Abstract
Objective
To investigate the effect of stereotactic radiofrequency thermocoagulation in the globus pallidus internus on refractory tic disorders.
Materials and methods
Forty patients with refractory tic disorders were enrolled between January 2015 and July 2017 to experience stereotactic radiofrequency thermocoagulation in the globus pallidus internus. All clinical data, Yale Global Tic Severity Scale (YGTSS) scores, serum dopamine (SDA), and 5-hydroxytryptamine (5-HT) were analyzed.
Results
Radiofrequency thermocoagulation was successfully performed in all patients. Periprocedural complications occurred in two patients (5.0%), one with fever (2.5%) and one with a urination disorder (2.5%); both returned to normal after treatment. After 12 months of follow-ups, excellent improvement was exhibited in 18 patients (45.0%), marked improvement in 10 (25.0%), good improvement in 9 (22.5%), and invalid in 3 (7.5%), with a total efficacy rate of 92.5% (37/40). Twenty-eight patients (70%) showed excellent or marked improvement without additional treatment after surgery. YGTSS scores were significantly (p < 0.05) decreased after compared with before thermocoagulation. SDA was significantly (p < 0.05) decreased 6 months (80.78 ± 18.82 ng/ml) and 12 months (75.65 ± 15.23 ng/ml) after compared with before (125.63 ± 35.26 ng/ml) surgery, whereas 5-HT was significantly (p < 0.05) increased 6 months (58.93 ± 16.88 ng/ml) and 12 months (62.63 ± 15.21 ng/ml) after compared with before (35.62 ± 3.41 ng/ml) surgery.
Conclusion
Stereotactic radiofrequency thermocoagulation can be safely applied in the globus pallidus internus to treat refractory tic disorders, resulting in significant tic symptom relief and a decrease in SDA but increase in 5-HT.
Introduction
Tics are repetitive, patterned, and disruptive motor behaviors involving most body muscles and may produce sounds when pharyngeal, laryngeal, or respiratory muscles are involved [Citation1]. Tics may negatively affect the quality of life or daily functions of patients and may consequently form tic disorders, which mostly consist of chronic neurodevelopmental conditions referred to as primary tic disorders. Tourette’s syndrome (TS) is one such neuropsychiatric disorder, with characteristics of multiple motor and vocal tics starting before age 18 and lasting over one year without influence of substance abuse or other medical conditions to cause secondary tics [Citation1–5]. The incidence of tics is nearly 3% in childhood and adolescence, and approximately 0.8% between 6 and 18 years [Citation6]. Tics may persist in 60%–80% of patients after the age of 18 years, but fewer than 25% of adult patients need medical surveillance. Patients with tics may present with other psychiatric comorbidities including anxiety, depression, sleep disorders, impulsivity, and self-injurious behaviors [Citation3]. As tics may cause a significant social and functional burden and affect normal educational development or professional activities, treatment is necessary using mostly behavioral therapy and oral medications. A subset of patients may not benefit from medication or behavioral interventions because of poor responses or unpleasant side effects that could cause them to become treatment-refractory or severely disabled [Citation2]. In this situation, deep brain stimulation may be used as a neuromodulation approach for symptom control because of its clinical benefits and low complication rates. The mechanism of deep brain stimulation has not been completely elucidated, but is thought to be mostly based on focalized high-frequency stimulation in the basal ganglia and thalamus, which are involved in movement disorders. Deep brain stimulation may induce ionic changes in the area around the electrode active tip, reversibly enhancing extracellular concentrations of potassium, affecting the dynamics of both cell bodies and axons, and disrupting local abnormal pathological activity and circuit irregularities [Citation7].
