Abstract
Background: Fever-range hyperthermia or fever-range temperature (hereafter FRT) improves survival and shortens disease duration in microbial infections. However, the mechanisms of these beneficial effects still remain elusive. We hypothesized that FRT might enhance cell responsiveness to infections by promoting cGAS-STING signaling to cause enhanced production of IFN-β.
Objective: To investigate the effect fever-range hyperthermia on cGAS-STING pathway.
Methods: RAW 264.7 and cGAS-/- RAW 264.7 cells, stimulated with 5μg/ml herring testis DNA (htDNA), were heated to 39.5°C and analyzed for the expression of cGAS, STING, IFN-β, and the synthesis of cGAMP and IRF3 phosphorylation. In vivo, wild type C57BL/6J mice were subjected to whole body hyperthermia (WBH) at 39.5°C. The mice were then challenged with influenza virus and analyzed for antiviral response in term of IFN-β expression, body weight and survival.
Results: We found that 39.5°C FRT upregulated the expression of cGAS and STING, and induced the synthesis of cGAMP and production of IFN-β in htDNA-transfected RAW 264.7 cells more potently as compared to 37°C. Moreover, FRT+DMXAA-treated cells were better protected from vesicular stomatitis virus (VSV)-induced cytotoxicity in vitro in contrast to the nonprotected control (no FRT and DMXAA) or DMXAA treatment alone. In vivo, FRT at 39.5°C, co-administered with DMXAA, significantly induced the expression of IFN-β, showed reduced weight loss mice and exhibited 25% more survival over the course of 14 days as compared to DMXAA treated mice 37°C.
Conclusion: We conclude that fever-range hyperthermia promotes cGAS-STING pathway to cause increased expression of IFN-β and mediate its antiviral effects.
Introduction
Temperature is considered to be closely associated with the host immune responses, which could establish an antiviral state for preventing viral infection [Citation1]. High temperatures have been observed to suppress viral replication whereas lower temperature favors viral replication [Citation2]. For example, hypothermia at 45 °C is effective in suppressing human rhinoviruses (HRV) multiplication by more than 90% [Citation3], high temperatures at 39 °C decreased RNA levels and/or viral titers of different influenza virus strains [Citation4] and the production of infectious particles decreased by >1000-fold in the non-transformed cells at 39.5 °C [Citation5]. This shows that fever-range temperature (FRT) is closely associated with improved host immune responses.
The beneficial effects of hyperthermia (HT) have been associated, in parts, with the improved interferon outcome. Temperatures higher than 37 °C have been shown to induce type-I interferon (IFN-I) expression whereas lower temperatures reduce IFN-I activity [Citation6–8]. The burst/suppression cytokine profile at febrile temperatures has been proposed to enhance early activation of host defenses and prevent prolonged exposure to potentially cytotoxic cytokines. Low temperatures, on the other hand, worsen disease outcome in infections by delaying and prolonging cytokine production [Citation9]. Despite its beneficial effects, the mechanism of these beneficial effects of elevated temperature is still not fully elucidated and needs to be uncovered.
cGAS–STING pathway is a component of the innate immune system that functions to detect the presence of cytosolic DNA. Upon binding DNA, cyclic GMP-AMP synthase (cGAS) triggers the reaction of GTP and ATP to form cyclic GMP-AMP (cGAMP) [Citation10]. cGAMP binds to stimulator of interferon genes (STING). STING then recruits TANK binding kinase 1 (TBK1) and activates transcription factor interferon regulatory factor 3 (IRF3), which then translocate into the nucleus to induce the transcriptional activation of IFN-I and other inflammatory cytokines, thus establishing an antiviral state in infected and uninfected neighboring host cells [Citation11,Citation12].
