Abstract
Introduction
Malignant peritoneal mesothelioma (MPM) is a lethal cancer, with approximately 2% of diagnoses occurring in patients less than 40 years of age. The purpose of this study is to report the only long-term follow up and survival of pediatric patients with MPM after multi-modality therapy including cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Methods
We retrospectively investigated a prospectively maintained database including patients <21 years old who underwent CRS and HIPEC from 1994 through 2014. Follow-up information was available through 2019 and is included in this report.
Results
Seven young patients underwent CRS and HIPEC. Final histology was epithelioid in all patients. Three patients had received neo-adjuvant systemic chemotherapy. At the time of the operation Peritoneal Cancer Index ranged from 6 to 25. Completeness of cytoreduction score after CRS was 0 in 4 patients, 1 in two patients, and 2 in one patient. Post-operative complications included acute kidney injury (n = 1), hyperbilirubinemia (n = 1), bilateral pleural effusions (n = 1) and pneumothorax requiring chest tube placement (n = 1). At last available follow-up, 71% of patients (n = 5) were alive with minimal or no evaluable disease. The remaining two patients had passed away from their disease at 14 and 26 months, respectively, following CRS and HIPEC. Overall survival ranged between 14 and 281 months.
Conclusion
Our surgical experience shows that CRS and HIPEC is a feasible and safe treatment option in pediatric patients, potentially improving overall survival.
Introduction
Malignant peritoneal mesothelioma (MPM) is a lethal cancer, with National Cancer Institute (NCI) review estimation of approximately 10% 5-year overall survival. This disease can affect both adults and children, who often present with nonspecific symptoms and are found to have advanced disease. The clinical features of abdominal distention, pain, and ascites often result in significant morbidity in these patients with an already established poor prognosis. Overall survival without treatment is <1 year [Citation1]. Collectively, response rates to systemic therapy, including pemetrexed and cisplatin, is about 30%, and result in a median overall survival of 6–11 months [Citation2–4]. Cytoreductive surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in patients with MPM has demonstrated promising clinical outcomes, with median survival range of 29–92 months [Citation1,Citation2,Citation5–7]. Unfortunately, limited information is available on the effects of CRS and HIPEC in young patients with MPM. The purpose of this study is to report long term follow up of young patients who underwent CRS and HIPEC with cisplatin and determine stability of treatment results.
Patients and methods
Patients and data acquisition
This study was an institutional review board (IRB) approved, retrospective investigation of a prospectively maintained database containing information on 109 patients with histologically proven MPM who underwent CRS and HIPEC at the NCI’s Surgery Branch between 1994 and 2011. Some results of this trial have been previously reported [Citation1,Citation8]. Because there is little data published for pediatric mesothelioma, we investigated long-term outcomes of seven patients in this cohort who were less than 21 years of age at the time of treatment and had available follow-up information through 2019. All patients underwent exploratory laparotomy, lysis of adhesions, cytoreduction, and HIPEC for 90 min with cisplatin. The objective of the CRS included render patients free of gross disease by systematically exploring the different regions of the abdomen and removing enough disease to not impact quality of life and normal digestive function. In females, significant attempt was made to preserve the reproductive structures when feasible. provides detailed information on PCI, CCS, surgery detail blood loss and intraoperative transfusion requirments. In summary, all patients underwent exploratory laparotomy, lysis of adhesions, tumor cytoreduction, omentectomy and peritonectomy. In addition, patient three had a peripancreatic mass that was resected. Patient five had a splenectomy. Patient two had extensive disease and thus underwent splenectomy, TAH/BSO, resection of pelvic mass, LAR and colorectal anastomosis and loop ileostomy. Patient four had less disease and underwent splenectomy along with TAB/BSO. Lastly, patient seven also underwent supracervical total abdominal hysterectomy and bilateral Salpingo-oophorectomy (TAH/BSO), resection of pelvic mass, resection of colonic mass, end colostomy and bilateral ureterolysis. After CRS, all patients received cisplatin at 250milligram/m2 through closed recirculatory system including reservoir, roller pump, and heat exchanger. Perfusion flow rate was maintained at 1.5 L/min primarily to warm the peritoneal tissues. All patients underwent constant manual agitation of abdomen to ensure diffuse and uniform distribution of chemotherapy and hyperthermia. Patients also received perioperative sodium thiosulfate. In selected patients (patients 1, 4, 6) a peritoneal dialysis catheter was inserted into the peritoneal cavity at the time of laparotomy and in the early postoperative period (day 7 − 10) intraperitoneal dwell chemotherapy with paclitaxel (125 mg/M2) and 5-FU (800 mg/M2) were administered.