Suppression of the brain activity associated with tics is connected to temporal blocking of spiking activity with the stimulation pulse, inducing various patterns of inhibition and excitation in the affected cells [Citation8]. Dysfunction in the pathways associated with the cortico-basal ganglia integrative network has been reported to relate to vocal and motor tics, and surgical targets have consequently been suggested for control of these motor and psychiatric symptoms [Citation9]. The thalamus, globus pallidus, and anterior limb of the internal capsule/nucleus have been reported in some studies as surgical targets for treatment of refractory TS and obsessive–compulsive disorder [Citation9]. High-frequency deep brain stimulation has been performed in the internal globus pallidus for treatment of medically intractable Parkinson’s disease, TS, and refractory obsessive–compulsive disorder [Citation10–13]; in the thalamus for treating medically refractory TS [Citation14]; and in the internal capsule and nucleus accumbens for treating refractory obsessive–compulsive disorder [Citation15]. For TS, it has been reported that when high-frequency stimulation is applied in the anteromedial globus pallidus internus, the amplitude of tic-associated phasic changes in the globus pallidus internus is reduced [Citation2]. However, the average effect of deep brain stimulation is approximately 40% improvement on tic scales of severity according to the outcomes of various case series, prospective cohorts, and controlled studies [Citation1]. Other stereotactic neurosurgical approaches should be evaluated for treating refractory tic disorders. Stereotactic neurosurgery was developed in the late twentieth century initially for localization of seizure onset zones and, more recently, for treatment of epilepsy. Several stereotactic approaches have been developed, focusing on treatments including radiosurgery, laser interstitial thermotherapy, high-intensity focal ultrasound, and stereoelectroencephalography-guided radiofrequency thermocoagulation [Citation16,Citation17]. In 1990, Kurlan et al. [Citation18] were the first to report the use of radiofrequency thermocoagulation for anterior cingulotomy in two patients with TS and disabling obsessive–compulsive symptoms, achieving limited but sustained improvement in behavioral symptoms and overall functional abilities. In 2005, Sun et al. reported the use of radiofrequency thermocoagulation guided by high-resolution magnetic resonance imaging (MRI) in bilateral anterior capsulotomy for the treatment of refractory tic disorder [Citation5], achieving 50% symptom improvement on the tic severity scale in patients receiving thermocoagulation in the posterior one-third of the anterior limb of the internal capsule, compared to 80% in the anterior one-third of the anterior limb of the internal capsule. As high-frequency deep brain stimulation in the globus pallidus internus is effective for the treatment of medically intractable Parkinson’s disease, TS, and refractory obsessive–compulsive disorder [Citation10–13], it was hypothesized that radiofrequency thermocoagulation to destroy the structure of this area could be more effective in treating refractory tic disorders. Because the globus pallidus internus is located in the basal-ganglia-thalamocortical circuit or pathway, blocking this circuit or pathway would interrupt the nervous transmission of vocal and motor tics, achieving the treatment effect. This study was consequently performed to prove this hypothesis by applying radiofrequency thermocoagulation in the posteroventral part of the globus pallidus internus for the treatment of refractory tic disorders.
Materials and methods
This study was approved by the ethics committee of our hospital, with all patients or their legal guardians giving their signed informed consent to participate. All methods were performed in accordance with the relevant guidelines and regulations. Between January 2015 and July 2017, patients with refractory tic disorders were prospectively enrolled. Refractory tic disorders refer to vocal and motor tics that cannot be controlled after regular drug therapy or psychiatry. The inclusion criteria were patients with refractory tic disorders that were intractable to treatment of medicines (tiopride, aripiprazole, clonidine, or haloperidol) or psychotherapy, with persistent symptoms severely affecting daily life, work, and social function, but without the presence of any other organic diseases. The exclusion criteria were patients younger than 14 years of age, with a short course of disease, or diagnosed as a transient tic disorder. Patients with other serious mental or physical diseases who did not cooperate or tolerate surgical treatment were also excluded.
After admission, all patients had a physical examination, chest radiography, electrocardiography, electroencephalography, head computed tomography (CT), MRI scan, and laboratory test to detect any possible contraindications for the surgery. Fasting blood samples of 20 ml in all patients were collected in the morning via the ulnar vein, with the patient in the sitting position, and sent to the laboratory for testing of serum dopamine (SDA) and 5-hydroxytryptamine (5-HT) using reversed-phase high-performance liquid chromatography (Waters 2-695, Welltech Inc, Milford, MA, USA) with the kit provided by Donglin Scientific Development Co. Ltd (Wuxi, China). The standard products of SDA and 5-HT were bought from Sigma-Aldrich (Merck, Germany). The blood sample was stored at 4 °C for 3–4 h before the supernatant was obtained and centrifuged at 2000 r/min for 15 min prior to analysis. Chromatographic separation was achieved on a chromatographic column of CAPCELL PAK C18 (4.6 mm × 250 mm, 5 μm), with the column temperature set at 35 °C. Elution was performed with the mobile phase of methanol and 0.1 mol/l potassium dihydrogen phosphate (15:85). The following chromatographic conditions were selected: pH 4.6, flow rate of 1.5 ml/min, excitation wavelength of 254 nm, and emission wavelength of 338 nm. The filtered and centrifuged sample of 20 μl/injection was measured and analyzed.
Prior to the radiofrequency thermocoagulation surgery, anti-tic medication was continuously applied to control the symptoms, primarily including tiopride, aripiprazole, clonidine, and haloperidol, and cefuroxime was applied once in a dose of 1.5 g to prevent possible infection. The radiofrequency thermocoagulation procedure was performed under local anesthesia in each patient. For patients with severe tics, the anti-tic medication should be used to control the tic symptoms appropriately for the procedure. After the patients were immobilized and positioned at the operation table, a Leksell localizer (Elekta AB, Stockholm, Sweden) was used, and a head MRI scan was performed for localization of the globus pallidus internus (). The MRI scan was performed using an MRI scanner with a field strength of 1.5 T. The conventional scanning parameters of our hospital were used, including a slice thickness of 2.0 mm, slice interval of 0.2 mm, matrix size 256 × 256, and field of view of 235 mm. T1 and T2, axial, and coronal images were obtained. The accuracy of the Leksell stereotactic system was high, as previously proven by repeated use in skull simulation scanning and correction, with the error being basically consistent with that in the manual.
