?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: Magnetic hydrogels (MHGs) have been proposed to avoid the redistribution and loss of magnetic nanoparticles (MNPs) when administrated by intratumoral injection. However, the requirement of complex cooling systems and temperature monitoring systems still hinder the clinical application of MHGs. This study investigates the feasibility of developing an MHG to realize the self-regulation of hyperthermia temperature.
Methods: The MHG was developed by dispersing the MNPs with self-regulating temperature property into the temperature-sensitive hydrogel through physical crosslinking. The MHG's gelation temperature was tested by measuring the storage modulus and loss modulus on a rotational rheometer. The biocompatibility of the MHG and MNPs was characterized by CCK-8 assay against HaCaT cells. The in vivo magnetic heating property was examined through monitoring the temperature in the MHG on mice back upon the application of the alternating magnetic field (400 ± 5 Oe, 100 ± 5 kHz) every week for successive six weeks.
Results: The gelation temperature of the MHG falls in 28.4°C-37.4°C. At in vivo applied concentration of 80 mg/mL, the MHG exhibits over 80% cell viability after 72 h, significantly higher than 50% cell viability of the MNPs (p<0.001). The MHG's stable magnetic hyperthermia temperatures in vivo are in the range of 43.4°C–43.8°C.
Conclusions: The developed MHG can be injected using a syringe and will solidify upon body temperature. The biocompatibility is improved after the MNPs being made into MHG. The MHG can self-regulate the temperature for six weeks, exhibiting application potential for self-regulating temperature hyperthermia.
1. Introduction
The magnetic hyperthermia based on magnetic nanoparticles (MNPs) has the potential for conformal therapy. However, the small size of MNPs also boosts the risk of the in vivo redistribution, incurred by the release of dead cell intracellular solution and the enhancement of vascular permeability after hyperthermia once [Citation1,Citation2], spreading to the peripheral healthy tissue and lowering curative effect [Citation3–5]. For instance, in the treatment of human prostate cancer on the mouse back, the 25 min hyperthermia resulted in the Fe3O4 MNPs spreading from the intratumoral injection site to tumor periphery [Citation2]. In the treatment of glioma in the rat thalamus, the dextran-coated Fe3O4 MNPs were also found to spread into the surrounding tissue shortly after intratumoral injection [Citation3]. Similarly, in the application of Fe3O4 MNPs on human ductal breast carcinoma on the mouse model, only 3/4 MNPs could be detected by magnetorelaxometry after 24 h [Citation4]. The migration of Fe3O4 MNPs in adenocarcinoma of the rat to the surrounding bone were also detected after the intratumoral injection of 6 h [Citation5]. The consequence of this risk is a significant reduction in therapy efficiency.
In response to this, thermosensitive and injectable hydrogels, such as poly(ethyleneglycol) diacrylate [Citation6], poly(organophosphazene) [Citation7,Citation8] and poly(Nisopropylacrylamide) [Citation9,Citation10] have been applied to carry MNPs, forming magnetic hydrogel (MHG). When the temperature is increased over body temperature, MHG experiences the solution-to-gel phase transition, and to a certain extent, the MNPs can be fixed at the location where they are injected. Another concern pertaining to the MNPs hyperthermia is the precise control of the temperature in tumor due to the complex biological environment [Citation11]. MNPs with self-regulating temperature characteristic offer a chance to solve this problem [Citation12–14]. Those unique MNPs can limit the upper hyperthermia temperature to a threshold without the involvement of the temperature control systems.
In the previous work, we developed the temperature-sensitive chitosan/hyaluronic acid/sodium glycerophosphate hydrogel, which behaves like a liquid at room temperature (10–23 °C) and quickly solidifies at human body temperature (36–37 °C) [Citation15]. Additionally, this biocompatible hydrogel has a good affinity for the CD44-expressed cancer, due to the specific binding of hyaluronic acid and CD44 [Citation15,Citation16]. Herein, we combine this hydrogel with the self-regulating temperature MNPs to develop the MHG, which has the following merits (i) the MNPs can self-regulate the hyperthermia temperature, (ii) the temperature-sensitive and injectable hydrogel can fix the MNPs at the location of injection, (iii) the MHG can be bonded to the cancer tissue through the interaction between antigen and antibody.
