Abstract
Objectives
To retrospectively compare the safety profile of percutaneous image-guided radiofrequency ablation (RFA) and cryoablation (CA) of bone metastases (BM) with and without a propensity score analysis.
Methods
Between January 2008 and April 2018, 274 consecutive patients (mean age 61.6 ± 12.1 years) with BM were treated at our Institution with RFA (53 patients; 66 BM) or CA (221 patients; 301 BM) and included in this study. Complications were assessed according to the type of ablation modality before and after applying a 1:1 propensity score method taking into account patient’s demographics, BM features, procedural details and follow-up findings.
Results
In the whole 9 BM (2.5%) reported major complications without significant difference between RFA (1/66; 1.5%) and CA (8/301; 2.7%; p = 1); 40 BM (10.9%) showed minor complications, which were more common with RFA (22/66; 33.3%) than with CA (18/301; 6.0%, p<.001) mainly due to post-procedural pain occurring more frequently with RFA than CA (20/66; 30.3% vs. 7/301; 2.3%, p<.001). Following 1:1 matching, similar results were obtained, since there were similar rates of major complications with RFA and CA (1/66 [1.5%] and 0/66 [0.0%], respectively; p = 1); and higher rates of minor complications with RFA compared to CA [33.3% (22/66) vs. 2/66 (3%); p<.001] due to preponderant postprocedural pain (90.9% [20/22] minor complications with RFA).
Conclusions
Similar low rates of major complications are expected with RFA and CA of BM. In the post-operative period, RFA appears more painful than CA, thus warranting for adoption of dedicated analgesic protocols for patients undergoing RFA.
Introduction
Bone metastases (BM) represent a frequently encountered complex clinical scenario [Citation1], often requiring a multidisciplinary approach to management, involving orthopedic surgeons, oncologists, histopathologists, as well as diagnostic and interventional radiologists. Treatment plans agreed by the tumor board are often conferred based on the patient’s demographics, histological grade and stage as well as on the symptomatic status of BM resulting from numerous skeletal-related events (SREs) such as pain, fractures, nerve root compression and hypercalcemia, warranting palliative treatment [Citation2]. Less often treatment is proposed with a curative intent of achieving effective locoregional tumor control in selected patients presenting with oligometastatic or oligoprogressive disease [Citation3].
In the last few decades, the therapeutic armamentarium for BM has broadened to include percutaneous thermal ablation (TA) techniques, available to the multidisciplinary tumor board to provide palliative or curative treatment. Radiofrequency ablation (RFA) and cryoablation (CA) [Citation4–7] are the two most commonly used and investigated techniques, and share comparable effective clinical results in the palliative [Citation4,Citation5,Citation8,Citation9], and curative [Citation9,Citation10] setting. Moreover, the safety of RFA and CA has been proven, with series reporting rates of overall/major complication of 16%/5% and 9.1%/2.5%, respectively [Citation11–15]. Nevertheless, there are no formal explicit analyses directly comparing RFA and CA, specifically in terms of safety; and two large recently published series specifically investigating the safety of bone tumor RFA and CA could not formally provide a definitive appraisal due to the lack of direct comparison between these two techniques; heterogeneous type of targeted tumors including primary/secondary, and benign/malignant tumors with variable histological grading; as well as due to the bias related to the selection of the TA technique, which was mainly operator’s driven, generally according to the radiological category of the index tumor (e.g., RFA not offered for sclerotic tumors; CA applied to sclerotic/mixed tumors, etc.) [Citation12,Citation15]. Therefore, we have hypothesized that a direct comparison among RFA and CA in 1:1 matched populations including BM with comparable features could provide more robust evidence regarding the safety profile of these two common ablation techniques. Therefore, the aim of this study was to retrospectively compare the safety profile of percutaneous image-guided RFA and CA of BM before and after applying a propensity score analysis with the intent to lessen the influence of patient’s, BM, procedural and follow-up factors. Our study specifically focused on BM since these definitely represent the most common type of bone tumors undergoing TA.
Materials and methods
This retrospective study was approved by our institutional review board with permission to perform chart review, with a waiver to written informed consent. All research was performed in accordance with relevant regulations.