IFNs-I play pivotal role in innate antiviral defense response and have been used in antiviral therapy [Citation13,Citation14]. Mice deficient in cGAS or STING exhibit lower IFN-I levels and are more susceptible to viral infection [Citation15]. Basal expression of IFNs is essential for priming the innate immune system to respond further to microbes and other stimuli. This basal IFN response requires cGAS activation [Citation16]. The expression levels of IFN-β correlates with the ability of the cells to express cGAS [Citation17]. Overexpression of cGAS activates transcription factor IRF3 and induces IFN-β in STING dependent manner whereas knockdown of cGAS inhibits IRF3 activation and IFN-β induction by DNA transfection or DNA virus infection [Citation17]. We hypothesized that FRT might enhance responsiveness of cells to viral infection by promoting cGAS/STING expression and the signaling pathway. We found that elevated temperature at 39.5° up-regulated the expression of cGAS protein and the synthesis of cGAMP in RAW264.7 cells in the presence of exogenous DNA, which in return induced the expression of IFN-β. IFN-beta ceased down to express in response to FRT in cGAS−/− cells or by inhibiting cells with cGAS inhibitor RU.521. In vivo, FRT synergized the antiviral efficacy of DMXAA (5,6-dimethylxanthenone-4-acetic acid), an agonist of the cGAS-STING pathway. We conclude that FRT enhances antiviral activity by up-regulating the expression of cGAS protein and promoting cGAS-STING pathway to induce elevated expression of IFN-β. These results might reveal a possible mechanistic explanation for the significance of fever in activating innate immune responses.
Materials and methods
Virus strains and cells
Vesicular stomatitis virus (VSV) was propagated as described previously [Citation18]. Mouse-adapted H1N1 influenza PR8 virus was grown in the allantoic fluid of 10-day-old embryonated chicken eggs, and virus titers were determined as previously described [Citation19].
Murine macrophage-like cell line RAW 264.7 and cGAS−/− RAW 264.7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 2 mM L-glutamine [Citation20]. For HT exposures, cells were grown at density 105 in 36-mm dishes. After 30-min preincubation at 37 °C, cells were stimulated with 5 µg/ml herring testis DNA (htDNA) using lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA) and maintained either at 39.5 °C (FRT) or 37 °C (normothermic control) in a water bath for 24 h. Cells supernatant was collected by gently centrifuging at 180 g for 3 min. ELISA on cell supernatant for IFN-β was performed using the IFN-β human ELISA Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. All animal studies were approved by the Institutional Animal Care and Use Committee of the Shanghai Jiao Tong University.
Gene expression analysis by real-time PCR
RNA was extracted using TRIzol (Ambion, Austin, TX) following the vendor protocol. A total of 800 ng of total RNA was reverse transcribed for cDNA synthesis in 20 μl final reaction volume using oligo (dT) primers at 2.5 μM and 10 U μl−1 SuperScript III (Thermo Fisher) for 50 min at 50 °C. Quantitative PCR was performed on a StepOne Plus qPCR System (Applied Biosystems, Foster City, CA) using 1/20th volume of the reverse-transcribed material as an input for each qPCR reaction. Expression levels of cGAS, STING and IFN-β mRNAs were measured in technical triplicate for each sample using primers shown in . Threshold cycle (CT) values obtained for the indicated mRNAs were normalized to GAPDH CT values. Relative mRNA expression levels were calculated using the ΔΔCT method (2^ΔΔCt).
Table 1. Primers for quantitative RT-PCR.
Western blot analysis
Cells were grown and treated as above. Cells were then collected and suspended in Laemmli sample buffers (Bio-Rad, Hercules, CA) containing 5% β-ME, followed by boiling for 5 min. The supernatants were collected as cell lysates, and the protein concentration was measured using BCA protein assay kit (Pierce, Appleton, WI). Equal amounts of protein samples (50 µg) were loaded on a 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in TBST buffer for 2 h at room temperature and subsequently incubated with primary antibodies at 4 °C overnight, respectively. It was followed by incubation with respective HRP-conjugated secondary antibodies, and the bands were developed with ECL substrate (Thermo Fisher Scientific, Waltham, MA and imaged. The following primary antibodies were used: anti-GAPDH, anti-cGAS, anti-IFN-β and anti-phospho-IRF3 (all from Cell Signaling Technology, Danvers, MA). Horseradish peroxidase-conjugated anti-mouse or anti-human IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a secondary antibody.