2 Experimental section
2.1. Preparation of MNPs
The chemicals were purchased from Shantou Xilong Chemical Co. Ltd., Guangdong, China. 1.214 g ZnCl2, 1.806 g CoCl2·6H2O, 2.638 g CrCl3·6H2O and 6.244 g FeCl3·6H2O were dissolved in 80 ml deionized water. Then, 18 g NaOH in 150 ml deionized water was added under stirring. The mixture was sealed in the high-pressure autoclave and heated to 180 °C for 6 h. The pressure within the autoclave was 0.60–0.62 MPa. Then, the resultant powder was washed with deionized water until neutral and dried at 80 °C for 8 h.
2.2. Characterization of MNPs
The morphology, size distribution and crystalline information of the MNPs were measured through the transmission electron microscopy (TEM, FEI Tecnai G2 F30). The crystalline structures were measured through the X-ray powder diffractometer (XRD, PANalytical Empyrean X-ray diffractometer with Cu-Kα radiation, λ = 1.54056 Å, Netherlands). The magnetization curve was measured on the vibrating sample magnetometer (VSM, Jilin University JDM-13, China) with the applied field of ±20 kOe at room temperature. The Curie temperature (Tc) of MNPs was tested by thermogravimetric analysis (TGA).
2.3. Preparation of MHGs
60 mg chitosan (Sinopharm Chemical Reagent Co. Ltd., China) and 11.44 μl acetate acid (Kermel, Tianjin, China) were added into 2 ml deionized water. Then, 0, 25, 50, 100, 150 and 200 mg nanoparticles were added into the solution. The mixtures were ultrasonicated for 30 min. 0.3 g hyaluronic acid (HA, Mw: 1500000 Shengjiade Bio Science and Technology Co. Ltd. China) and 5 mg β-glycerophosphate (Bomei, Hefei, Anhui, China) were dissolved in 0.5 ml deionized water to make the solution. The mixture and the solution were kept in ice-bath to maintain a temperature under 4 °C. Then 0.5 ml solution was dropped into the 2 ml mixture under stirring in the ice-bath to make the MHGs. The components of MHGs are given in . Considering the high density of MNPs, the volumes of MNPs per unit of MHGs are negligible. Thus, the concentrations of the MHGs are marked as the mass of MNPs over the volume of hydrogel.
Table 1. The components of magnetic hydrogels.
2.4. Rheological measurement
The rheological properties were tested on a rotational rheometer (Anton Paar MCR302, Austria) with a PP25 plate indenter (platform plate configuration). The MHGs were piped between the plates with mineral oil on the periphery of the hydrogel, preventing water evaporation during the tests. Dynamic oscillatory mode measurements were performed at the strain of 5% to ensure the storage modulus (G') and the loss modulus (G'') are independent of the strain amplitude [Citation15]. In dynamic temperature sweep, the frequency was set as 10 rad/s and the temperature was increased from 10 °C to 45 °C with a rate of 2.5 °C/min. The sweep time was set as 1000s. In dynamic time sweep, the sample plate was pre-heated to 37 °C. Then the MHGs were dropped onto the plate and pre-heated for 30 s. Following this, the time sweep at 37 °C was conducted.
2.5. SEM observation and EDS analysis
After the gelation, the MHGs were frozen in liquid nitrogen for 20 min and then fractured to obtain the cross-section. The obtained sample was immediately transferred to a freeze drier (FD-1A-50, China Shanghai Xinweng) and then lyophilized for 24 h. After coated with gold on a magnetron ion sputter metal coating device (Vacuum Device MSP-1s, Japan), the microstructures were observed by SEM (FEI Quanta 450, FEI), and the element contents were analyzed by an energy dispersive spectrometer, EDS (X-Max, Oxford Instruments, Abingdon, UK).
2.6. Biocompatibility
The biocompatibility of MNPs and MHGs with different concentrations were tested through CCK-8 assay against HaCaT cells (China Center for Type Culture Collection, Wuhan). The MHGs of 200 μg/ml and 80 mg/ml were made. Then, the MNPs and MHGs were sterilized with 75% (v/v) ethanol/deionized water for 24 h and rinsed with phosphate buffer saline (PBS) three times. Then the MNPs were suspended in cell culture medium with concentrations of 200 μg/ml and 80 mg/ml, while the MHGs were soaked in the cell culture medium.