Study population
All consecutive patients with BM undergoing percutaneous RFA and CA in a single tertiary university hospital between January 2008 and April 2018 were identified by research performed in the institutional radiological information system. Three different key-words were simultaneously entered (‘bone metastases’, ‘cryoablation’ and ‘radiofrequency ablation’); and a total of 320 consecutive unpaired patients were identified: 81 had received RFA, and 239 had received CA; 28 patients treated with RFA and 18 with CA were excluded due to loss of follow-up data subsequent to hospital discharge. Consequently, the final study population included 274 patients (150 [54.7%] males; 124 [45.3%] females; mean age 61.6 ± 12.1 years; range 18–86) receiving RFA (53 patients; 66 BM; 54 interventional sessions) or CA (221 patients; 301 BM; 269 interventional sessions) (). A subset of the study population has been previously reported by Auloge et al. and Cazzato et al. [Citation12,Citation15].
The decision to perform TA was established following discussion in a multidisciplinary tumor board meeting including oncologists, orthopedic surgeons, anesthesiologists and interventional radiologists. Patients were referred for consideration of percutaneous TA if they required either a palliative treatment due to painful BM not previously treated with, or refractory to standard treatments (including analgesics and radiotherapy [RT]); either a curative treatment due to an oligometastatic (<3 metastases with tumors size ≤3 cm) or oligoprogressive disease (1–2 metastases not responding to systemic therapy despite an overall stable systemic disease) [Citation9,Citation10].
Patients were not offered TA if they presented with an irreversible coagulopathy, signs of active infection, life expectancy less than 1 month, mechanically unstable spinal lesions, focal neurological deficit related to the BM or tumors for which TA was deemed technically unfeasible due to the high risk of damage to adjacent structures (nerves, vessels, solid/hollow organs, cartilage or skin) in spite of extensive application of thermo-protective measures [Citation16].
Thermal ablation procedures
Procedures were performed by seven interventional radiologists with variable (1–13) years of experience.
Patients underwent either CA () or RFA () at the discretion of the interventional radiologist performing the procedure. All interventions were performed under conscious sedation, spinal or general anesthesia in a strict sterile environment under computed tomography (CT) or cone-beam CT (CBCT)-guidance. Additional fluoroscopic guidance was applied when required.
Figure 2. Curative cryoablation of a solitary scapular metastasis of a papillary thyroid cancer in a 63-year-old male patient. Axial Short Tau Inversion Recovery (STIR) (A) and coronal T1 weighted (T1W) contrast enhanced (B) MR acquisitions (A, B) show the metastatic lesion of the scapular spine (white arrows). Multiplanar reconstructed CT images (C) display the positioning of 4 cryoprobes along the long axis of the lytic lesion (white arrows). Axial CT image (D) shows the maximal iceball (arrow heads); in this case, the supraspinatus muscle was hydrodissected (black arrow). A hydrodissection of the suprascapular nerve was also performed with a 22 G needle placed in the suprascapular notch (not shown). In this patient, a symptomatic post-ablation fracture occurred 1 month after the procedure as shown on coronal CT reconstructions (arrows in E). This fracture was treated by percutaneous screw fixation (arrows in F).