In vitro effect of FRT on the efficacy of DXMAA-induced antiviral response
RAW264.7 macrophages were cultured as described previously [Citation20] and seeded at 1 × 105 cells/well in a 96-well plate. After overnight incubation at 37 °C, cells were treated with medium containing DMXAA (100 µg/ml), and FRT group was subjected to 39.5 °C. After 3 h, the culture medium was replaced with serum-free DMEM containing VSV at the MOI of 0.1 for 1 h. Cells were then maintained in complete DMEM with 10% FBS. Twenty-four hours later, cells were washed with PBS, fixed with 10% buffered formalin (Sigma-Aldrich, St. Louis, MO), and rinsed thoroughly with distilled H2O. Adherent cells were stained with crystal violet. Virus-induced cell death was measured as decreased crystal violet staining of cell monolayers as a result of the detachment of dead macrophages [Citation21].
In vivo effect of FRT on the efficacy of DXMAA-induced antiviral response
To test the whether HT synergizes the antiviral efficacy of DMXAA in vivo; we followed the experimental design of Shirey et al. [Citation22]. Six- to 8-week-old WT C57BL/6J mice, 10 mice per group, were injected i.p. DMXAA (25 mg/kg) and housed at 37 °C or subjected to whole body HT at 39.5 °C. Fever-range whole body hyperthermia (WBH) was induced for 2 h in mice using a Wisconsin oven incubator (East Troy, WI) [Citation23]. Mice were heated until the core temperature reached 39.5 °C. Afterwards, incubator temperatures were adjusted in order to maintain core temperatures between 39.5 ± 0.5 °C. To avoid the possible influence of diurnal cycling, all experiments were started at approximately the same time each day (9:00–10:00 am). After 2 h, mice were anesthetized with isoflurane and infected i.n. with 50 µl PR8 influenza (200 pfu/mouse) as this titer is enough to induce death with initial infections. A second dose DMXAA was given i.p at 48 h. Survival was monitored daily for two weeks. Weight loss was monitored on individual mice after infection. In some experiments, mice were killed on day 5 after treatment to harvest lungs for gene expression analysis or viral load in the tissue. Lungs were homogenized using an Omni tissue homogenizer (Omni International, Kennesaw, GA) in Opti-MEM I medium containing 25% sucrose and (Life Technologies, Carlsbad, CA). The levels of IFN-β in lungs homogenate were measured by ELISA. Virus infectious titers were determined by standard plaque assay.
Statistical analyses
All numerical data are expressed as mean ± SD of at least three technical replicates or otherwise mentioned. An unpaired two-tailed Student’s t-test was used to compare the differences between two groups using GraphPad Prism version 7.01 (Graphpad Inc., San Diego, CA).
Results
Fever-range hyperthermia upregulated the expression of INF-β in the presence of htDNA
Cells induce IFN-β expression in response to infection or cytosolic DNA. To investigate the effect of fever-range hyperthermia (FRT) on the expression of INF-β, RAW 264.7 cells with and without htDNA transfection were heated at 39.5 °C and analyzed for the expression of IFN-β. HT at 39.5 °C in the presence of htDNA led to robust production of IFN-β protein as compared to the basal IFN-β level at 37 °C as well as the htDNA transfected cells at 37 °C. In contrast, heating the cells alone at 39.5 °C did not induce IFN-β upregulation (). Quantitative PCR showed >15 times increase in the expression of INF-β at 39.5 °C in htDNA transfected cells as compared to the basal expression level at 37 °C, and >10 times increase as compared to htDNA 37 °C (). This was further proved by WB ().
Figure 1. Effect of hyperthermia on the expression of INF-β. Pre-warmed RAW264.7 cells were stimulated with 5 µg/ml htDNA and maintained either at 37 °C or 39.5 °C for 24 h. A) IFN-β level in the supernatant was determined by ELISA. B) IFN-β relative expression level. C) Western blot analysis of IFN-β. D) Time course experiment showing the upregulation of IFN-β across 12 h.