The HaCaT cells were cultivated in a humidified incubator (Thermos HERAcell150) at 37 °C and 5% CO2 with high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco). Meanwhile, with the supplement of 1% penicillin-streptomycin (Gibco) and 10% fetal bovine serum (FBS, Gibco), HaCaT cells were seeded in 96-well plates with a density of 5 × 104 cells per ml. After 3 h culture, 70 μL cell culture medium with MNPs or MHGs were separately added for contact with cells in each well.
After 24 h, 48 h and 72 h incubation, the culture medium in each well was replaced by 100 μL new mediums with 10 μL CCK-8 and incubated for another 3 h. Then, a microplate reader (SpectraMax M2e, Molecular Devices) was used to measure the absorbance of the formazan product at 450 nm, and the cell viability was calculated [Citation17].
2.7. SLP measurements
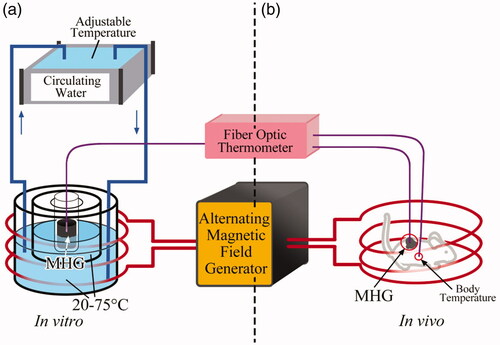
The specific loss powers (SLPs, representing the heat production efficiency) of MHG80 at 20–75 °C, under 400 ± 5 Oe and 100 ± 5 kHz, were tested on the experimental setup illustrated in . The setup consists of alternative magnetic field generating unit (GUF-30T, Shenqiu Yongda High Frequency Equipment Co. Ltd, Henan, China), circulating water unit with adjustable temperature and thermometer unit. The central temperature of 2 ml MHG80 (80 mg/mL) was monitored by the fiber optic thermometer (Fotemp-Trafo FTT-0100, Optocon, Dresden, Germany). Through tuning the temperature of circulating water, the startup temperature of MHG80 was set at 20–75 °C with 5 °C interval before SLP measurements. Then, the alternative magnetic field generator was switched on for 10 min (100 ± 5 kHz, 400 ± 5 Oe) to obtain the time-dependent temperature curves. The experiments were performed for five times under each condition. Details about the experiment setup are given in the Supporting information.
The SLP, which describes the energy converted into heat per time and mass, was calculated using the formula (1) [Citation18]:
(1)
(1)
where, cpMHG is the specific heat capacity (2.30 ± 0.18 J·g−1·°C−1) of MHG, determined through differential scanning calorimetry method in reference [Citation19]; T is the temperature; t is the time; dT/dt is the initial slope of the time-dependent temperature curve when t→0, provided the temperature distribution within the sample is homogeneous, and the initial thermal losses are negligible [Citation20]; mMHG is the mass of MHG, mMNP is the mass of MNPs in the MHG. The experiments were performed for five times under each condition. The data were analyzed by mean ± combined standard uncertainty.
2.8. In vivo experiments
Ten male Kunming mice were purchased from Dalian Medical University (Dalian, China), five for carrying MHGs to test the magnetic heating property and five as the control group to test the body temperature changes under the alternating magnetic field. The MNPs and the transparent solution of chitosan and acetate acid, as well as the transparent solution of hyaluronic acid and β-sodium glycerophosphate were sterilized by ultraviolet irradiation for 12 h. After the five mice in MHG group were anesthetized by intraperitoneal injection of 10% chloral hydrate (10 mg/kg), 500 μL MHG80 (80 mg/mL) was subcutaneously injected on the right back of the mice.
The in vivo experiments were conducted by the experiment setup in . Details about the experiment setup are given in the Supporting information. For the MHG group, the thermometer probes were fixed in the MHG and the back of mice on the other side for security consideration. For the control group, the thermometer probes were fixed subcutaneously in the back on the both sides. The temperatures were recorded by the fiber optic thermometer. The intensity of the alternating magnetic field was 400 ± 5 Oe, and the frequency was 100 ± 5 kHz, which matched the safety criterion for human being [Citation21]. The temperature rising measurements for MHG group were performed weekly for successive six weeks to examine the stability of the in vivo heating capacity of MHG, and the measurements for control group were performed for one time. The thermal infrared camera (Ti25, Fluke, Washington) was used to measure the distribution of temperature.
All of the experimental procedures were performed in accordance with protocols approved by the Biology and Medical Ethics Committee of Dalian University of Technology. The photocopy of the approved application form is attached to the Supporting information.
