Abstract
Objectives
To investigate the feasibility and efficacy of liver-specific magnetic resonance imaging (MRI) with gadolinium-containing contrast agent guidance for microwave ablation (MWA) of recurrent small hepatocellular carcinoma (HCC).
Materials and methods
The Ethics Committee of the First Affiliated Hospital of Fujian Medical University approved this study. Eighteen patients presented with 30 recurrent small HCCs, at least one lesion per patient was undetectable on unenhanced MRI, but this was clearly demonstrated in the hepatobiliary phase after liver-specific MRI contrast agent administration. Gd-BOPTA (16 cases) or Gd-EOB-DTPA (2 cases) were injected half an hour before the procedure, and MWA was performed by percutaneous puncture of the target lesion with a magnetic resonance-compatible microwave antenna under 1.5 T MRI guidance.
Results
The technical success rate was 100%. The mean maximum diameter of the lesions was 9.7 ± 2.8 mm (5.0–15.4 mm). The mean follow-up time was 11.6 ± 4.7 months (range, 4–19 months), and no local recurrence was observed.
Conclusions
MWA of small HCCs guided by enhanced liver-specific MRI contrast agent is a safe and effective technique.
Introduction
Image-guided thermal ablation of small hepatocellular carcinoma (HCC) (2 cm or smaller) has a similar efficacy to surgical treatment [Citation1]. Microwave ablation (MWA) is an optimal thermal ablation treatment because of its rapid temperature rise, large ablation range, and minor influence by heat sink effect [Citation2–4]. CT and US are the most used guiding methods, but there are many limitations in lesion display and efficacy evaluation [Citation5,Citation6]. Magnetic resonance imaging (MRI) has the advantages of using no ionizing radiation, high resolution of soft tissue, imaging using any orientation, sensitivity to temperature changes, and accurate evaluation of efficacy. Therefore, it is an excellent guiding method for liver tumor MWA [Citation7].
Whether using CT, US, or MRI for displaying the lesions of small HCC, contrast-enhanced examination can improve the detection rate. However, the conventional contrast agent enhancement scan only has a short window of time for detecting the lesion. Moreover, because of the limited amount of contrast agent applied, it cannot be reused within a short period of time. Thus, the need to guide MWA cannot always be successful.
The sensitivity of liver lesions displayed by MRI is significantly higher than that of CT and US, particularly liver-specific MRI gadolinium-containing contrast agents, which are taken up by the hepatocytes and excreted by the bile. The hepatobiliary phase clearly shows the lesion for up to 2 h and provides a sufficient time window for guiding MWA [Citation8]. We conducted MWA guided by an enhanced liver-specific MRI gadolinium-containing contrast agent in 18 patients with recurrent small HCC from August 2017 to September 2019 to evaluate its feasibility and short-term therapeutic efficacy.
Materials and methods
Patients
The Ethics Committee of the First Affiliated Hospital of Fujian Medical University approved this study, and all patients provided written informed consent. Our study included 18 patients (17 men and 1 woman) with recurrent small HCC, including a total of 30 lesions, four patients with two lesions, and four patients with three lesions. The average age of patients was 60 ± 12.7 (35–80) years. Prior to MRI-guided MWA, 8 patients underwent hepatectomy, and 10 patients underwent ablation (7 MWA and 3 RFA, the mean recurrent time was 7.2 ± 3.4 months). All patients were diagnosed with HCC based on the image, the diagnostic criteria consistent with ‘Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition)’ [Citation9].
Inclusion criteria included lesions <2 cm and total number of lesions ≤3, liver function of Child A or B, no extrahepatic metastasis, and a > 50,000/ul platelet count. All patients underwent liver-specific MRI gadolinium-containing contrast agent enhancement examination. At least one lesion per patient was undetectable on unenhanced MRI. However, it was hyperintense at the arterial phase and hypointense in the delayed phase and the hepatobiliary phase.
Exclusion criteria included lesions ≥2 cm, patients with >3 lesions, extrahepatic metastases, Child C liver function, uncorrectable coagulopathy, hyperbilirubinemia, claustrophobia, and contraindications for MRI in patients with cardiac pacemakers or metal implants. The general condition of the patient is shown in .