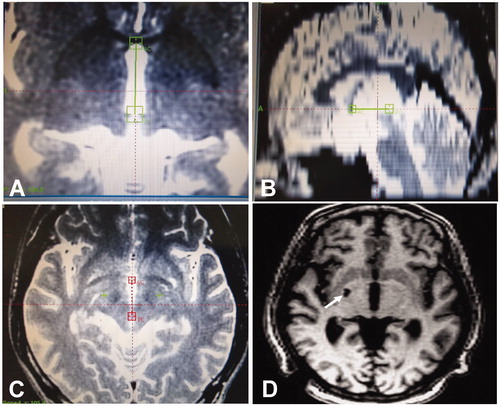
Figure 1. Seventeen-year-old man with refractory tic disorders treated with stereotactic radiofrequency thermocoagulation. A. Magnetic resonance imaging (MRI) scan performed for localization of the globus pallidus internus on the axial plane. B. Localization of the globus pallidus internus on the coronal plane of MRI. C. Targeted globus pallidus internus on both sides of the brain. D. Head MRI scan one year after the thermocoagulation, revealing the lesion of thermocoagulation in the right globus pallidus internus. The patient exhibited excellent improvement with no tic symptoms. The arrow indicates the damaged right globus pallidus internus.
Once the target of the posteroventral part of the globus pallidus internus had been determined, the patient was sent to the operation room for radiofrequency thermocoagulation. Subsequently, a twist drill hole was made on the top of the forehead, with the dura being cauterized and opened and an anchor bolt secured in the drill hole. An Elekta electrode was inserted into the target area and secured. The radiofrequency lesion generator system used was a Leksell LNG30-1 (Elekta AB, Stockholm, Sweden). The radiofrequency needle for thermocoagulation was 1.6 mm × 3.0 mm at the tip of the electrode. Before radiofrequency thermocoagulation, electrical stimulation was applied with a NeuroNav microelectrode recording system (Alpha Omega, Alpharetta, GE, USA) at a frequency of 2–100 Hz and voltage of 0–10 V to observe the reaction symptoms of the optic and corticospinal tracts. The target position was confirmed by electrical stimulation, with a low frequency of 2 Hz and a high frequency of 100 Hz. In electrical stimulation by the most experienced neurosurgeon, the voltage began at 0 V and was gradually increased, and the main purpose was to observe whether there were movement or visual symptoms caused by stimulation of the pyramidal or optic tract, respectively. Specific attention was paid to the visual symptoms. After the electrical stimulation had proven the safety of radiofrequency thermocoagulation, microelectrodes were used for globus pallidus lesioning. When the electrode for lesioning was close to the target of the globus pallidus internus, which was near the optic tract, noise could be generated by light stimulation, and high-frequency stimulation could produce light perception on the opposite side. In the lesioning process, the visual acuity was checked on the visual dial for possible damage. Motor function was mainly tested using low-frequency stimulation of 2 Hz to verify whether there was movement corresponding to the relevant frequency of electrical stimulation. In the lesioning process, the patients were asked to raise their hands and repeatedly clench their fists to determine whether the corresponding motor function was damaged. If the corticospinal tract and optic tract symptoms occurred when the stimulation intensity was less than 3 V, the target for radiofrequency thermocoagulation should be adjusted to the lateral area to prevent injury to normal tissues. At the beginning of radiofrequency, the temperature of the radiofrequency needle was set at 45 °C for reversible thermocoagulation, at which temperature the radiofrequency effect was reversible and the damaged tissue could be recovered. If no abnormal symptoms or effects occurred, the needle was set at a temperature of 60–80 °C for permanent radiofrequency thermocoagulation lasting 30–60 s. Generally, the globus pallidus internus on one side was chosen, whereas for patients with more severe tic symptoms, the bilateral globus pallidus internus was chosen for thermocoagulation. Patients with bilateral body symptoms or accompanied by phonation underwent bilateral lesioning ablation. In patients with chronic movement or vocal twitches in whom the limb twitch was relatively fixed and only unilateral, unilateral therapy was applied. When lesioning the bilateral globus pallidus internus, it was necessary to be very cautious in the selection of patients because they could have a large postoperative reaction and severe brain edema, leading to symptoms similar to pseudobulbar palsy or an impact on their state of consciousness. The temperature for radiofrequency thermocoagulation was maintained at 60–80 °C lasting 30—50 s. During the operation, a sedative dosage of propofol was pumped in intravenously to maintain the sedative status of the patient. After the operation, the consciousness state, limb movement, and language ability of the patient were observed, and head CT was performed to confirm the exact location of coagulation ablation. The patient was then sent back to the Intensive Care Unit for monitoring of their vital signs, state of consciousness, limb movement, and other neurological functions. The patient was administered with low-flow oxygen inhalation at 1 l/min and kept in the supine