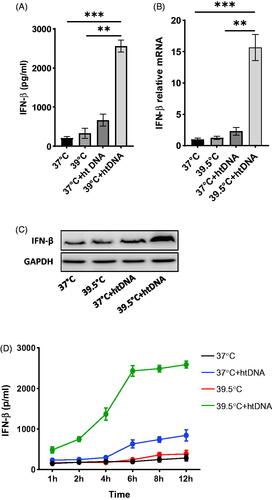
We performed the same experiments on cGAS−/− RAW264.7 cells. No significant increase in the level of INF-β was observed in cGAS−/− cells even in the presence of DNA as well as in cells inhibited with cGAS-STING pathways specific inhibitor RU.521 (data not shown). From these observations, we concluded that FRT induces the expression of IFN-β only in the presence of exogenous DNA.
Time course experiment showed that upregulation in IFN-β in htDNA cells at 39.5 °C started sharply after the treatment and reached the peak value at 6 h time point. However, htDNA transfected cells at 37 °C showed a delayed raise in IFN-β expression and reached peak value at 12 h time point. Thus FRT not only causes increase in IFN-β expression but also induces an earlier expression earlier at 39.5 °C as compared to 37 °C ().
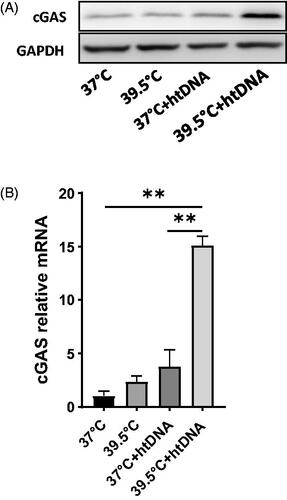
Fever-range hyperthermia upregulated the expression of cGAS in the presence of DNA
The ability of cells to induce IFN-β expression in response to cytosolic DNA correlates with the level of cGAS in different cell types [Citation17,Citation24]. Therefore, we sought the effect of FRT on the expression of cGAS. FRT upregulated the expression of cGAS in the presence of htDNA (). PCR showed more than 10-fold increase in mRNA expression of cGAS at 39.5 °C in htDNA transfected cells as compared to the cGAS basal level expression at 37 °C without exogenous DNA (). However, we could not see any significant increase in un-transfected cells at 39.5 °C. This shows that the upregulation in cGAS expression is not reliant on only heating; rather, the increase in cGAS expression is subject to sensing DNA in the cytosol. This increase in the expression of cGAS at 39.5 °C correlated well with the level of IFN-β at the same temperature.
Figure 2. Effect of hyperthermia on cGAS expression. Pre-warmed RAW264.7 cells were stimulated with 5 µg/ml htDNA and maintained either at 37 °C or 39.5 °C for 24 h. Total RNA and proteins were extracted from the cell pellet after gentle centrifugation. A) Western blot analysis of cGAS protein expression. B) qRT-PCR analysis of cGAS gene expression. Data represent ± SD of n = 5. **p < .01.
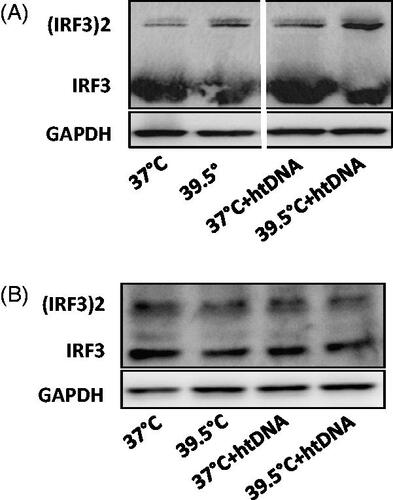
Hyperthermia induced IRF3 phosphorylation
Cyclic GMP-AMP Synthase (cGAS) triggers the reaction of GTP and ATP to form cGAMP. To test if the increased expression of cGAS and hence FRT, could also increase the production of cGAMP in the cells; cells with and without htDNA transfection were heated at 39.5 °C and the synthesis of cGAMP was assessed by its ability to induce IRF3 phosphorylation and thus its dimerization, using native gel electrophoresis. HT at 39.5 °C showed higher IRF3 phosphorylation in htDNA transfected cells indicating increased production of cGAMP as compared to 37 °C. No significant difference in the degree of phosphorylation was seen in cells with or without htDNA transfection at 37 °C or in cells at 39.5 °C without DNA transfection showing no increased production of cGAMP (. As expected, cGAS−/− did not show such difference in the degree of phosphorylation among the four groups with or without htDNA ().