Table 1. Patient Characteristics.
Equipment
Magnetic resonance-compatible microwave therapy equipment (2450 MHz, MTC-3C, Vision Medical, Nanjing, China), a microwave generator, and a connecting cable (2.5 m) were modified by MRI shielding, placed in a scanning room, and worked in a 1.5 T MRI environment without disturbing the imaging.
The MRI-compatible microwave antenna (MTC-3CA-II, 2.0 mm, 15 cm) consisted of a copper alloy and ceramics with a cool tip.
1.5T MRI scanner (Signa Infinity TwinSpeed with Excite, GE, Milwaukee, WI, USA) consisted of 8-channel cardiac coils with operating holes. The scan sequences and parameters were as follows:
Fat-suppressed fast-recovery fast spin echo (fs FRFSE) T2WI with repetition time (TR) of 8500.0 ms, echo time (TE) of 85.0 ms, 90° flip angle (FA), 17 echo train length (ETL), 5.0 mm slice thickness (ST), 1.0 mm GAP, 280 × 380 field of view (FOV), 1 excitation (NEX), and 70–90 s scanning time (T).
Three-dimensional dynamic T1 weighted imaging (3D Dyn T1WI) with 4.8 ms TR, 1.1 ms TE, 45° FA, 1 ETL, 3.0 mm ST, 280 × 380 FOV, 1 NEX, and 12 s T.
Oblique T1WI with 160 ms TR, 3.3 ms TE, 45° FA, 1 ETL, 5.0 mm ST, 330 × 430 FOV, 1 NEX, and 18 s T.
The fs FRFSE T2WI sequence used respiratory gating-controlled scanning, and the 3D Dyn T1WI and Oblique T1WI sequences used breath-holding scanning.
Operative method
Preoperative preparation
During the 2 weeks before the procedure, MRI liver-specific gadolinium-containing contrast agent enhancement examination was performed. In addition to conventional dynamic enhancement scan, images of the hepatobiliary phase were collected to confirm the lesion location, size, and number.
A liver-specific MRI gadolinium-containing contrast agent was injected intravenously 30 min before the procedure. Gadobenate dimeglumine (Gd-BOPTA, Multihance, Bracco, Milan, Italy) at 0.1 mmol/kg body weight was used in 16 patients, and gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA, Primovist Bayer Schering Pharma, Berlin, Germany) at 0.025 mmol/kg body weight was used in two patients.
The patients were placed in an appropriate position according to optimal percutaneous lesion access. Blood pressure, heart rate, and breathing were monitored. A preoperative intramuscular injection of 10 mg of morphine hydrochloride was used for sedation.
Procedure
First, the fs FRFSE T2WI and 3D Dyn T1WI scans were performed, and the mark was used as the body surface marker to determine the puncture path (the puncture path was as short as possible and avoided important structures, such as large blood vessels and intestinal loops). Conventional disinfection, local anesthesia, drape, and sterile cover were used to wrap the cardiac coils. Under MRI guidance, the microwave antenna gradually penetrated the center of the tumor, with the tip 0.5–1 cm beyond the distal end of the tumor. The 3D Dyn T1WI and oblique T1WI were scanned several times during the puncture to ensure optimal position of the antenna. The oblique T1WI scanning direction was in the oblique coronal or oblique sagittal position parallel to the microwave antenna. When the antenna was thin and its position uncertain, 1 ml of contrast agent was injected into the water circulation pipeline to thicken the antenna artifact. After reaching the target point, it was replaced with normal saline, and the antenna artifact was restored to its original state. The cable and water pump were connected, the power and time were set, and the ablation was started. After completion of the ablation, the fs FRFSE T2WI and 3D Dyn T1WI sequences were scanned to evaluate the technical success rate (this term simply addresses whether the tumor was treated according to protocol and covered completely by the ablation zone) [Citation10]. The ablation zone completely covered the original lesion when it exceeded the edge of the lesion by 0.5–1 cm for complete ablation. Complementary ablation was performed in residual lesions immediately after the post-procedure scan. After complete ablation, antenna path ablation was performed and the antenna was withdrawn.