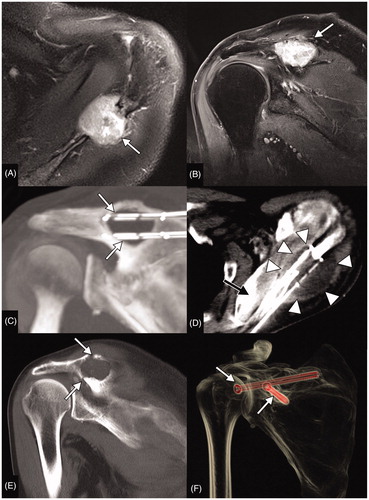
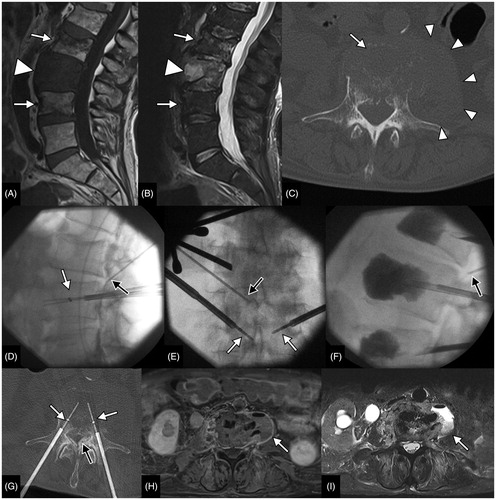
Figure 3. Palliative bipolar radiofrequency ablation of an L3 lung cancer metastasis in a 67-year-old male complicated with early post-procedural spondylodiscitis and para vertebral abscess. Sagittal T1W (A) and STIR (B) acquisitions show enlargement of the metastatic infiltration of the L3 vertebral body (arrowhead); there were also benign vertebral compression fractures of the adjacent vertebral bodies (arrows). Pre,-procedural CT (C) shows the lysis of the L3 body (arrow) but also enlargement of the left psoas muscle (arrowheads) secondary to soft tissue metastasis. Per procedural fluoroscopic (D, E, F) and CT (G) images show the positioning of the RF electrodes (white arrows), an 18 G epidural needle (black arrow) allowing dissection of the epidural space (black arrow in G) and the final acquisition prior to needle removal and after cementoplasty of L3 and the adjacent fractured vertebra (F). Contrast-enhanced axial T1W (H) and axial T2W (I) acquisitions obtained 3 d after the procedure show an abscess within the left psoas muscle communicating with the L2/L3 disk (arrows).
One or more cryoprobes (IceSphereVR, IceRodVR or IceForceVR and Galil medical) and RFA electrodes (Cool-tip or Osteo-Cool, Medtronic) were used for each BM according to its size and goal of the procedure. When multiple probes/electrodes were applied, simultaneous activation was performed to achieve a synergistic effect.
For curative treatments, the ablation area was planned to cover the entire BM volume with an additional 5–10 mm safety margin. For palliative treatments, the minimal goal set was to achieve effective ablation of the BM/normal bone interface and, whenever possible, complete ablation of the entire BM.
Whenever necessary, adjuvant thermo-protective measures including carbo- or hydro-dissection ( and ), nerve root electrostimulation or thermal monitoring through thermocouples were applied to avoid iatrogenic damage to nearby non-target critical structures located 1 cm within the BM [Citation16]. Subsequent mechanical stabilization was offered during the same interventional session with osteoplasty and/or osteosynthesis (depending on the location of the tumor and the regional biomechanics [Citation17]) for BM considered at high risk of secondary fracture.
Data collection
Chart review was performed by PA and PDM (interventional radiologists with 2 and 1 years of experience, respectively), who were blinded to procedural, clinical and BM details during data collection. Chart review was always performed by single authors. Intra- and inter-observer variability was not specifically assessed.
The following data were collected from each patient’s electronic records: patient characteristics (age, sex, previous RT to the index BM, Eastern Cooperative Oncology Group Performance Status [ECOG-PS], goal of treatment curative or palliative]); BM characteristics (histology, size [largest diameter on pre-operative imaging], location [spine, pelvis, long bone or others], radiological features of the tumor [lytic, sclerotic, mixed; presence of cortical disruption], presence of nearby vital structures located within 1 cm from the BM [nerves, vessels, solid organs, bowel, cartilage, or skin]); procedural details (type of TA [RFA or CA], need for thermoprotective measures, subsequent bone consolidation); as well as subsequent imaging and clinical follow-up findings and complications.
Complications were classified according to the Common Terminology Criteria for Adverse Event version 5.0 (CTCAE 5.0) into major (CTCAE grade 3–5), and minor (CTCA grade 1–2) [Citation18]. Among the assessed complications, postprocedural pain was recorded in our study when an increase of 3 or more points on the 0–10 visual analog scale (VAS) was noted at the treated site compared to the patient’s preprocedural status. The complication rate was reported as the number of complications divided by the number of BM.
Statistical analysis
Descriptive statistics were used to present the results. Mean and standard deviation or proportions were reported for continuous or categorical variables, respectively.
Complication rate analysis was performed per tumor according to the intention-to-treat principle.