3 Results and discussions
3.1. Magnetic properties of MNPs
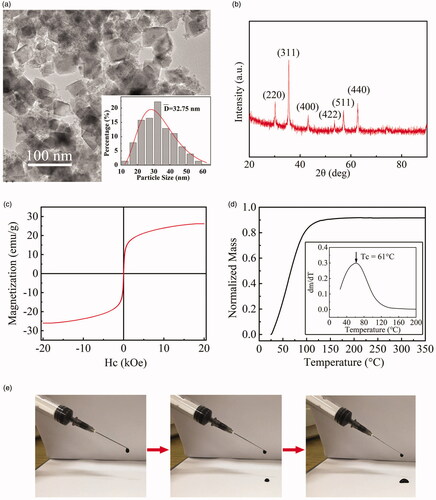
The magnetic nanoparticles (MNPs) were made through the hydrothermal method. The manufactured Zn0.54Co0.46Cr0.6Fe1.4O4 MNPs have nanocube shape with a size range of 12.5–62.5 nm, which can be seen through the TEM images with size distribution in . The determination of the MNPs element ratio is shown in the Supporting information. The size distribution of the MNPs exhibits lognormal form (Mean diameter of 32.75 nm), as shown in the inset of . The XRD pattern of the MNPs is exhibited in . The crystallite size calculated by the Scherer formula is 29.8 nm. The Bragg reflections of the MNPs can be indexed as (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) without obvious impurity peaks, compared with the standard data of CoFe2O4 (JCPDS-ICDD database no. 22-1086), confirming the spinel crystal structures of the MNPs. The magnetization curve of the MNPs is shown in , exhibiting the specific saturation magnetization (σs) of 26.2 emu·g−1. The Curie temperature (Tc) of MNPs was tested through the TGA method [Citation22]. The normalized mass as the function of temperature was depicted in . The first derivative of the obtained curve was plotted, and the maximum value is referred as Tc of the MNPs, i.e., 61 °C. The temperature of MNP suspension is not over Tc, proved by in vitro magnetic heating experiments, shown in the Supporting information.
Figure 2. (a) The TEM image of MNPs and the size distribution (the inset); (b) the XRD pattern of MNPs; (c) the magnetization curve of MNPs; (d) the normalized mass-temperature curve of MNPs from TGA and the corresponding 1st derivative (its inset); (e) MHG can be injected through a commercial 5 ml syringe with a 22-gauge needle.
3.2. Rheological properties and morphology of MHGs
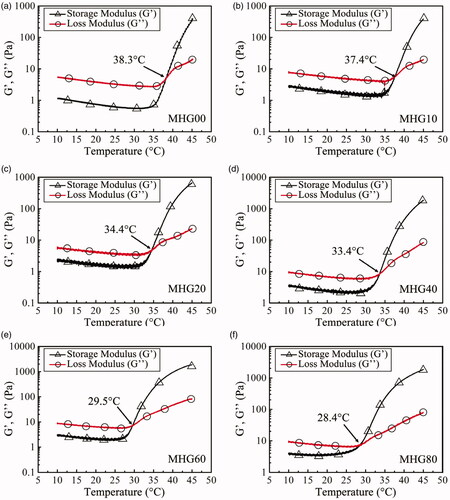
We used thermal-sensitive hydrogel to carry MNPs and fabricated magnetic hydrogels (MHGs). The newly formed MHGs showed fluid characteristics and could be injected through a commercial 5 ml syringe with a 22-gauge needle, as shown in , due to the fact that the Brownian movement of MNPs becomes sluggish in viscous medium. The MHGs with different MNPs concentrations were tested on the rheometer, as shown in . The viscoelastic properties of MHGs were assessed by measuring the storage modulus (G’), representing the elastic behavior of the material, and loss modulus (G’’), indicative of the viscous behavior. When the temperature is at 10 °C, the G’ is lower than G’’ and the MHG behaves more like a solution. As the temperature increases, the G’’ increases slightly and G’ increases abruptly over a certain temperature, and the temperature where G’ equals G'' is defined as the gelation temperature. demonstrates that the gelation temperature for MHGs ranges from 37.4 to 28.4 °C, with the MNP concentration of 0-80 mg/mL, suggesting that the gelation of MHG can be induced by increasing the temperature to human body temperature. The decrease of gelation temperature with increasing MNP concentration indicates that the physical crosslinking of MNPs with matrix facilitates the sol-gel transition, which may be attributed to the interactions between the hydroxyl group on the surface of MNPs and hydrogel matrix.