position to prevent the possible formation of low intracranial pressure caused by loss of cerebrospinal fluid during the procedure. On the day after the radiofrequency procedure, cefuroxime was applied once in a dose of 1.5 g, and 25 g of mannitol was applied once every 12 h to prevent possible cerebral edema around the globus pallidus internus. If severe cerebral edema existed around the radiofrequency coagulation area, methylprednisolone was administered twice daily at a dose of 30 mg/kg. Ringer’s solution of sodium acetate at a dose of 1000 ml/d was also administered to prevent formation of low intracranial pressure. After radiofrequency ablation, the anti-tic medication was continued, and the dosage of medication was maintained at the same level as before the ablation operation. If the lesioning effect was evaluated to be good, the maintenance drug was reduced in the dosage, with an initial reduction of one-third, and then the drug dosage could be further reduced by one-third until complete withdrawal. If the condition of the patient was stable with good control of the tic symptoms but without any abnormalities in the nervous or psychic systems, the patient could be discharged home. One week later, a further head imaging examination was performed on the coagulation area, and clinical evaluation was performed for the control of symptoms. Subsequent assessment was conducted at three and six months to evaluate the effect of radiofrequency thermocoagulation. If the tic symptoms were well controlled at six months, the anti-tic medication was gradually reduced until complete withdrawal.
The Yale Global Tic Severity Scale (YGTSS), SDA, and 5-HT were evaluated in all patients before and after surgery. Clinical efficacy and complications were also assessed after treatment. The SDA and 5-HT were measured using reversed-phase high-performance liquid chromatography with the kit provided by Donglin Scientific Development Co. Ltd (Wuxi, China). The clinical efficacy was divided into excellent (total YGTSS score 0–20), marked (total YGTSS score 21–35), good (total YGTSS score 36–50), and invalid (total YGTSS score >51) based on the clinical global impressions scale of the Chinese psychiatric surgery cooperation group [Citation19]. Excellent improvement was represented by complete disappearance of vocal and motor tics and resumption of function, without any additional treatment. Marked improvement was represented by apparent resolution of vocal and motor tics, without affecting daily life and with no additional treatment. Good improvement was represented by some resolution in vocal and motor tics but with defective function, affecting daily life and needing additional treatment. Invalid indicated no resolution or aggravation of the tic symptoms. The total efficacy rate was the sum of excellent, marked, and good improvement rate.
Statistical analysis was conducted with an SPSS 21.0 (IBM, Chicago, IL, USA). Continuous data was expressed as mean ± standard deviation (SD) and tested via the student t-test with p < 0.05 set as statistically significant.
Results
A total of 40 patients met the inclusion criteria and were enrolled, including 23 males and 17 females, with an age range of 15–35 years (mean 21.36 ± 1.69) and a disease course of 5–13 years (mean 9.85 ± 1.75). There were 25 patients with TS and 15 with refractory chronic motor or vocal tic disorders. Thirty-eight patients were unilateral, whereas the other two were bilateral in the case of motor disease. All patients had been treated with tiopride, aripiprazole, clonidine, or haloperidol without significant effects before the surgical ablation procedure.
Radiofrequency thermocoagulation was successfully performed in all patients. Periprocedural complications occurred in two patients (5.0%), including one with fever (2.5%) and the other with a urination disorder (2.5%), both of whom returned to normal after proper treatment. After 12 months of follow-ups (), excellent improvement was exhibited in 18 patients (45.0%), marked improvement in 10 (25.0%), good improvement in 9 (22.5%), and invalid in 3 (7.5%), with a total efficacy rate of 92.5% (37/40). Twenty-eight patients (70%) exhibited excellent or marked improvement without any additional treatment after surgery. YGTSS scores were significantly (p < 0.05) decreased 6 months (21.28 ± 6.92) and 12 months (19.05 ± 2.32) after compared with before (75.68 ± 5.71) thermocoagulation (). SDA was significantly (p < 0.05) decreased 6 months (80.78 ± 18.82 ng/ml) and 12 months (75.65 ± 15.23 ng/ml) after compared with before (125.63 ± 35.26 ng/ml) surgery (), whereas 5-HT was significantly (p < 0.05) increased 6 months (58.93 ± 16.88 ng/ml) and 12 months (62.63 ± 15.21 ng/ml) after compared with before (35.62 ± 3.41 ng/ml) surgery (). No subtle neuropsychiatric changes, including mood or behavioral changes, were observed in these patients.