Follow-up
One month after MWA, MRI liver-specific gadolinium-containing contrast agent enhancement examination was used for review, and the technical efficacy was evaluated (technique efficacy should therefore refer to a prospectively defined time point at which point ‘complete ablation’ of macroscopic tumor, as evidenced by imaging follow-up, was achieved) [Citation10], followed by follow-up every 3 months.
Statistical processing
Statistical analyses were performed using SPSS version 22.0 (IBM, Chicago, IL, USA). Continuous variables were shown as mean ± standard deviation.
Results
Duration of MWA and complications
The puncture and ablation of lesions were successfully completed in all patients. All lesions were completely ablated, with a 100% technical success rate. The average ablation power was 81.7 ± 3.7 W (range, 80–90 W), and the average ablation time was 6.7 ± 1.7 min (range, 4–10 min). The average procedure time was 65.5 ± 22.6 min (range, 29–126 min). The 11th and 14th patient in received complementary ablation. There were no major complications, such as massive hemorrhage, biliary fistula, diaphragmatic perforation, or jaundice. After conventional treatment for improving liver function, all patients were discharged within 3 days.
Therapeutic effect
The average maximum diameter of the tumors was 9.7 ± 2.8 mm (5.0–15.4 mm); the maximum diameter of the ablated zone was 23.4 ± 3.1 mm (17.2–29.5 mm). The average follow-up duration was 11.6 ± 4.7 months (4–19 months). During follow-up, all patients were alive, and three patients with new HCC lesions were found. No local recurrence was found, and the rate of technique efficacy was 100%.
MRI findings
Pre-ablative imaging
Preoperative imaging: On unenhanced MRI, 23 lesions (at least one lesion per patient) was undetectable. Seven lesions showed slightly hypo-intensity on unenhanced T1WI and slightly hyper-intensity with unclear boundaries. Lesions in the arterial phase showed nodular-like enhancement and showed low or slightly lower signals in the portal or delayed phase. The lesions at the hepatobiliary phase showed significant hypointensity with clear boundaries. All patients showed normal biliary excretion of the hepatobiliary contrast agent. ( and ).
Figure 1. Male, 66 years old, postoperative hepatocellular carcinoma, recurrence after TACE and microwave ablation. Preoperative scan: S4 shows a nodule; TSE fs T2WI sequence (a) and fs-T1 Vibe (b) shows isointensity.
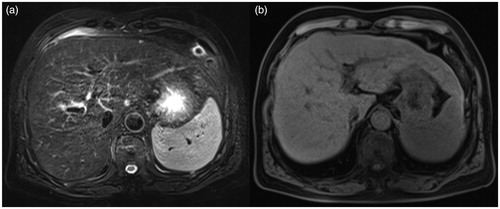
Figure 2. The enhanced scanning arterial phase (a) shows nodular enhancement (white arrow). In the delayed phase (b), the enhancement is weakened, and the low signal intensity (white arrow) is visible in the center of the lesion. The hepatobiliary phase (c) shows a hypointense lesion with a clear boundary (white arrow).
Microwave antenna
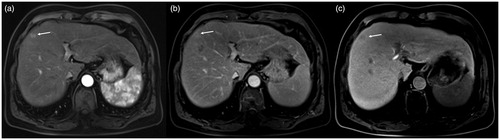
The microwave antenna showed as signal void on all sequences, with a diameter of approximately 1.9 mm. The antenna artifact was thickened after injecting the contrast agent into the water circulation pipeline, and the diameter was approximately 5.2 mm. The original size was restored after replacing it with normal saline ().
Figure 3. Intraoperative scan: The scanned image of the oblique sagittal position parallel to the long axis of the microwave antenna (a) and the microwave antenna shows a thin line signal void (white arrow). After injecting 1 ml of contrast agent into the water circulation pipeline, the artifact of the microwave antenna shows thickening (b).