Figure 3. The dynamic temperature sweep tests of the MHGs with varying MNP contents: (a) MHG00; (b) MHG10; (c) MHG20; (d) MHG40; (e) MHG60; (f) MHG80.
Meanwhile, a higher MNP concentration does not impact the complex viscosity of MHGs when they were fluids. As shown in , the complex viscosities of magnetic hydrogels with 0 mg/mL, 40 mg/mL and 80 mg/mL MNP concentrations are all around 1 Pa·s at 10 °C, decreasing slightly with increasing temperature until the gelation temperatures. This means the MHGs at 0–80 mg/mL remain injectable, as illustrated in .
Figure 4. (a) Plot of the complex viscosity of the magnetic hydrogels with varying MNPs content: MHG00, MHG40, MHG80; (b) plot of the elastic modulus (G’) and viscous modulus (G’’) of the magnetic hydrogels versus time at a frequency of 10 rad/s; (c) SEM image and EDS images of the cross-section of freeze-dried MHG80.

The gelation time is the time elapsed before the storage modulus of the fluid turns out to be higher than the loss modulus at a given temperature, reflecting the gelation rate of the MHG. As exhibits, for MHG00 and MHG10, the gelation times are about 60 s and 10 s, while the gelation time of MHG20, MHG40, MHG60 and MHG80 cannot be observed. This is because the precursor solution forms a gel in the 30 s pre-heating. These results show that the introduction of MNPs can shorten the gelation time of the hydrogel. After 1000s at 37 °C, G’ remains stable for all specimens and higher than G’’, indicating a stable gel state at a temperature above body temperature.
In , the SEM images of MHG and the corresponding element distribution mapping are shown. The Fe, Co, Cr and Zn result from the MNPs, and the C, O, Na and P arise from the components of hydrogel. The uniform distribution of elements reflects the homogeneous distribution of MNPs.
3.3. Biocompatibility and SLPs
One possible benefit of using hydrogel to encapsulate MNPs is to reduce the potential cytotoxicity of MNPs. The biocompatibilities of MNPs and MHGs with concentrations of 200 μg/mL and 80 mg/mL were tested on HaCaT cells. As shown in , although the 200 μg/mL MNPs showed good biocompatibility, when the concentration reaches 80 mg/mL, which is at the same level as therapeutically required dose [Citation23], the cell viability drops down to 50% after 72 h. On the contrary, all the hydrogels and MHGs exhibit over 80% cell viability after 72 h. This is because the MNPs were embedded in hydrogel, as shown in , avoiding the direct contact between cells and MNPs. Thus, it seems the combination of MNPs with hydrogel can increase the safe dose threshold of MNPs.
Figure 5. (a) The cytotoxicity of MNPs at 200 μg/mL and 80 mg/mL, hydrogel, and MHGs at 200 μg/mL and 80 mg/mL (n = 5, mean ± SD), at 24, 48 and 72 h (**: p < .01, ***: p < .001); (b) the SLPs of MHG80 at 20–75 °C, 5 °C interval, under 400 ± 5 Oe and 100 ± 5 kHz (n = 5, mean ± combined standard uncertainty).

The SLPs of MHG80 at 20–75 °C under 400 Oe and 100 kHz are shown in . The measured SLP is higher at higher magnetic field intensity and decreases with increasing temperature over 35 °C. The SLP of MHG80 drops from 3.88 ± 0.37 W/g at 35 °C to 0.78 ± 0.13 W/g at 75 °C. The decrease of SLP is caused by the reducing magnetization of MNPs near the Curie temperature [Citation22,Citation24].
3.4. In vivo heating stability
The in vivo heating of MHG was repeated weekly for successive 6 weeks, and the results are shown in . For all the six weeks, the MHG stabilizes at 43.4 ± 0.5 °C −43.8 ± 0.7 °C after about 10 min, indicating the good heating stability of MHG in vivo. This temperature can induce the hyperthermia therapy effect [Citation25]. The stability in heating ability may result from the fact that the hydrogels cover and lock up the MNPs, as shown in . The dorsum temperatures of the mice in control group remain around 37 °C (35.9 ± 1.1–37.2 ± 0.9 °C), which are the normal body temperatures of the mice, indicating the increasing temperature resulting mainly from the MHG rather than the alternating magnetic field on the living body. The peak temperatures are lower than the Tc of MNPs (61 °C). This may be ascribed to the heat dissipation, such as heat conductivity of living tissue and blood perfusion, as well as the decreasing SLP of MNPs over 35 °C, shown in . Similar gaps between Curie temperature and in vivo peak temperature exists in other self-regulating applications [Citation12,Citation26].