Initial and follow-up
Prior to therapy, all patient underwent a full medical history, physical examination, routine laboratory studies, and a computed tomographic (CT) or magnetic resonance imaging (MRI) scan of the abdomen, and pelvis. The extent of residual disease or completeness of cytoreduction (CCR) after surgery was assessed as previously described [Citation1] and as follows: CCR 0, no gross residual disease; CCR 1, fewer than 100 total lesions all smaller than 5 mm; CCR 2, more than 100 total lesions all less than 5 mm or any one greater than 5 mm; and CCR 3, residual tumor larger than 1 cm. The National Cancer Institute common toxicity criteria (version 2.0) was used to assess toxicity. Patients were evaluated 3–6 weeks postoperatively and then every 3 months for 1 year, every 4 months for 1 year, and then every 6 months with blood work, physical examination, and CT of the chest, abdomen, and pelvis to assess for potential disease progression including development of ascites or soft tissue masses. Following intervention with CRS and HIPEC, all patients were considered to have stable disease until they had radiographic evidence of recurrence. Five out of 7 patients went on to receive second- or third-line treatments at the NCI at some point in their disease course.
Results
Analysis of 109 patients with MPM, who were enrolled and underwent CRS followed by HIPEC at the Surgery Branch, revealed a total of seven patients less than 21 years of age at time of treatment. Patient demographics and clinical characteristics are described in . The median age of these patients was 16 (range of 12–18 years of age, mean: 15.7 years). The majority of the patients, 57% (n = 4), were female. Clinical presentation at time of initial diagnosis was obtained by reviewing outside medical records. Approximately, 30% of the patients (n = 2) presented with abdominal pain, 43% (n = 3) of the patients presented with abdominal distention/ascites, 14% (n = 1) presented with fevers/night sweats, and 14%, (n = 1) with bilateral peripheral edema (14%, n = 1). Except for patient 1 who presented with abdominal pain, all signs and symptoms, including ascites resolved following CRS and HIPEC (, ).
Figure 1. (A) Pre and (B) four years post CRS and HIPEC imaging in patient seven demonstrated significant durable reduction in disease burden.
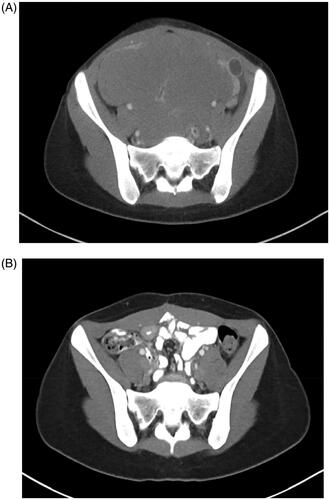
Table 1. Patient demographic information (n = 7).
Table 2. Patient specific information including including Peritoneal Cancer Index (PCI) score, Completeness of Cytoreductive score (CCR), Surgical information, perfusion volume, total dose of cisplatin, maximum temperature, EBL, and intraoperative transfusion.
Tumor histology was confirmed by the Department of Pathology and revealed epithelioid type in all patients. Histology is a known predictor of outcomes, with epithelioid type being associated with best prognosis [Citation1,Citation5].