Table 1. YGTSS scores before and after surgery (mean ± SD).
Table 2. SDA and 5-HT before and after surgery (mean ± SD, ng/ml).
Discussion
Although the majority of tic patients typically experience a decrease in severity and frequency of tics between 15 and 17 years of age, and around three-quarters of children with tics will show considerable improvement in early adulthood, some patients still have refractory tics that are difficult to manage with oral medications and behavioral therapy [Citation2]. Application of radiofrequency thermocoagulation in the treatment of refractory tic disorders achieved good results in this study, with a total efficacy rate of 92.5%. Twenty-eight patients (70%) exhibited excellent or marked improvement after surgery without any additional treatment. This effect was superior to that of other approaches, such as deep brain stimulation, which has an average efficacy rate of approximately 40% [Citation1]. Moreover, radiofrequency thermocoagulation in the globus pallidus internus also caused a significant decrease in SDA but a significant increase in 5-HT, which may reflect the possible mechanism of this treatment mode.
Various neurotransmitters may be involved in the genesis of tic disorders, including dopamine, glutamate, γ-aminobutyric acid, 5-HT, and acetylcholine [Citation20]. Dopamine may be the most important factor in tic disorders, and dopamine D2 receptor antagonists can aggravate tics, probably indicating the hypersensitivity of dopamine receptors in tic patients [Citation21]. Studies have shown that tic patients have abnormal distribution of dopaminergic nerves in the ventral striatum, changes in the structure of the globus pallidus internus, and increased uptake of dopamine in the striatum [Citation20,Citation22]. All these results support the enhancement of activity of the dopamine system in tic patients. The majority of options for treating tics are pharmacological, with the most commonly prescribed agent being dopamine antagonists, including neuroleptics (haloperidol), benzamides (sulpiride), or atypical antipsychotics (risperidone) [Citation23]. Dopamine antagonists may result in the most reliable treatment response, but may have various side effects. After electric high-frequency stimulation of the globus pallidus internus in a rat model of TS, Zhao et al. [Citation20] found that the concentrations of dopamine and dopamine transporter were significantly (p < 0.05) decreased, as were the tic severity and scores. Deep brain stimulation of the globus pallidus internus can affect the metabolism and transport of corresponding neurotransmitters and subsequently change the biological activity of the basal ganglia nerve circuits. The tic symptoms are best controlled if dopamine is released least in the striatal target neurons. To test this hypothesis, Vernalaken et al. [Citation24] used [18F] fallypride positron emission tomography scanning in the steady phase of deep brain stimulation treatment in a tic patient and reported a significant increase in endogenous dopamine in the period without deep brain stimulation. However, bilateral thalamic stimulation decreases dopamine production in the striatum [Citation24]. Corroborating this outcome, a similar study enrolling three patients also demonstrated that deep brain stimulation acts by modulating dopamine transmission [Citation25]. Stimulation of the centromedian nucleus and substantia periventricularis is likely to suppress excitatory feedback projections to the motor and limbic circuits of the striatum, consequently decreasing tics while improving behavioral disorders [Citation26]. Chronic circuit abnormalities presenting in tic disorders are probably associated with the failure of cortical inhibition to the basal ganglia “filter”, which may in turn lead to thalamic hyperactivity feeding the pathological loop, generating tics. In our study, radiofrequency thermocoagulation in the globus pallidus internus significantly decreased SDA, and its mechanism may involve the dopamine system, regulate the concentration of synaptic neurotransmitters, and change the biological activity of the basal ganglia nerve circuits.
Evidence generally suggests that the neurotransmitter 5-HT has an inhibitory effect in the brain and is deeply involved in the regulation of emotions and behavior, including inhibition of aggression [Citation27–29]. Dysfunction of 5-HT has been reliably related to the pathophysiology of TS [Citation22,Citation30,Citation31]. After studying the brain 5-HT transporter binding capacity in the midbrain and thalamus in patients with TS, Müller-Vahl et al. [Citation30] found that the binding capacity was significantly decreased in these patients compared with normal controls, indicating that 5-HT concentration is decreased in the midbrain and thalamus. After studying the effectiveness of the Chinese herbal medicine Ningdong granule in treating patients with TS, Wang et al. [Citation28] found that the treatment effect occurred through regulating dopamine/5-HT and gamma-aminobutyric acid. In our study, radiofrequency thermocoagulation in the globus pallidus internus significantly increased the serum concentration of 5-HT compared with that before surgery, which may indicate the role of 5-HT in the pathophysiology of tic disorders. However, the exact mechanism requires further study for elucidation.