The Student t- (for continuous variables) and the Chi-squared (for categorical variables) or Fischer’s tests (when the number of events was too low) were used to compare the two unmatched RFA and CA populations to highlight BM and procedural characteristics, as well as safety outcomes. Nevertheless, to confirm or reject the findings achieved by this analysis derived from such heterogeneous non-comparable populations, we have matched 1:1 BM treated with RFA and CA according to a propensity score method taking into account patient’s demographics (sex, age, ECOG-PS, previous RT and goal of the treatment), BM features (size, location, radiological features, cortical disruption and presence of nearby critical structures), procedural details (n. probes, thermo-protective measures and subsequent bone consolidation) and follow-up findings. The nearest neighbor approach was used. A p value <.05 was set to be statistically significant. Statistical analysis was performed using R statistical software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristic in the unmatched population
Baseline characteristics of the study population are summarized in . There were no differences between RFA and CA in terms of patients’ gender (female/male ratio 25/28 [89%] vs. 99/122 [81%], respectively; p=.76); and age (mean age 62.0 ± 11.0 years [range 31–81] vs. 61.5 ± 12.3 years [range 18–86], respectively; p=.77). RFA and CA were also comparable for the goal of treatment (curative/palliative ratio 14/52 [26.9%] vs. 47/254 [18.5%], respectively; p=.27), number of BM treated per interventional session (1.1 ± 0.4 [range 1–4] vs. 1.2 ± 0.5 [range 1–3], respectively; p = 0.07), and the length of follow-up (mean 18.5 ± 25.6 months [range 0–123] vs. 17.8 ± 23.7 months [range 1–112], respectively; p=.83).
Table 1. Patient demographics and tumor characteristics in the entire unmatched population.
Nonetheless, RFA was more frequently performed in patients with better performance status (223/301 [74.1%] CA patients with ECOG-PS ≤2 vs. RFA 59/66 (89.4%); p=.02), for spinal BM (CA 63/301 [20.9%] vs. RFA 40/66 [60.6%]; p<.001), with smaller size (mean size with CA 4.5 ± 2.2 cm [range 0.6–14.0] vs. mean size with RFA 3.0 ± 1.5 cm [range 0.9 − 0.0]; p<.001), and with less frequent closeness to critical structures (CA 200/301 [66.5%] vs. RFA 15/66 [23.1%]; p<.001). As a result, thermo-protective measures were more frequently used with CA compared to RFA (CA 223 [74.1%] vs. RFA 40 [60.6%]; p=.03). In addition, BM treated with RFA always presented with a lytic or mixed radiographic aspect, which was not the case with BM treated with CA that could present also with a sclerotic aspect (RFA 45/66 [68.2%] lytic, 0/66 [0.0%] sclerotic, 21/66 (31.8%) mixed vs. CA 194/301 [64.4%] lytic, 67/301 [22.3%] sclerotic, 40/301 (13.3%) mixed; p<.001).
BM treated with RFA were less likely to have received previous RT when compared to BM treated with CA (6/66 [9.1%] vs. 63/301 [20.9%], respectively; p=.02); and were more frequently treated in combination with bone consolidation during the same interventional session (20/66 [30.3%] vs. 64/301 [21.3%], respectively; p<.001).
Complications in the unmatched population
Within the entire unmatched population, complications occurred in 49/367 (13.4%) BM. In particular, there 9/367 [2.5%] major and 40/367 [10.9%] minor complications.
Complications were more likely to occur with RFA compared to CA (23/66 [34.8%] vs. 26/301 [8.6%], respectively; p<.001); which could be attributed to the higher incidence of minor complications occurring with RFA (22/66 [33.3%]) compared to CA (18/301 [6.0%]; p<.001), mainly in the form of immediate post-procedural pain requiring analgesic treatments (RFA 20/66 [30.3%] vs. CA 7/301 [2.3%]; p<.001].
Major complication rates were similar among RFA and CA (RFA1/66 [1.5%] vs. CA 8/301 [2.7%]; p = 1).
Major and minor complications and their management are detailed in and .
Table 2. Major complications in the entire unmatched population according to the applied thermal ablation technique.
Table 3. Minor complications in the entire unmatched population according to the applied thermal ablation technique.