Figure 6. (a) In vivo magnetic hyperthermia experiments. MHG: the temperature rises of MHGs beneath the back skin of mice (n = 5) under 400 ± 5 Oe and 100 ± 5 kHz for 6 weeks. Control: the subcutaneous temperatures of the mouse back (n = 5) without MHG injection under the magnetic field of 400 ± 5 Oe and 100 ± 5 kHz for 1 time. Data: mean values and standard deviations (SD); (b) the mouse carrying MHG (in red dotted circle) in magnetic coil with a reference ruler; (c) skin temperature of the mouse by thermal infrared camera; (d) zooming at the MHG region of (b).
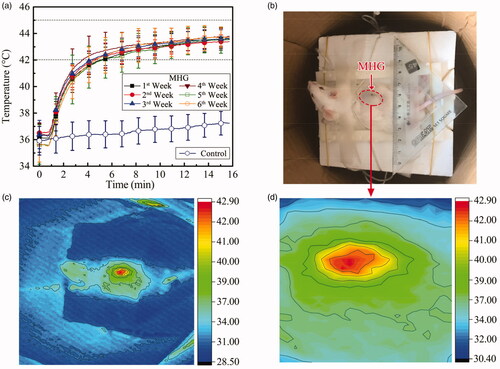
The skin temperature map of the mouse captured on a thermal infrared camera is shown in . The temperature-rising region is consistent with the area of injected MHG. The highest temperature obtained on the skin was 42.9 °C. While the highest temperature sensed by fiber-optic thermometer was 43.8 °C. The 0.9 °C temperature difference between the inner temperature and surface temperature might be caused by the cover of the skin.
In hyperthermia therapy, when the MNPs are heated by the alternating magnetic field, the heat elevates the temperature in the tumor, forming temperature gradients with surrounding tissue, driving the heat to the surrounding tissue. The heat dissipation increases with temperature differences until it equals the heating power of the MNPs, resulting in a stable temperature gradient, similar to that in . However, the individual differences in heat dissipation parameters among patients add uncertainties to the preplan. Thus, in the clinical trials of MNP hyperthermia, thermometers and cooling systems are applied to avoid overheat [Citation27]. As for the MHG with self-regulating temperature, fabricated here, its SLP drops over 35 °C, providing additional insurance for preventing overheat.
The MHG here also suites for long-term intratumoral therapy, considering its injectability and long-term heating stability. Further, with the help of low Curie temperature MNPs, the MHG has the potential to self-regulate temperature to the therapeutic temperature range and avoids the application of thermometers and cooling systems. Another possible treatment route is to paste the MHG on the wall of the brain cavity after surgical resection for recurrent cancer [Citation28].
4. Conclusions
A novel magnetic hydrogel (MHG) with self-regulating temperature and long in situ retention is developed. The MHG consists of the temperature-sensitive hydrogel with low gelation temperature (28.4 °C) and the self-regulating magnetic nanoparticles (MNPs) with low Curie temperature (61 °C). The MHG can be injected using a syringe and in situ and solidify upon body temperature. The gelation temperature and gelation time reduce with increasing concentration of MNPs, while the complex viscosity of the MHG increases marginally, indicating the good mobility of MHG even at 80 mg/mL. The MHG has higher biocompatibility at 80 mg/mL (p < .001) than MNPs and self-regulates temperature to 43.4–43.8 °C in vivo. No significant loss of heating ability can be detected for at least one month. This MHG may offer a new route for applying magnetic nanoparticles based hyperthermia more precisely, safely and effectively.
Supplemental Material
Download PDF (2.5 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Attaluri A, Ma R, Qiu Y, et al. Nanoparticle distribution and temperature elevations in prostatic tumours in mice during magnetic nanoparticle hyperthermia. Int J Hyperthermia. 2011;27(5):491–502.
- Gu Q, Joglekar T, Bieberich C, et al. Nanoparticle redistribution in PC3 tumors induced by local heating in magnetic nanoparticle hyperthermia: in vivo experimental study. J Heat Transfer. 2019;141(3):032402.
- Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006;78(1):7–14.
- Richter H, Kettering M, Wiekhorst F, et al. Magnetorelaxometry for localization and quantification of magnetic nanoparticles for thermal ablation studies. Phys Med Biol. 2010;55(3):623–633.
- Zadnik PL, Molina CA, Sarabia-Estrada R, et al. Characterization of intratumor magnetic nanoparticle distribution and heating in a rat model of metastatic spine disease: laboratory investigation. J Neurosurg Spine. 2014;20(6):740–750.
- Lee H, Thirunavukkarasu GK, Kim S, et al. Remote induction of in situ hydrogelation in a deep tissue, using an alternating magnetic field and superparamagnetic nanoparticles. Nano Res. 2018;11(11):5997–6009.
- Zhang Z-Q, Song S-C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials. 2016;106:13–23.
- Kim JI, Chun CJu, Kim B, et al. Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials. 2012;33(1):218–224.
- Campbell S, Maitland D, Hoare T. Enhanced pulsatile drug release from injectable magnetic hydrogels with embedded thermosensitive microgels. ACS Macro Lett. 2015;4(3):312–316.
- Campbell SB, Patenaude M, Hoare T. Injectable superparamagnets: highly elastic and degradable poly(N-isopropylacrylamide)-superparamagnetic iron oxide nanoparticle (SPION) composite hydrogels. Biomacromolecules. 2013;14(3):644–653.
- Zhang W, Wu C, Silva SRP. Proposed use of self-regulating temperature nanoparticles for cancer therapy. Expert Rev Anticancer Ther. 2018;18(8):723–725.
- Tang Q, Zhang D, Cong X, et al. Using thermal energy produced by irradiation of Mn-Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expression. Biomaterials. 2008;29(17):2673–2679.
- Saito H, Mitobe K, Ito A, et al. Self-regulating hyperthermia induced using thermosensitive ferromagnetic material with a low Curie temperature. Cancer Sci. 2008;99(4):805–809.
- Ito A, Saito H, Mitobe K, et al. Inhibition of heat shock protein 90 sensitizes melanoma cells to thermosensitive ferromagnetic particle-mediated hyperthermia with low Curie temperature. Cancer Sci. 2009;100(3):558–564.
- Zhang W, Jin X, Li H, et al. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr Polym. 2018;186:82–90.
- Thomas RG, Moon MJ, Lee H, et al. Hyaluronic acid conjugated superparamagnetic iron oxide nanoparticle for cancer diagnosis and hyperthermia therapy. Carbohydr Polym. 2015;131:439–446.
- Zhang W, Zuo X, Niu Y, et al. Novel nanoparticles with Cr3+ substituted ferrite for self-regulating temperature hyperthermia. Nanoscale. 2017;9(37):13929–13937.
- Hilger I, Frühauf K, Andrä W, et al. Heating potential of iron oxides for therapeutic purposes in interventional radiology. Acad Radiol. 2002;9(2):198–202.
- Abdel-Sayed P, Moghadam MN, Salomir R, et al. Intrinsic viscoelasticity increases temperature in knee cartilage under physiological loading. J Mech Behav Biomed Mater. 2014;30:123–130.
- Bordelon DE, Cornejo C, Grüttner C, et al. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J Appl Phys. 2011;109(12):124904.
- Hergt R, Dutz S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J Magn Magn Mater. 2007;311(1):187–192.
- Zhang W, Yu X, Li H, et al. Magnetic nanoparticles with low Curie temperature and high heating efficiency for self-regulating temperature hyperthermia. J Magn Magn Mater. 2019;489:165382.
- Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–324.
- Yu X, Yang R, Wu C, et al. Effect of chromium ion substitution of ZnCo ferrites on magnetic induction heating. J Alloys Compd. 2020;830:154724.
- Pang CLK. Hyperthermia in oncology. Boca Raton, FL: CRC Press; 2015.
- Deger S, Taymoorian K, Boehmer D, et al. Thermoradiotherapy using interstitial self-regulating thermoseeds: an intermediate analysis of a phase II trial. Eur Urol. 2004;45(5):574–580.
- Johannsen M, Gneveckow U, Eckelt L, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperthermia. 2005;21(7):637–647.
- Grauer O, Jaber M, Hess K, et al. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J Neurooncol. 2019;141(1):83–94.