SDA and 5-HT are the two most significant neurotransmitters to assist signaling between neurons in the central nervous system [Citation32]. Dopamine is an excitatory neurotransmitter in nature in the class of catecholamine neurotransmitters, whereas 5-HT is an inhibitory neurotransmitter. Dopamine is primarily synthesized in areas such as the substantia nigra pars compacta, arcuate nucleus of the hypothalamus, and ventral tegmental area, with a significant effect on several pathways including motor control arousal, activeness, reward-based learning, motivation, and upper cognition function. 5-HT also has the functions of psychomotor inhibition and adjustment of emotions and mood, including impulsivity, analgesia, aggression, addition, eating disorders, adaptation to stressors, and cognition [Citation32]. Two serotonergic pathways originating from the dorsal and medial raphe nuclei are able to innervate the cortical and subcortical structures through serotonergic release. Although the two systems have opposite functions, they are closely related. The dopaminergic system is mainly centralized in the midbrain, with two primary pathways of the nigrostriatal pathway and the ventral tegmental area projection [Citation33]. Neurons arising from the substantia nigra pars compacta join the striatum through the nigrostriatal pathway and act as modulators of behavior. The ventral tegmental area enters the mesocortical and mesolimbic pathways, influencing the cortical and limbic areas, and is involved in behavioral adjustment. The 5-HT pathway also originates from the midbrain, mainly in the medial and dorsal raphe nuclei. The dorsal raphe nuclei have projections toward the striatal and cortical regions, and the medial raphe nuclei enter the limbic regions. The inhibitory function of 5-HT affects dopaminergic neurons in two locations: the forebrain and the midbrain. Because the dorsal raphe neurons are projected directly into the substantia nigra pars compacta, 5-HT displays inhibitory effects on the dopamine neurons located in the substantia nigra.
The treatment of TS and other tic disorders comprises a variety of psychoactive medications interacting with dopamine and non-dopamine systems and psychoeducative interventions. Despite advancements in medical and behavioral therapies, there is a subset of patients with refractory tic disorders that do not respond to conventional clinical management, with clinical treatment efficacy and good responses to these measures ranging from 30% to 85% [Citation2]. In this setting, deep brain stimulation has been used as an additional therapeutic approach for symptom control ever since it was first reported in 1999 by Vandervalle et al. [Citation26]. The targets for deep brain stimulation in the treatment of TS mostly resemble those used earlier in focal ablative procedures in the thalamus, pallidum, and ventral striatum/ventral capsule, for the control of motor and psychiatric symptoms. However, deep brain stimulation has an effect of approximately 40% improvement on the tic severity scale based on case series, prospective cohorts, and controlled studies [Citation1]. Johnson et al. [Citation34] reported a 40% improvement in tic severity in a cohort of 123 patients with TS at a median follow-up time of 13 months after deep brain stimulation. Again, some patients with TS or tic disorders were refractory to this treatment. Radiofrequency thermocoagulation has been applied clinically in the nervous system primarily for the treatment of epilepsy [Citation35,Citation36]. Because Kurlan et al and Sun et al had applied radiofrequency thermocoagulation in the gyri cinguli and anterior capsule interna for the treatment of TS or refractory tic disorders [Citation5,Citation18], we hypothesized that the use of radiofrequency thermocoagulation in the globus pallidus internus could be more effective for refractory tic disorders. This is because high-frequency deep brain stimulation in the globus pallidus internus is effective in the treatment of medically intractable Parkinson’s disease, TS, and refractory obsessive–compulsive disorder [Citation10–13], and destruction of this structure can block the basal-ganglia-thalamocortical circuit or pathway, thus achieving better treatment effects. Our study revealed that a higher proportion of patients with refractory tic disorders benefited from this approach, with a total efficacy rate of 92.5% and a rate of 70% of excellent or marked improvement.
Various brain targets have been suggested as potential targets of deep brain stimulation therapy in tic disorders along the basal-ganglia-thalamocortical circuit [Citation2]. Because tic disorders are a complex disease combining psychiatric and movement disorders with anxiety and compulsive symptoms, both the sensory-motor and associative/limbic areas may serve as targets of therapy. Among these options, areas of the globus pallidus internus and medial thalamus are the most frequently used targets because of their involvement in the motor and limbic pathways [Citation2], both of which are affected by deep brain stimulation in the globus pallidus internus, especially for motor symptoms in Parkinson’s disease and dystonia. Good outcomes have been achieved in stimulating the anteromedial part of the globus pallidus internus, which involves the limbic loops in tic expression [Citation37,Citation38]. However, approximately 50% of patients did not show significant improvement. In contrast, stimulation in the posteroventral part of the globus pallidus internus achieved much better results in controlling tic symptoms, with an improvement rate of 73%–87% [Citation39–42]. The effect of stimulating the posteroventral part appears superior to that of stimulating the anteromedial part of the globus pallidus internus, which is why we chose the posteroventral part of the globus pallidus internus as the target in our study.