Post-ablative imaging
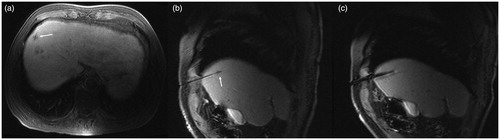
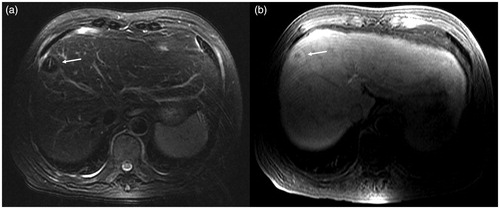
In the center of the ablation lesion, we observed the antenna path as a strip-shaped long T1 long T2 signal. The ablation lesions were spherical-like, with hypointensity on T2WI and thin layer hyperintensity on the edge. The signal of ablated lesions on T1WI was more complicated; according to the different background signals of the liver parenchyma at the hepatobiliary phase, it was slightly higher, equal, or slightly lower signal ().
Figure 4. Scan after ablation: TSE fs T2WI (a) shows hypointensity at the ablation lesion. The central microwave antenna needle shows a strip-like hyperintensity (white arrow), and a thin layer of higher signal ring is visible in the periphery of the ablation lesion. In the 3D Dyn T1WI sequence (b), the ablation lesions show a ‘target sign’. The central lesions are still hypointense, and the surrounding ablation area shows hyperintensity, but there is no obvious difference between the high signal intensity ablation lesion and the higher signal intensity of normal liver parenchyma in the hepatobiliary phase.
Discussion
The therapeutic effect of MWA on small HCC is excellent, but small HCCs (<2 cm), especially <1 cm lesions, are difficult to identify by imaging, and off-target phenomenon occurs during ablation. Yu et al. [Citation11] analyzed image data of 638 patients who underwent liver transplantation and found that in 225 patients with HCC, the sensitivity of the lesions showed by ultrasound, CT, and MRI was 46%, 65%, and 72%, respectively; if the size of the lesion was <2 cm, the sensitivity was significantly reduced to 21%, 40%, and 47%, respectively. A previous study showed that the sensitivity of Gd-EOB-DTPA dynamic enhanced MRI diagnosis of ≤1 cm small HCC was 46%, and the sensitivity of lesions >1 cm was 95% [Citation12]. Image-guided ablation generally uses an unenhanced scan; however, the sensitivity of small lesions is significantly reduced. The size of tumors in our study was <2 cm (including 16 lesions <1 cm) and could not be clearly displayed by conventional ultrasound, CT, or MRI unenhanced scans.
The liver-specific gadolinium-containing contrast agents are taken up by hepatocytes and excreted via the biliary tract. Therefore, after intravenous injection, in addition to the conventional arterial, portal vein, and delayed phases, there is a hepatobiliary phase, which provides useful information for the display and diagnosis of liver nodular lesions, especially small lesions, and for evaluating hepatocyte function [Citation13]. In the hepatobiliary phase, the HCC lesions demonstrated hypointensity with a clear boundary [Citation14]. Commonly used liver-specific gadolinium-containing contrast agents include Gd-BOPTA and Gd-EOB-DTPA. Gd-BOPTA is 2–4% metabolized by the liver, and the optimal scanning time of the hepatobiliary phase is 40–120 min after injecting the contrast agent; whereas Gd-EOB-DTPA is 50% metabolized by the liver, and the optimal scanning time of the hepatobiliary phase is 20–120 min after injecting the contrast agent [Citation8]. The average operation time of our group of patients was 65.5 min; the longest was 126 min, and all MWA procedures were performed with the lesions clearly displayed. Despite differences in uptake rates, the two contrast agents can effectively improve the diagnostic sensitivity of small HCC. A previous finding showed that in <2 cm HCC lesions, two groups of diagnostic physicians observed Gd-EOB-DTPA enhanced MRI hepatobiliary images and found that the sensitivity of the lesions significantly improved compared to that of the conventional dynamic enhanced MRI (74.6% and 83.1% vs. 57.7% and 66.2%, respectively) [Citation15]. Becker-Weidman DJ et al. retrospectively analyzed MRI Gd-BOPTA enhanced images in 35 patients with HCC from 101 patients who underwent liver transplantation. When compared with conventional contrast agent enhanced MRI control, the sensitivity increased from 77.8% to 91.4%, and the sensitivity of HCC <2 cm increased from 55.6% to 87.5% [Citation16]. In this study, 23 lesions were undetectable during the unenhanced scan; the lesions at the hepatobiliary phase had clear boundaries and could effectively guide MWA. Hoffmann et al. [Citation7] reported 15 cases of MRI-guided MWA treatment of HCC, two of which used liver-specific contrast agents.