All patients had undergone treatment for MPM prior to CRS and HIPEC. Four (57%) of the patients had received previous surgical therapy including exploratory laparotomy and debulking. One patient had received systemic therapy including two cycles of cisplatin and pemetrexed, and 29% (n = 2) of the patients received both exploratory laparotomy and debulking followed by systemic chemotherapy with ifosfamide, cisplatin, taxol, and etopiside prior to presentation and evaluation at the NCI ().
At the time of the operation, the Peritoneal Cancer Index (PCI) ranged from 6 to 25 (). Post PCI score ranged 0–10. The CCR after CRS was determined as 0 in 4 patients, and 1–2 in 3 patients. All patients underwent omentectomy, tumor debulking, and parietal peritonectomy. In addition, three patients (2, 4 and 5) had involvement of spleen and underwent splenectomy. Patent two and three had resection of pelvic mass and peripancreatic mass, respectively. Despite every effort to save the reproductive structures, three of the four female patients underwent TAH/BSO. Patient two required LAR and loop ileostomy. EBL ranged from 300 ml to 6 l, with EBL >1 L requiring transfusion of blood products ().
No operative mortalities occurred, and post-operative adverse events (AE) included acute kidney injury (n = 1), hyperbilirubinemia (n = 1), both managed conservatively, respiratory distress including bilateral pleural effusions (n = 1), and pneumothorax requiring chest tube placement (n = 1) (). Two patients developed postoperative anemia and coagulopathies requiring packed red blood cell (PRBC) and fresh frozen plasma (FFP) administration (). The average length of hospital stay was 18.6 days with range of 13–24 days.
Table 3. Specific patient related information including post-operative adverse events (A.E.), follow up duration and clinical status.
Overall, at last available follow-up, 71% of the patients (n = 5) were alive with stable disease (). The remaining patients (29%, n = 2) had passed away from their disease at 14 and 27 months from CRS and HIPEC. One patient (patient 2) underwent repeat CRS and HIPEC for recurrence 120 months after initial surgical intervention. At initial evaluation following the second treatment, she was noted to have a fluid collection along the greater curvature of the stomach which has since remained stable more than four years from second CRS and HIPEC (). This patient was alive with disease at last follow up, 281 months from initial cytoreductive surgery and chemotherapy. Patient five also developed recurrence and underwent CRS only followed by six cycles of systemic chemotherapy with adjuvant cisplatin and pemetrexed, and, as of last available follow-up, 80 months from CRS and HIPEC, was alive with stable disease. Patient six also experienced recurrence 48 months following initial surgical and chemotherapy intervention and went on to receive CRS in addition to completing six cycles of systemic pemetrexed and cisplatin. He had stable disease with ongoing survival of 253 months from CRS and HIPEC. Patient seven completed two cycles of adjuvant systemic chemotherapy with cisplatin and pemetrexed following CRS and HIPEC. She had stable disease for four months at which time the disease demonstrated progression in L3/L4 vertebra and pelvis. She was enrolled on a clinical trial with PLX3397, a CSF1R and Kit inhibitor, through the Pediatric Oncology Branch and was alive with disease 40 months from initial CRS and HIPEC.
Discussion
MPM is a lethal cancer, with an estimated historic median survival of 11 months in young patients. No additional benefit has been reported with addition of systemic chemotherapy, with reported 11-month median survival [Citation9–15]. To date, our report constitutes of the largest and longest follow-up cohort of young patients with MPM, who received multimodal therapy including CRS and HIPEC. It is the only report of pediatric MPM without other histologies. Here, we have demonstrated a trend toward better survival, with five out of the seven patients alive more than five years from treatment, suggesting that combination therapy in these selected pediatric patients with MPM can potentially prolong survival.