Sun et al [Citation5] studied the effects of radiofrequency ablation of bilateral anterior capsulotomy in 12 patients with TS. In seven patients, the target was the anterior one-third of the anterior limb of the capsula interna, whereas in the remaining five, the posterior one-third of this structure was targeted. After radiofrequency thermolesions were created at 80 °C for 60 s, a reduction of >50% in TS symptoms (motor and vocal tics and/or obsessive–compulsive behavior) was achieved in all patients, regardless of the target. A comparison of the two lesion sites indicated that patients with lesions of the posterior one-third of the anterior limb had a greater reduction in TS symptoms (>80%) than those receiving lesions of the anterior one-third of the anterior limb (>50%). No severe or permanent side effects were observed in any patient. Kurlan et al [Citation18] reported two patients with TS and disabling obsessive–compulsive and ritualistic behaviors who underwent bilateral radiofrequency anterior cingulotomy. After surgery, both exhibited a limited but sustained improvement in behavioral symptoms and overall functional abilities. The study suggested that the procedure should be considered for patients with TS complicated by resistant obsessive–compulsive disorder. Radiofrequency ablation of the fast-spiking interneurons in the dorsal striatum has been applied in animal studies in mice [Citation43,Citation44]. The disruption of the dorsolateral striatum—roughly analogous to the putamen—produced abnormal tic-like movements after either acute stress or amphetamine treatment, and ablation of the specific interneurons caused behavioral changes in an animal model that resembled aspects of a movement disorder [Citation43,Citation44].
Limitations that may exist in this study include one center design, non-control subjects, non-blinded and non-randomized planning, and only Chinese patients enrolled in the cohort. Moreover, neuropsychological assessment was not performed on these patients. In addition, the degree of obsessive–compulsive disorder was not recorded. These factors may all affect the publication bias. Thus, the interpretation of these results should be cautious and future studies are required to solve these issues.
In conclusion, stereotactic radiofrequency thermocoagulation can be applied in the globus pallidus internus for the treatment of refractory tic disorders, resulting in significant relief of tic symptoms, a decrease in SDA, and an increase in 5-HT.
Disclosure statement
The authors declared that there are no conflicts of interest in publication of this article.
References
- Martino D, Pringsheim TM. Tourette syndrome and other chronic tic disorders: an update on clinical management. Expert Rev Neurother. 2018;18:125–137.
- Casagrande SCB, Cury RG, Alho EJL, et al. Deep brain stimulation in Tourette's syndrome: evidence to date. Neuropsychiatr Dis Treat. 2019;15:1061–1075.
- Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in tourette syndrome. Front Psychiatry. 2016;7:97.
- Jankovic J. Tourette's syndrome. N Engl J Med. 2001;345:1184–1192.
- Sun B, Krahl SE, Zhan S, et al. Improved capsulotomy for refractory Tourette's syndrome. Stereotact Funct Neurosurg. 2005;83:55–56.
- Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(1):68–87.
- Florence G, Sameshima K, Fonoff ET, et al. Deep brain stimulation: more complex than the inhibition of cells and excitation of fibers. Neuroscientist. 2016;22(4):332–345.
- McCairn KW, Iriki A, Isoda M. Deep brain stimulation reduces Tic-related neural activity via temporal locking with stimulus pulses. J Neurosci. 2013;33:6581–6593.
- Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, et al. Deep brain stimulation for Tourette syndrome: target selection. Stereotact Funct Neurosurg. 2012;90:213–224.
- Azriel A, Farrand S, Di Biase M, et al. Tractography-guided deep brain stimulation of the anteromedial globus pallidus internus for refractory obsessive-compulsive disorder: case report. Neurosurgery. Neurosurgery. 2020;86(6):E558–E563.
- McCairn KW, Iriki A, Isoda M. High-frequency pallidal stimulation eliminates tic-related neuronal activity in a nonhuman primate model of Tourette syndrome. Neuroreport. 2012;23:206–210.
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol. 2009;101(4):1941–1960.
- McCairn KW, Turner RS. Pallidal stimulation suppresses pathological dysrhythmia in the parkinsonian motor cortex. J Neurophysiol. 2015;113:2537–2548.
- Jo HJ, McCairn KW, Gibson WS, et al. Global network modulation during thalamic stimulation for Tourette syndrome. Neuroimage Clin. 2018;18:502–509.
- Park HR, Kim IH, Kang H, et al. Electrophysiological and imaging evidence of sustained inhibition in limbic and frontal networks following deep brain stimulation for treatment refractory obsessive compulsive disorder. PLoS One. 2019;14:e0219578.