Figure 3. Effect of hyperthermia on IRF3 phosphorylation. Pre-warmed RAW264.7 and cGAS−/− cells were stimulated with 5 µg/ml herring testis DNA (htDNA) and maintained either at 37 °C or 39.5 °C for 24 h. IRF3 dimerization was analyzed by native gel electrophoresis. A) IRF3 phosphorylation in RAW 264.7 cells. B) IRF3 phosphorylation in cGAS−/− RAW 264.7 cells.
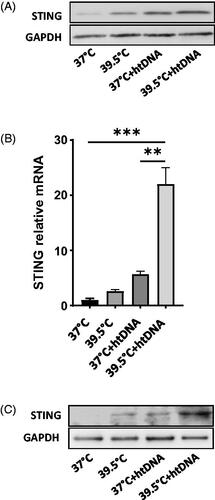
Hyperthermia upregulated the expression of STING protein in cGAS dependent manner
Treatments of cells with higher concentration of c-di-nucleotides (cDNs) have been shown to increase the levels of STING protein and the increased levels of STING and phosphorylated IRF3 is associated with increased production of IFN-β [Citation25,Citation26]. Therefore, we assessed whether the increased concentration of cGAMP caused by HT could upregulate the expression of STING protein. FRT significantly increased the expression of STING at 39.5 °C in htDNA transfected cells However, cells at 39.5 °C without exogenous DNA and DNA transfected cells at 37 °C did not show significant increase in the expression level of STING (). This correlated well with the IRF3 phosphorylation pattern. qPCR showed >15-fold increase in the expression of STING at 39.5 °C in htDNA transfected cells as compared to 37 °C (). However, in cGAS−/− cells, STING expression did not increase significantly even in the htDNA transfected cells in response to 39.5 °C, showing that upregulation in the expression of STING in response to the increased production of cGAMP caused by HT is dependent on cGAS protein ().
Figure 4. Effect of hyperthermia on STING expression. Pre-warmed RAW264.7 cells were stimulated with 5 µg/ml herring testis DNA (htDNA) and maintained either at 37 °C or 39.5 °C for 24 h. Total RNA and proteins were extracted from the cell pellet after gentle centrifugation. A) Western blot analysis of STING protein expression. B) qRT-PCR analysis of STING gene expression. C). STING expression in cGAS−/− RAW264.7 cells. Data represent ± SD of n = 5. **p < .01 and ***p < .001.
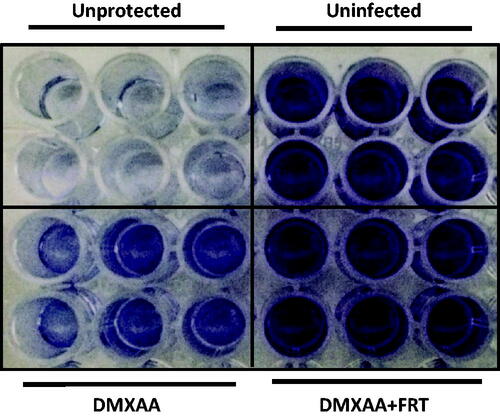
FRT synergizes DMXAA-induced antiviral activity in vitro
DMXAA is an agonist of the mouse STING pathway which stimulates an antiviral state and protects mice against viral infection [Citation22,Citation27,Citation28]. To assess the effect of FRT on the cGAS-STING mediated IFN production and hence antiviral activity, we sought the effect of FRT on the ability of DMXAA to induce antiviral activity against VSV infection in the mouse macrophage cell line RAW 264.7 as the replication of VSV is markedly inhibited by treatment with type-I IFN [Citation29]. RAW 264.7 cells were pretreated with DMXAA for 2 h with or without FRT and then infected with VSV at a MOI of 0.1 for 24 h. Virus-induced cell death was measured as decreased crystal violet staining of cell monolayers as a result of the detachment of dead macrophages [Citation21]. As shown in , FRT + DMXAA-treated cells were better protected from VSV-induced cytotoxicity in contrast to the nonprotected control (no FRT and DMXAA) or DMXAA treatment alone. This provided the evidence that FRT could synergize DMXAA-induced antiviral state in vitro.