When the artifact of the MRI-compatible microwave antenna is large, it is easy to cover small lesions. However, when the artifact is too small, the antenna is difficult to track. In this study, the contrast agent was injected into the water circulation pipeline, causing local magnetic field unevenness and expanding the artifacts, which helped easily identify the antennas during puncture. After reaching the lesion, the contrast agent was replaced with normal saline, which reduced the artifact, and the lesion was not covered.
Limitations
After the completion of MWA under the guidance of an unenhanced MRI, the ablation zone showed a characteristic target sign on T1WI, the central lesions still showed a low signal, and the normal liver tissue surrounded by ablation showed a high signal, which could visually show the relationship between the ablation zone and the lesion and accurately judge the therapeutic effect. The ablation zone on T2WI showed a low signal with clear boundaries, but the original lesion often could not be displayed. After injecting the liver-specific contrasting agent, the signal intensity increased after hepatocyte uptake of the contrasting agent on T1WI, and the normal liver tissue showed a high signal after ablation. Furthermore, the signal difference between the ablation zone and normal tissue was narrowed, the ablation boundary was unclear, and the advantage of using T1WI to evaluate the therapeutic effect of ablation was weakened. We could only observe the extent of the ablation lesion that showed a low signal on T2WI. Therefore, liver-specific gadolinium-containing contrast agent enhancement can only be used to guide the ablation of unclear lesions on unenhanced MRI, and conventional use is not recommended.
Because of the small lesions and stable ablation range, our study did not scan to monitor the ablation process. Our cohort of patients was small, and the follow-up time was relatively short. In future studies, we will increase the sample size and patients will be followed for a longer period of time to further explore clinical significance.
In conclusion, this study used liver-specific MRI gadolinium-containing contrast agent enhancement to guide recurrent small HCC MWA, with the following advantages: no ionizing radiation, clear lesions, accurate positioning, and accurate evaluation of efficacy; thereby, achieving good efficacy with few complications, proving to be a safe and effective technology.
Ethical approval
The study complied with the standards of the Declaration of the Helsinki and current ethical guidelines, and was approved by our institutional ethics board.
Informed consent
Patient consent was obtained.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Peng ZW, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033.
- Lucchina N, Tsetis D, Ierardi AM, et al. Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol. 2016;29:460–465.
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol. 2012;198:W46–W50.
- Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83:552–558.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34:863–869.
- Wang G, Sun Y, Cong L, et al. Artificial pleural effusion in percutaneous microwave ablation of hepatic tumors near the diaphragm under the guidance of ultrasound. Int J Clin Exp Med. 2015;8:16765–16771.
- Hoffmann R, Rempp H, Keßler DE, et al. MR-guided microwave ablation in hepatic tumours: initial results in clinical routine. Eur Radiol. 2017;27:1467–1476.
- Morana G, Salviato E, Guarise A. Contrast agents for hepatic MRI. Cancer Imaging. 2007;7:S24–S27.
- Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer. 2017;11:317–370.
- Ahmed M, Solbiati L, Brace CL, et al.; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update . Radiology. 2014;273:241–260.
- Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167.
- Yu MH, Kim JH, Yoon JH, et al. Small (≤1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014;271:748–760.
- Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–429.
- Jeong WK, Byun JH, Lee SS, et al. Gadobenate dimeglumine-enhanced liver MR imaging in cirrhotic patients: quantitative and qualitative comparison of 1-hour and 3-hour delayed images. J Magn Reson Imaging. 2011;33:889–897.
- Di Martino M, Anzidei M, Zaccagna F, et al. Qualitative analysis of small (≤2 cm) regenerative nodules, dysplastic nodules and well-differentiated HCCs with gadoxetic acid MRI . BMC Med Imaging. 2016;16:62.
- Becker-Weidman DJ, Kalb B, Sharma P, et al. Hepatocellular carcinoma lesion characterization: single-institution clinical performance review of multiphase gadolinium-enhanced MR imaging–comparison to prior same-center results after MR systems improvements. Radiology. 2011;261:824–833.