Multimodality therapy, including surgery, HIPEC and systemic chemotherapy, may be key in helping these young patients live longer. Prior to evaluation and treatment at NCI, six patients had received surgical intervention in the form of diagnostic laparotomy, appendectomy and/or cytoreduction, two of which also went on to receive systemic chemotherapy. Only one patient received systemic chemotherapy alone. Of the five patients alive with disease at the end of this report analysis, four had developed recurrence and went on to receive CRS and HIPEC and/or additional systemic therapy. Overall, five out of seven of our patients received some type of second- or third-line therapy, suggesting that compared to historically reported survival with systematic therapy alone, multimodality treatment may have contributed to improvement in survival. A randomized control trial evaluating CRS and HIPEC compared to systemic chemotherapy alone would help answer this question, but it will be difficult to conduct given the overall rarity of this disease.
Studies suggest that younger age alone in patients undergoing CRS and HIPEC is an independent prognostic factor for favorable outcomes in MPM, thus, this variable may have played a role in the improvement in survival in our patient cohort. Alexander et al. analyzed 104 patients who underwent CRS and cisplatin based HIPEC for MPM and determined that favorable outcomes included age <60 years (p < 0.01) [Citation1]. Similarly, Thomas et al. examined the clinical characteristics and outcomes of young patients with mesothelioma using the Surveillance, Epidemiology, and End Results database and found that patients less than 40 had significantly longer survival compared to older patients, with 5-year survival rates of 38% vs. 3%, respectively [Citation3]. In addition to age, better overall performance status and physiological reserve may have played a significant role in improvement of overall survival, but more research is needed to definitively link this association.
In addition to age, PCI and completeness of cytoreduction is another factor that has independently been shown to be associated with favorable outcomes [Citation1,Citation16]. Hayes-Jordan et al. analysis of 50 pediatric patients with different peritoneal malignancies including mesothelioma, who had received CRS and HIPEC concluded that completeness of cytoreductive surgery determined outcome, with median overall survival for patients with CR score 0 or CCR1 being 34 months compared with 7.1 months for those > CCR2 [Citation15]. In our more homogenous patient cohort, the maximum CCR score was noted in patient four (CCR of 2), who passed away 14 months after CRS and HIPEC. Interestingly, patient three had a CCR of 0 but passed away 26 months after intervention. Thus, no definitive conclusion can be made on role of CCR score in our patient cohort, with lack of concordance likely due to small number of patients.
The most notable immediate impact in our patients following CRS and HIPEC was reduction in disease burden and improvement in signs and symptoms including pain and ascites, all benefits previously described by other groups [Citation5,Citation10,Citation17]. All patients presented with either abdominal pain, fever, night sweats or ascites. Six out of seven of the patients achieved resolution in their symptoms, including complete resolution of ascites in all three patients with pre-operative ascites. Furthermore, the reported 1.6–2% mortality [Citation10,Citation15], associated with HIPEC was not observed in our patient cohort, although this may be due to small sample size. The most severe HIPEC associated toxicity observed was Grade 3 renal insufficiency, which resolved following conservative management. One patient did require chest tube placement post-operatively, but overall, our experience confirms the previously established low treatment morbidity, further supporting the safety and feasibility in young patients.
The role of immunotherapy and molecular targeted therapies in patients with malignant mesothelioma are currently being investigated in clinical trials. A phase I/II trial at NCI Pediatric Oncology Branch is evaluating PLX3397, a CSF1R and Kit inhibitor, in treatment of refractory cancers including peritoneal mesothelioma. Similarly, immune system stimulation using monoclonal antibodies toward PD-1 is actively being investigated in a phase II trial (Clinicaltrials.gov). Results are still pending. Perhaps in the near future, immunotherapy can be included in the multimodality therapy of this lethal cancer affecting young patients. Until then, our experience suggests that multi-modality therapy including CRS and HIPEC can perhaps improve long-term outcomes.