Figure 5. In vitro effect of hyperthermia on the efficacy of DXMAA-induced antiviral response. RAW 264.7 cells were treated with DMXAA (100 µg/ml) for 2 h, washed, and infected with VSV at 0.1 MOI. After 24 h, cells were washed with PBS, fixed in 10% buffered formalin, and stained with crystal violet. The results are derived from a single representative experiment n = 3).
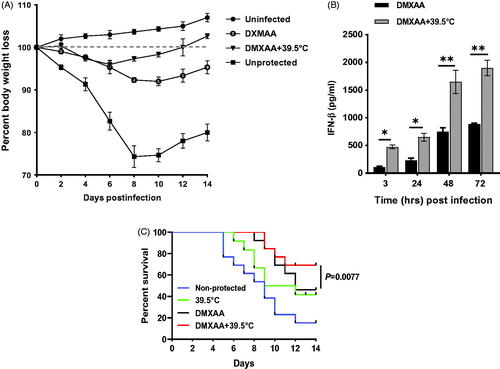
FRT synergizes DMXAA-induced antiviral activity in vivo
We next investigated the cGAS-STING mediated antiviral effects of FRT in vivo. We sought to determine if whole body HT application synergizes the efficacy of DMXAA to protect mice against lethal infection with a mouse-adapted H1N1 influenza virus, strain PR8. Intraperitoneal DMXAA and WBH were administered 2 h prior to i.n. infection with the influenza virus, followed by the same treatment 2 d later. Weight loss was used as a surrogate marker for influenza-induced disease in mice. shows that influenza infection caused a marked decrease in weight in non-protected control mice that reached its nadir 8 d after infection, the point at which control mice begin to die and after which survivors regain weight. FRT and DMXAA co-administration led to significantly less weight loss in influenza-infected mice. ELISA analyses showed that the lung homogenates of DMXAA-treated mice at 39.5 °C contained significantly elevated levels of IFN-β as compared to the DMXAA-treated mice (). DMXAA-treated mice infected with mouse-adapted influenza PR8 virus exhibited 64% survival and non-protected 39.5 °C exhibited 41% over the course of 14 d; whereas DMXAA + FRT co-treatment protected 69% mice over the same course of time (p = .0077; ). This correlated with the level of IFN-β determined by ELISA. This shows that HT synergizes the effect of DXMAA-induced antiviral immunity and supports our hypothesis that HT antiviral effects are mediated via cGAS-STING pathway.
Figure 6. Effect of hyperthermia on the efficacy of DXMAA-induced antiviral response. WT C57BL/6J mice were injected i.p. with DMXAA (25 mg/kg). Three hours later, mice were anesthetized with isoflurane and infected i.n. with 50 µl mouse-adapted H1N1 influenza virus. Mice received a second i.p. dose DMXAA (25 mg/kg) at 48 h after infection. A) Percent body weight loss. B) IFN-β level in lungs homogenate. C) Survival analysis over 14 d for n = 13. *p <.05 and **p < .01.
Discussion
A better understanding of the mechanism of the beneficial effects of FRT in host immunity could be useful in applying artificial fever as an antiviral and anticancer therapy in combination with other therapeutic modalities. In this study, we analyzed the effect of FRT (39.5 °C) on the production IFN-β, and the underlying pathway in vitro and in vivo. A previous study reports that exposure to 39.5 °C did not change the expression level of IFN-β as compared to 37 °C [Citation30]. We found that FRT induced enhanced production of IFN-β in RAW264.7 cells only in the presence exogenous DNA and no such increase in the expression of IFN-β was observed by heating up cells alone at 39.5 °C in the absence of htDNA. This explains the raise in temperature after natural infection. Sustained increased production of type-I IFNs is associated with inflammation [Citation31]. Thus heating without exogenous DNA would lead to inflammation. Panchanathan et al. report that treatment of cells with type-I IFN suppresses the expression of the adaptor protein STING [Citation26]. We found that prolong heating caused IFNs level to decline (data not shown). This mechanism might operate just to prevent the overexpression of IFNs which triggers inflammation. This explains the situation in natural infections where body temperature drops down after the clearance of infection and so the IFN-β level. Time course experiments showed an early induction of IFN-β at 39.5 °C. Thus FRT not only causes increase in the expression of IFN-β but also induces an earlier expression of the cytokines. This is what Fairchild et al. hypothesized that the burst cytokine profile at febrile temperatures enhances early activation of the host defense and prevents prolonged exposure to potentially cytotoxic cytokines [Citation9].