Complication rates in 1:1 matched population
After applying the propensity score matching algorithm, 66 BM treated with RFA were matched to 66 BM treated with CA to provide homogeneous and comparable populations in terms of patient’s, BM and procedural characteristics (except for proximity to critical non-target structures, which remained less common in the RFA population compared to the CA population; 17/66 [23.1%] vs. 32/66 [48.5%]; p=.002; ), as well as follow-up.
Table 4. Patient demographics and tumor characteristics in the 1:1 matched population.
Analysis of matched populations confirmed the results noted in the study population without applying the propensity score method. In fact, there was no statistically significant difference in the incidence of major complications between RFA and CA (1/66 [1.5%] vs. 0/66 [0.0%], respectively; p = 1); and minor complications were still more common with RFA (22/66 [33.3%] vs. 2/66 [3.0%], respectively; p<.001) with immediate post-operative pain accounting for 20/22 (90.9%) of minor complications encountered with RFA.
Discussion
In this study, the overall complication rate for TA applied for the curative or palliative treatment of BM was 13.4%, with minor complications being largely more common (10.9%) than major ones (2.5%). In the unmatched population, the incidence of major complications was similar with RFA and CA (1.5 vs. 2.7%); which was not the case for minor complications, that were more likely to occur with RFA when compared to CA (33.3 vs. 6.0%) primarily due to the excessive incidence of immediate post-procedural pain with RFA compared to CA (30.3 vs. 2.3%). Such trend remained substantially unchanged in the RFA and CA populations resulting from the propensity matching that we have applied to lessen the influence of several confounding factors dealing with patients, BM, procedural and follow-up features. In fact, after matching the two populations, the major and minor complication rates with RFA and CA were 1.5% and 0.0%, and 33.3 and 3.0%, respectively; with post-operative pain accounting for 90.9% of the minor complications noted in the RFA group. Except for the high rate of postprocedure pain, which in our series was considered as a complications since it required an increased level of care, these results of ours are in line with literature on TA of BM reporting rates of minor and major complications ranging between 16 and 24%, and 7 and 8%, respectively [Citation11,Citation12,Citation14,Citation15,Citation19–21].
Coming to the analysis of the type of complications encountered in our study, pathological fracture was the most common major complication occurring in the CA group, with fractures occurring (in two acetabulae, one iliac bone and one scapula) approximately 2 months after the treatment, and requiring percutaneous fixation. This is recognized following bone tumor TA and results from loss of structural and mechanical integrity of the bone due to tumor and post-TA necrosis, which necessitates immediate bone consolidation during the same interventional session of TA to prevent such sequela [Citation13–15,Citation17]. This represents a major advantage of percutaneous TA when compared to RT [Citation13,Citation14,Citation22], which is also affected from a non-negligible risk of secondary fracture particularly when stereotactic techniques are used. Indications to perform bone consolidation should be based on Mirel’s score for long bone, SINS score for spine and should also take into account the size of the target tumor and the cortical involvement [Citation17,Citation23,Citation24]. Cementoplasty is the most adapted consolidation technique for bones mainly undergoing compressive stresses (e.g., vertebral body). On the other hand, screw-mediated osteosynthesis should be applied in bones mainly undergoing shearing stress, or whenever there is a need to bridge a fracture [Citation17,Citation25]. Other consolidation techniques have been proposed; nevertheless, many of them lack long-term biomechanical assessment [Citation26–29]. Accordingly, their use should be evaluated case by case within the multidisciplinary tumor board. Thus, interventional radiologists may promote TA of BM in the multidisciplinary tumor board by levering on the potential to integrate TA with percutaneous consolidation to prevent secondary pathological fractures, which carries high morbidity and mortality risks independently of the tumor type/stage.
On the other hand, the sole major complication experienced in the RFA population was a lethal septic shock developing at an RFA site. Therefore, strict sterile intra and postprocedural conditions and avoidance of any percutaneous procedure in case of suspected local or systemic infection are strongly recommended throughout literature [Citation5,Citation15,Citation16,Citation30].