- Bourdillon P, Devaux B, Job-Chapron AS, et al. SEEG-guided radiofrequency thermocoagulation. Neurophysiol Clin. 2018;48:59–64.
- Bourdillon P, Rheims S, Catenoix H, et al. Surgical techniques: stereoelectroencephalography-guided radiofrequency-thermocoagulation (SEEG-guided RF-TC). Seizure. 2019;77:64–68.
- Kurlan R, Kersun J, Ballantine HT, Jr, et al. Neurosurgical treatment of severe obsessive-compulsive disorder associated with Tourette's syndrome. Mov Disord. 1990;5:152–155.
- Wu W-Y. Clinical global impression. Shanghai Archives of Psychiatry. 1990;23:70–71.
- Zhao M, Wang X, Deng J, et al. Globus pallidus internus electric high-frequency stimulation modulates dopaminergic activity in the striatum of a rat model of tourette syndrome. World Neurosurg. 2019;127:e881–e887.
- Singer HS, Butler IJ, Tune LE, et al. Dopaminergic dsyfunction in Tourette syndrome. Ann Neurol. 1982;12:361–366.
- Wong DF, Brašić JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–1251.
- Eddy CM, Rickards HE, Cavanna AE. Treatment strategies for tics in Tourette syndrome. Ther Adv Neurol Disord. 2011;4:25–45.
- Vernaleken I, Kuhn J, Lenartz D, et al. Bithalamical deep brain stimulation in tourette syndrome is associated with reduction in dopaminergic transmission. Biol Psychiatry. 2009;66:e15–e17.
- Kuhn J, Janouschek H, Raptis M, et al. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette's syndrome. Biol Psychiatry. 2012;71:e11–e13.
- Vandewalle V, van der Linden C, Groenewegen HJ, et al. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724.
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616.
- Wang S, Qi F, Li J, et al. Effects of Chinese herbal medicine Ningdong granule on regulating dopamine (DA)/serotonin (5-TH) and gamma-amino butyric acid (GABA) in patients with Tourette syndrome. Biosci Trends. 2012;6:212–218.
- Yan Z. Regulation of GABAergic inhibition by serotonin signaling in prefrontal cortex: molecular mechanisms and functional implications. Mol Neurobiol. 2002;26:203–216.
- Muller-Vahl KR, Meyer GJ, Knapp WH, et al. Serotonin transporter binding in Tourette Syndrome. Neurosci Lett. 2005;385:120–125.
- Oades RD. Differential measures of 'sustained attention' in children with attention-deficit/hyperactivity or tic disorders: relations to monoamine metabolism. Psychiatry Res. 2000;93:165–178.
- Manciu F, Manciu M, Ciubuc J, et al. Simultaneous detection of dopamine and serotonin-a comparative experimental and theoretical study of neurotransmitter interactions. Biosensors (Basel). 2018;9(1):3.
- Ichinose T, Tanimoto H, Yamagata N. Behavioral modulation by spontaneous activity of dopamine neurons. Front Syst Neurosci. 2017;11:88.
- Johnson KA, Fletcher PT, Servello D, et al. Image-based analysis and long-term clinical outcomes of deep brain stimulation for Tourette syndrome: a multisite study. J Neurol Neurosurg Psychiatry. 2019;90(10):1078–1090.
- Chipaux M, Taussig D, Dorfmuller G, et al. SEEG-guided radiofrequency thermocoagulation of epileptic foci in the paediatric population: feasibility, safety and efficacy. Seizure. 2019;70:63–70.
- Mullatti N, Landre E, Mellerio C, et al. Stereotactic thermocoagulation for insular epilepsy: lessons from successes and failures. Epilepsia. 2019;60(8):1565–1579.
- Cannon E, Silburn P, Coyne T, et al. Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette's syndrome. Am J Psychiatry. 2012;169:860–866.
- Martinez-Fernandez R, Zrinzo L, Aviles-Olmos I, et al. Deep brain stimulation for Gilles de la Tourette syndrome: a case series targeting subregions of the globus pallidus internus. Mov Disord. 2011;26:1922–1930.
- Dehning S, Leitner B, Schennach R, et al. Functional outcome and quality of life in Tourette's syndrome after deep brain stimulation of the posteroventrolateral globus pallidus internus: long-term follow-up. World J Biol Psychiatry. 2014;15:66–75.
- Dehning S, Mehrkens JH, Muller N, et al. Therapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord. 2008;23:1300–1302.
- Diederich NJ, Kalteis K, Stamenkovic M, et al. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20(11):1496–1499.
- Shahed J, Poysky J, Kenney C, et al. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology. 2007;68:159–160.
- Xu M, Kobets A, Du JC, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA. 2015;112:893–898.
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329.