Review of the literature revealed two additional investigations that evaluated the role of CRS and HIPEC in young patients. Dhir et al., a retrospective analysis of prospectively maintained database compared perioperative and oncological outcomes between young (defined as <40 years of age) and middle aged patients with a variety of histological types of which included 25 patients with MPM. One of their conclusions included that younger patients with MPM derived similar improvement in overall median survival (young patients 260.1 months v.s. 54.9 months, p = 0.016). Similar to our study, the strength of their findings was in the availability of long-term follow-up on young patients. Unlike our study, these investigators defined young patients as those <40 years of age, therefore, it is unclear which, if any, were <21 years of age [Citation16]. Moreover, as previously mentioned Hayes-Jordan et al. reported their single institution clinical trial outcomes in a variety of different histology, which included three patients with MPM who were <21 years of age at the time of intervention. Unlike our study, which included a follow up duration of up to 281 months, their median follow-up was only 21 months [Citation15]. Overall, our experience provides the longest duration of follow up in a homogenous population of young patients with MPM. Lastly, we provide the most detailed information of intraoperative and immediate post operative A.E., allowing for other investigators to perhaps extrapolate this information to their practice.
Limitations of this study include the retrospective nature of the analysis, with the inherit biases that may result and a small sample size. In addition, postoperative intraperitoneal chemotherapy administration was not done in all of our patients and potentially may have an impact on the overall outcome. Lastly, Ki-67 expression was not performed in our patient cohort because a majority of our patients underwent intervention prior to routine analysis. Ki-67 has been shown to correlate with outcomes [Citation18], and an important adjunct to consider in future studies.
In summary, we report long-term follow-up of seven pediatric patients with MPM who received combination therapy including CRS and HIPEC, which likely resulted in longer survival. Our experience at the NCI suggests that until there are better therapies, it is reasonable to continue offering young patients these combination modalities in an effort to reduce disease burden, improve symptoms and possibly extend survival.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery. 2013;153:779–786.
- Eltabbakh GH, Piver MS, Hempling RE, et al. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70:6–12.
- Thomas A, Chen Y, Yu T, et al. Distinctive clinical characteristics of malignant mesothelioma in young patients. Oncotarget. 2015;6:16766–16773.
- Cioffredi L-A, Jänne PA, Jackman DM. Treatment of peritoneal mesothelioma in pediatric patients. Pediatr Blood Cancer. 2009;52:127–129.
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–4567.
- Sugarbaker PH, Yan TD, Stuart OA, et al. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:686–691.
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol. 2006;13:405–412.
- Alexander HR, Bartlett DL, Libutti SK. National Cancer Institute Experience with regional therapy for unresectable primary and metastatic cancer of the liver or peritoneal cavity. In: Markman M, editor. Regional chemotherapy: clinical research and practice. Totowa (NJ): Humana Press; 2000. p. 127–150.
- Talerman A, Montero JR, Chilcote RR, et al. Diffuse malignant peritoneal mesothelioma in a 13-year-old girl. Report of a case and review of the literature. Am J Surg Pathol. 1985;9:73–80.
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242.
- Sugalski A, Davis M, Prasannan L, et al. Clinical, histologic, and genetic features of mesothelioma in a 7-year-old child. Pediatr Blood Cancer. 2013;60:146–148.
- Gutman SI, Steinherz PG, Gray GF. Malignant peritoneal mesothelioma in a child. Am J Dis Child. 1976;130:1268–1269.
- Oberto C, Schwarz KB, Zambidis E, et al. Malignant peritoneal mesothelioma in a pediatric patient mimicking inflammatory bowel disease. Dig Dis Sci. 2004;49:434–437.
- Haliloglu M, Hoffer FA, Fletcher BD. Malignant peritoneal mesothelioma in two pediatric patients: MR imaging findings. Pediatr Radiol. 2000;30:251–255.
- Hayes-Jordan A, Green H, Ludwig J, et al. Toxicity of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric patients with sarcomatosis/carcinomatosis: early experience and phase 1 results. Pediatr Blood Cancer. 2012;59:395–397.
- Dhir M, Ramalingam L, Shuai Y, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion in adolescent and young adults with peritoneal metastases. Ann Surg Oncol. 2017;24:875–883.
- Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol. 2014;21:1474–1479.
- Kusamura S, Torres Mesa PA, Cabras A, et al. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2016;23:1468–1473.