As DNA viruses and different types of DNA like HT-DNA, E. coli DNA and interferon stimulatory DNA (ISD) induce the production of IFN-β using cGAS-STING pathway, we hypothesized that FRT might mediate its effect via cGAS-STING pathway. The expression levels of IFN-β in different cell lines correlate with the ability of these cells to produce cGAS and induce the synthesis of cGAMP in response to cytosolic DNA [Citation17]. In our study, FRT at 39.5 °C in the presence of htDNA induced the expression of cGAS and cGAMP. The expression of IFN-β correlated well with the expression of cGAS and cGAMP, this implies that HT induces the increase synthesis of cGAMP by cGAS which in return induces increased STING expression and IRF3 phosphorylation. No increase in the level of cGAS or IFN-β was seen in cGAS−/− cells or by blocking the cells with cGAS inhibitor RU.521. This proves that the increase in IFN-β level in response to FRT is mediated via cGAS-STING pathway. These results show that febrile range temperature exerts its antiviral effect via cGAS-STING pathway.
DMXAA (5,6-dimethylxanthenone-4-acetic acid) is an agonist of the cGAS-STING pathway. Stimulation of the cGAS-STING pathway with DXMAA establishes antiviral state and provides protection against viral infection by inducing a better interferon response. FRT better-protected mice against the mouse-adapted H1N1 influenza PR8 virus by strengthening the antiviral efficacy of DXMAA through synergizing the IFN-β production both in vitro and in vivo.
Long-term (72 h) exposure to high temperatures (39 °C and/or 40 °C) has been found to negatively correlate with RNA levels and/or viral titers of eight influenza virus strains [Citation32]. In our study, viral titer in the lungs of mice housed at 37 °C increased gradually over time. However, in 39.5 °C group, viral titer did not increase and was well suppressed. This correlated well with the expression level of IFN-β in DMXA and DMXAA + FRT groups. In mice at 39.5 °C, the higher level of IFN-β was able to keep viral replication suppressed and provided better protection against morbidity as compared to DMXAA alone.
We conclude that HT enhances antiviral activity by up-regulating the expression of cGAS protein and promoting cGAS-STING pathway. These results might reveal a possible mechanistic explanation for the significance of artificial fever in activating innate immune responses and could indicate new strategies in the treatment of viral infections by using HT in combination with other antiviral agents.
Acknowledgments
The authors are also thankful to Shanghai Sorrento Medical Technology Co. Ltd. for providing financial and technical support.
Disclosure statement
The authors declare that there is no conflict of interest related to this article.
Additional information
Funding
References
- Zhang J, Tang X, Sheng X, et al. The influence of temperature on viral replication and antiviral-related genes response in hirame rhabdovirus-infected flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2017;68:260–265.
- Prow NA, Tang B, Gardner J, et al. Lower temperatures reduce type I interferon activity and promote alphaviral arthritis. PLoS Pathog. 2017;13(12):e1006788.
- Conti C, De Marco A, Mastromarino P, et al. Antiviral effect of hyperthermic treatment in rhinovirus infection. Antimicrob Agents Chemother. 1999;43(4):822–829.
- Yamaya M, Nishimura H, Lusamba Kalonji N, et al. Effects of high temperature on pandemic and seasonal human influenza viral replication and infection-induced damage in primary human tracheal epithelial cell cultures. Heliyon. 2019;5(2):e01149.
- Thorne SH, Brooks G, Lee YL, et al. Effects of febrile temperature on adenoviral infection and replication: implications for viral therapy of cancer. J Virol. 2005;79(1):581–591.