Concerning minor complications, postprocedural pain was the salient adverse event after RFA, which was not the case with CA. This may be partially attributed to the analgesic properties of the ice-ball, which may significantly contribute to lowering postprocedural pain after bone tumor CA; and conversely by the pro-inflammatory properties of the necrosis associated with RFA [Citation31–34]. Aside from the pathophysiology of such event, there are practical concerns of poorly managed post-RFA pain, which has driven us to consider pain as a possible post-operative complication. In fact, we share the view from Thacker et al. who have reported that the intense post-RFA pain developing within the first few post-operative hours and often requiring active analgesia, may negatively impact the length of the in-hospital stay [Citation35]. Moreover, intense post-operative pain represents a major element considered whilst grading complications in the most common complication scales used in the field of interventional radiology (SIR, CIRSE and CTCAE scales) [Citation18,Citation36,Citation37]. To overcome such consequence, we advocate the adoption of dedicated institutional analgesic protocols for patients undergoing RFA, and we present in our recently adopted protocol, which is based on a standard systemic intraprocedural multimodal analgesia administered by the anesthesiologist, which is further reinforced by a central or peripheral nerve block performed by the interventionalist immediately following the RFA. Although we do not dispose of large prospective data on the presented analgesic protocol of ours due to its recent introduction, preliminary results from the first five consecutive patients treated with it revealed interesting results, with 24-h post-ablation pain never reaching levels >2/10 on the 0–10 VAS compared to baseline.
Table 5. Analgesic protocol recently adopted at authors’ institution.
Other common minor complications were almost equally distributed in RFA and CA populations and included post-ablation neural damage, which needed conservative management with steroids with subsequent remission. Many of these minor complications were attributed to damages to adjacent non-target structures, which occurred despite the large use (71.4%) of thermo-protective measures, often in the form of hydrodissection (79%), thermal monitoring (40%) or a combination of both of them.
Limitations of this study include its retrospective design, which did not allow prospective randomization of the population according to the TA technique resulting in unmatched groups of treatment that were mainly selected on a per tumor basis by each operator. To overcome this limitation, we have applied the propensity score method to match the RFA and CA populations; and although similar safety profiles were obtained in the unmatched and matched populations, results obtained with the latter allowed more robust appraisal of the safety profiles of these two commonly used TA techniques. Moreover, we did not report the in hospital stay; however, since post-procedural pain was systematically graded as minor, it is unlikely that it had resulted in a significant prolongation of the hospital stay [Citation18,Citation36,Citation37]. Furthermore, some patients referred to our institution for TA of BM have only benefited from 1-month follow-up consultation in our department. Therefore, we could not entirely rule out the potential for further complication detected in further follow-up reviews. Lastly, we compared only the most established ablation techniques (RFA and CA) despite there are emerging techniques such as microwave ablation and high-focused ultrasound. Nevertheless, experience with these new techniques is limited, and mainly focus on palliative treatments, often without large agreement on ablation protocols [Citation38].
In conclusion, CA and RFA of BM performed for curative or palliative reasons are both safe given the very low rate of major complications. Nevertheless, minor complications are more common after RFA due to high rates of post-operative pain, which should encourage the adoption of dedicated institutional analgesic protocols for patients receiving RFA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
- Wilkinson AN, Viola R, Brundage MD. Managing skeletal related events resulting from bone metastases. BMJ. 2008;337:a2041.
- Mastier C, Gjorgjievska A, Thivolet A, et al. Musculoskeletal metastases management: the interventional radiologist’s toolbox. Semin Intervent Radiol. 2018;35(04):281–289.
- Kurup AN, Morris JM, Callstrom MR. Ablation of musculoskeletal metastases. AJR Am J Roentgenol. 2017;209(4):713–721.
- Gangi A, Tsoumakidou G, Buy X, et al. Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol. 2010;33(4):706–713.
- Moynagh MR, Kurup AN, Callstrom MR. Thermal ablation of bone metastases. Semin Intervent Radiol. 2018;35(04):299–308.
- Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol. 2010;27(03):276–284.
- Nomiya T, Teruyama K, Wada H, et al. Time course of pain relief in patients treated with radiotherapy for cancer pain: a prospective study. Clin J Pain. 2010;26(1):38–42.
- Deschamps F, Farouil G, Ternes N, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24(8):1971–1980.
