stage HCC [Citation33,Citation34]. In our series, since the presence of liver cirrhosis or the CP grade was not associated with the prognosis of early-stage HCC, we speculated that patients in the low-AAPR group might exhibit more aggressive tumor behaviors, indicated by AAPR or the ALBI grade, due to the direct development of HCC in the absence of liver cirrhosis. However, this speculation warrants further validation, and the underlying mechanism should be explored in future biological studies.
The present study had several limitations: 1) as a retrospective research study, potential selection bias was inevitable; 2) this study was conducted among a large Asian cohort, and 79.6% of the patients tested positive for HBV infection. Therefore, the findings cannot be directly applied to patients from the United States and Europe before external validation, since HCV infection and alcohol abuse are predominant causes of HCC in these geographic areas; 3) the cutoff values of AAPR vary between studies, and no consensus has been proposed; thereby, the present cutoff value of AAPR, derived using the X-tile software, warrants further validation; and 4) given that elevated levels of AKP could be observed in several malignancies in addition to HCC, AAPR should be used in HCC patients without concomitant tumors.
Conclusions
In conclusion, the present study demonstrates that pretreatment AAPR can serve as an excellent predictor of both RFS and OS in patients with early-stage HCC undergoing RFA as initial therapy. The developed AAPR-based nomogram may help in individualized prediction of clinical outcomes and facilitate further clinical decision making. However, large-scale prospective studies and external validation are warranted to confirm our findings.
Supplemental Material
Download PDF (823.8 KB)Acknowledgments
The authors gratefully acknowledge the statistical guidance of Li-Hong Huang in conducting this research.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
Due to the nature of this research, the participants of this study did not agree to have their data shared publicly; hence, supporting data are not available.
Additional information
Funding
References
- Benson AB, D'Angelica MI, Abbott DE, et al. Nccn guidelines (r) insights hepatobiliary cancers, version 1.2017 featured updates to the nccn guidelines. J Natl Compr Canc Netw. 2017;15:563–573.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology. 2018;67:381–400.
- Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287:461–472.
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–1784.
- Kim CG, Lee HW, Choi HJ, et al. Development and validation of a prognostic model for patients with hepatocellular carcinoma undergoing radiofrequency ablation. Cancer Med. 2019;8:5023–5032.
- Allaire M, Rekik S, Layese R, et al. Virologic control and severity of liver disease determine survival after radiofrequency ablation of hepatocellular carcinoma on cirrhosis. Dig Liver Dis. 2019;51:86–94.
- Cai XR, Chen ZH, Chen J, et al. Albumin-to-alkaline phosphatase ratio as an independent prognostic factor for overall survival of advanced hepatocellular carcinoma patients without receiving standard anti-cancer therapies. J Cancer. 2018;9:189–197.
- Chen ZH, Zhang XP, Cai XR, et al. The predictive value of albumin-to-alkaline phosphatase ratio for overall survival of hepatocellular carcinoma patients treated with trans-catheter arterial chemoembolization therapy. J Cancer. 2018;9:3467–3478.
- Chan AWH, Chan SL, Mo FKF, et al. Albumin-to-alkaline phosphatase ratio: A novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057–564010.
- Xiong JP, Long JY, Xu WY, et al. Albumin-to-alkaline phosphatase ratio: A novel prognostic index of overall survival in cholangiocarcinoma patients after surgery. WJGO. 2019;11:39–47.
- Pu N, Gao SS, Xu YD, et al. Alkaline phosphatase-to-albumin ratio as a prognostic indicator in pancreatic ductal adenocarcinoma after curative resection. J Cancer. 2017;8:3362–3370.
- Tan P, Xie N, Ai JZ, et al. The prognostic significance of albumin-to-alkaline phosphatase ratio in upper tract urothelial carcinoma. Sci Rep. 2018;8:12311.
- Wu SJ, Lin YX, Ye H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36:143–151.
- Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumour progression and prolongs overall survival in patients with intermediate (bclc b) hepatocellular carcinoma. Bmc Cancer. 2014;14:849.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update . Radiology. 2014;273:241–260.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259.
- Chun YS, Pawlik TM, Vauthey JN. 8th edition of the ajcc cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–847.
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: The cancer of the liver italian program (clip) investigators. Hepatology (Baltimore, Md.). 1998;28:751–755.
- Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928.
- Kudo M, Chung HB, Osaki Y. Prognostic staging system for hepatocellular carcinoma (clip score): Its value and limitations, and a proposal for a new staging system, the japan integrated staging score (jis score). J Gastroenterol. 2003;38:207–215.
- Farinati F, Vitale A, Spolverato G, et al.; ITA.LI.CA study group. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLoS Med. 2016;13:e1002006.
- Leung TWT, Tang AMY, Zee B, et al. Construction of the chinese university prognostic index for hepatocellular carcinoma and comparison with the tnm staging system, the okuda staging system, and the cancer of the liver italian program staging system - a study based on 926 patients. Cancer. 2002;94:1760–1769.
- European Assoc Study L. Easl clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- D'Amico G, Morabito A, D'Amico M, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Inter. 2018;12:S34–S43.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the albi grade. J Clin Oncol. 2015;33:550–545. U
- Yamamoto K, Awogi T, Okuyama K, et al. Nuclear localization of alkaline phosphatase in cultured human cancer cells. Med Electron Microsc. 2003;36:47–51.
- Iwadate Y, Matsutani T, Hirono S, et al. Transforming growth factor-β and stem cell markers are highly expressed around necrotic areas in glioblastoma . J Neurooncol. 2016;129:101–107.
- Yi JK, Mehrazarin S, Oh JE, et al. Osteo-/odontogenic differentiation of induced mesenchymal stem cells generated through epithelial-mesenchyme transition of cultured human keratinocytes. J Endod. 2014;40:1796–1801.
- Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407.
- Wang L, Li Q, Zhang J, et al. A novel prognostic scoring model based on albumin and γ-Glutamyltransferase for Hepatocellular Carcinoma Prognosis . Cancer Manag Res. 2019;11:10685–10694.
- Pinato DJ, Kaneko T, Saeed A, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: Adjunctive role of the albi grade. Cancers. 2020;12:1862.
- Xiang X, You XM, Zhong JH, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis b virus infection. J Hepatol. 2017;67:885–886.
- Ince V, Akbulut S, Otan E, et al. Liver transplantation for hepatocellular carcinoma: Malatya experience and proposals for expanded criteria. J Gastrointest Cancer. 2020;51:998–1005.