- Foxman EF, Storer JA, Vanaja K, et al. Two interferon-independent double-stranded RNA-induced host defense strategies suppress the common cold virus at warm temperature. Proc Natl Acad Sci USA. 2016;113(30):8496–8501.
- Foxman EF, Storer JA, Fitzgerald ME, et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci USA. 2015;112(3):827–832.
- Ikaheimo TM, Jaakkola K ,Jokelainen J, et al. A decrease in temperature and humidity precedes human rhinovirus infections in a cold climate. Viruses. 2016;8(9):244.
- Fairchild KD, Viscardi RM, Hester L, et al. Effects of hypothermia and hyperthermia on cytokine production by cultured human mononuclear phagocytes from adults and newborns. J Interferon Cytokine Res. 2000;20(12):1049–1055.
- Yang H, Wang H, Ren J, et al. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA. 2017;114(23):E4612–E4620.
- Hall J, Ralph EC, Shanker S, et al. The catalytic mechanism of cyclic GMP-AMP synthase (cGAS) and implications for innate immunity and inhibition. Protein Sci. 2017;26(12):2367–2380.
- Gao D, Wu J, Wu YT, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–906.
- Lin FC, Young HA. Interferons: success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):369–376.
- Zhou JH, Wang YN, Chang QY, et al. Type III interferons in viral infection and antiviral immunity. Cell Physiol Biochem. 2018;51(1):173–185.
- Cheng WY, He XB, Jia HJ, et al. The cGas-sting signaling pathway is required for the innate immune response against ectromelia virus. Front Immunol. 2018;9:1297.
- Hartlova A, Erttmann SF, Raffi FA, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42(2):332–343.
- Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791.
- Vogel SN, Friedman RM, Hogan MM. Measurement of antiviral activity induced by interferons alpha, beta, and gamma. Curr Protoc Immunol. 2001.
- Larkin J, Rankin AL, Picca CC, et al. CD4 + CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J Immunol. 2008;180(4):2149–2157.
- Roberts ZJ, Goutagny N, Perera PY, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204(7):1559–1569.
- Falk LA. Measurement of interferon-mediated antiviral activity of macrophages. Curr Protoc Immunol. 2001.
- Shirey KA, Nhu QM, Yim KC, et al. The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-beta-mediated antiviral activity in vitro and in vivo. J Leukoc Biol. 2011;89(3):351–357.
- Eng JWL, Reed CB, Kokolus KM, et al. Housing temperature influences the pattern of heat shock protein induction in mice following mild whole body hyperthermia. Int J Hyperthermia. 2014;30(8):540–546.
- Wu J, Sun L, Chen X, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830.
- Sooreshjani MA, Gursoy UK, Aryal UK, et al. Proteomic analysis of RAW macrophages treated with cGAMP or c-di-GMP reveals differentially activated cellular pathways. RSC Adv. 2018;8(64):36840–36851.
- Panchanathan R, Liu H, Xin D, et al. Identification of a negative feedback loop between cyclic di-GMP-induced levels of IFI16 and p202 cytosolic DNA sensors and STING. Innate Immun. 2014;20(7):751–759.
- Guo F, Han Y, Zhao X, et al. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother. 2015;59(2):1273–1281.
- Ceron S, North BJ, Taylor SA, et al. The STING agonist 5,6-dimethylxanthenone-4-acetic acid (DMXAA) stimulates an antiviral state and protects mice against herpes simplex virus-induced neurological disease. Virology. 2019;529:23–28.
- Basu M, Maitra RK, Xiang Y, et al. Inhibition of vesicular stomatitis virus infection in epithelial cells by alpha interferon-induced soluble secreted proteins. J Gen Virol. 2006;87(Pt 9):2653–2662.
- Cooper ZA, Ghosh A, Gupta A, et al. Febrile-range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-{kappa}B recruitment to cytokine gene promoters. Am J Physiol Cell Physiol. 2010;298(1):C171–C181.
- Sunthamala N, Thierry F, Teissier S, et al. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-κ transcription in keratinocytes. PLoS One. 2014;9(3):e91473.
- Moriyama M, Koshiba T, Ichinohe T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat Commun. 2019;10(1):4624.