- Cazzato RL, Auloge P, De Marini P, et al. Percutaneous image-guided ablation of bone metastases: local tumor control in oligometastatic patients. Int J Hyperth. 2018;35(1):493–499.
- Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116(4):989–997.
- Auloge P, Cazzato RL, Rousseau C, et al. Complications of percutaneous bone tumor cryoablation: a 10-year experience. Radiology. 2019;291(2):521–528.
- Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22(2):300–306.
- Cazzato RL, Garnon J, Caudrelier J, et al. Percutaneous radiofrequency ablation of painful spinal metastasis: a systematic literature assessment of analgesia and safety. Int J Hyperth. 2018;34:1272–1281.
- Cazzato RL, Palussière J, Auloge P, et al. Complications following percutaneous image-guided radiofrequency ablation of bone tumors: a 10-year dual-center experience. Radiology. 2020;296(1):227–235.
- Tsoumakidou G, Buy X, Garnon J, et al. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol. 2011;14(3):170–176.
- Cazzato RL, Garnon J, Shaygi B, et al. Percutaneous consolidation of bone metastases: strategies and techniques. Insights Imaging. 2019;10(1):14.
- U.S.D. of H. and H. Services, common terminology criteria for adverse events (CTCAE), version 5.0; 2017. Avilable from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
- Gardner CS, Ensor JE, Ahrar K, et al. Cryoablation of bone metastases from renal cell carcinoma for local tumor control. J Bone Joint Surg Am. 2017;99:1916–1926.
- Ma Y, Wallace AN, Waqar SN, et al. Percutaneous image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Intervent Radiol. 2017;41:726–733.
- Cazzato RL, Garnon J, Caudrelier J, et al. Low-power bipolar radiofrequency ablation and vertebral augmentation for the palliative treatment of spinal malignancies. Int J Hyperth. 2018;34(8):1282–1288.
- Oh D, Huh SJ. Insufficiency fracture after radiation therapy. Radiat Oncol J. 2014;32(4):213–220.
- Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop. 1989;249:256–264.
- Campos M, Urrutia J, Zamora T, et al. The Spine Instability Neoplastic Score: an independent reliability and reproducibility analysis. Spine J. 2014;14(8):1466–1469.
- Deschamps F, de Baere T, Hakime A, et al. Percutaneous osteosynthesis in the pelvis in cancer patients. Eur Radiol. 2016;26(6):1631–1639.
- Cornelis FH, Deschamps F. Augmented osteoplasty for proximal femur consolidation in cancer patients: biomechanical considerations and techniques. Diagn Interv Imaging. 2017;98(9):645–650.
- Aebi M, Maas C, Pauli von Treuheim TD, et al. Comparative biomechanical study of a new transpedicular vertebral device and vertebroplasty for the treatment or prevention of vertebral compression fractures. Clin Biomech. 2018;56:40–45.
- Kelekis A, Filippiadis D, Anselmetti G, et al. Percutaneous augmented peripheral osteoplasty in long bones of oncologic patients for pain reduction and prevention of impeding pathologic fracture: the rebar concept. Cardiovasc Intervent Radiol. 2016;39(1):90–96.
- Gausepohl T, Pennig D, Heck S, et al. Effective management of bone fractures with the IlluminOss® photodynamic bone stabilization system: initial clinical experience from the European Union registry. Orthop Rev. 2017;9:6988.
- Tsoumakidou G, Koch G, Caudrelier J, et al. Image-guided spinal ablation: a review. Cardiovasc Intervent Radiol. 2016;39(9):1229–1238.
- Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33(12):140.
- Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology. 2005;237(1):366–370.
- Palussière J, Pellerin-Guignard A, Descat E, et al. Radiofrequency ablation of bone tumours. Diagn Interv Imaging. 2012;93(9):680–684.
- Rozenblum N, Zeira E, Bulvik B, et al. Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology. 2015;276(2):416–425.
- Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol. 2011;197(2):510–515.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146.
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28(10):1432–1437.e3.
- Cazzato RL, de Rubeis G, de Marini P, et al. Percutaneous microwave ablation of bone tumors: a systematic review. Eur Radiol. 2020. [Epub ahead of print].