Abstract
Introduction
Hyperthermic Ιsolated Limb Perfusion using melphalan and TNFα (TM-HILP) is a regional chemotherapy method for advanced melanoma.
Purpose
To explore the feasibility of the study of Circulating Melanoma Cells (CMCs) in the context of acute physiological changes induced by TM-HILP and their association with oncological outcomes.
Methods
The study included 20 patients undergoing TM-HILP for unresectable in-transit melanoma of the limbs, stage III(B/C/D). CMCs in the peripheral blood were analyzed at 5-time points from the preoperative day until day 7 from surgery using the following biomarkers: MITF, Tyrosinase mRNA, Melan-A and S100b, through quantitative RT-PCR.
Results
No CMCs according to Tyrosinase and Melan-A biomarkers were found in any sample. Friedman test showed significant alterations perioperatively for MITF (p < .001) and S100b (p = .001). Pairwise tests showed a significant increase of MITF levels on postoperative day 7 compared with postoperative day 1, intraoperative and preoperative levels (p < .05). Pairwise tests for S100b showed a significant difference between intraoperative sample and postoperative day 7 (p < .0001). Patients who experienced a complete response to TM-HILP (n = 12) had higher mean levels of MITF and the difference was significant at the time point immediately after the operation (0.29 ± 0.27 vs. 0.06 ± 0.06, p = .014) and on postoperative day 1 (1.48 ± 2.24 vs. 0.41 ± 0.65, p = .046). There was no association of MITF or S100b levels with 4-year disease specific survival.
Conclusion
TM-HILP is associated with increased levels of CMCs, but there was no association of this increase with survival. Patients with complete response to HILP demonstrate higher values of MITF shortly after the operation.
Introduction
Approximately 10% of the patients with skin melanoma will develop subdermal lymphatic metastases occurring between the primary tumor and its draining lymph basin [Citation1]. These are called in transit or satellite metastases and represent a therapeutic challenge because of their great extent and resistance to chemotherapy and radiation therapy. Hyperthermic isolated limb perfusion (HILP) is the regional chemotherapy method using antineoplastic agents at high concentrations, avoiding systemic toxicity [Citation2]. The use of TNFα in HILP targets tumor endothelium and induces apoptosis of endothelial cells, but it does not affect the healthy endothelium because the latter lacks TNFR1 receptors [Citation3]. Additionally, tumor endothelium does not consist of a continuous lining of endothelial cells; it lacks pericyte coverage and is characterized by a thickened basement membrane. This tumor vessel phenotype results in greater permeability of the vessel. The procedures and structures as described here result in an enormous induction of vessel permeability. As a result, the chemotherapy drug is well distributed throughout the tumor, and strong extravasation of erythrocytes results in massive hemorrhagic tumor necrosis. The selectively targeted tumor’s vessels are no longer functional and regress. This process of ‘lysis’ of the melanoma tumor leads to tumor regression [Citation4,Citation5]. We hypothesize that at the same time, melanoma cells are released in the systemic circulation.
Circulating Melanoma Cells (CMCs) have been the object of intense research in the last years. Several studies attempt to correlate CMCs with the prognosis of melanoma [Citation6,Citation7], diagnosis and monitoring [Citation8], disease recurrence and progression [Citation9,Citation10], evaluation of adjuvant therapy [Citation11], and survival [Citation12]. Exfoliating cells from the primary tumor that enter the blood system is considered a necessary step in the development of disseminated malignancy. It is estimated that up to 106 tumor cells per gram of tumor can be introduced into circulation every day, but they do not survive long in the peripheral blood as they are frequently undergoing apoptosis [Citation13]. During HILP, it is plausible to expect a large load of melanoma cells to spill over into the patient’s peripheral blood with unknown effects. Herein, the feasibility of the study of CMCs in the context of acute physiological changes induced by TM-HILP and their association with oncological outcomes are explored.
Methods
Demographic data, presentation of the disease, prior treatment history, treatment details, and outcome were retrieved from a prospectively maintained database of the Department of Surgical Oncology. Treatment response was evaluated two months after the surgical procedure according to the response evaluation criteria in solid tumors (RECIST) [Citation14]. No biopsies of limb lesions were routinely performed for the assessment of response. Follow-up was performed every 3–6 months by the surgical oncologists and/oncologists. The study was conducted with the formal approval of the Institutional Review Board and Bioethics Committee of the University Hospital of Heraklion. Informed consent was obtained from all patients for being included in the study.
TM-HILP technique
The technique has been previously described in detail [Citation15]. Briefly, following superficial and/or deep inguinal lymph node dissection, HILP was carried out at the external iliac or common femoral vessels level. Systemic heparinization was administered 5 min before the occlusion of vessels, catheters were inserted, and a limb tourniquet was applied at the inguinal ligament level [Citation16] to achieve occlusion of the superficial dermal vessels. The flow rate was adjusted to 35–40ml/l of limb volume/min. Perfusate consisted of ∼250ml Ringer's lactate, 1–2 units of red blood cell concentrate, and 0.5 ml of 2500–5000 IU heparin/ml. The whole procedure was performed under mild hyperthermia (38.5–40° C). Subsequently, 2 mg of TNF-α-1a (Tasonermin, Beromun®, Boehringer-Ingelheim) as bolus was injected into the extracorporeal circuit for 30 min followed by 10 mg/l limb volume Melphalan infusion for 60 min. Finally, the limb was flushed out with 4–6 liters of Gelofusine, Esmarch tourniquet was released, catheters were removed, and the vessels were reconstructed. Continuous intraoperative external monitoring of perfusate leakage was performed for all patients using a collimated Nal (T.I.) scintillator, and Tc-99m labeled patients' red blood cells [Citation17].
Samples of 20 ml peripheral venous blood were collected at specific time points, from each patient, for analyzing the concentrations of CMCs. The 5 time points were: The day before surgery (preop), during the operation just before cannulation of the vessels (intraop1), 20 min after vessels' reconstruction and returning the limb to the systemic circulation (intraop2), in postoperative day 1 (postop1) and postoperative day 7 (postop7). Preop sample represents the baseline values against which any increases of CMCs are measured. Intraop1 sample represents the effect of surgical maneuvers over CMCs, and intraop2 represents the early TNFα effect within 20 min from the administration. Postop1 sample represents the effect of TM-HILP at 24 h and postop7 the late impact of TM-HILP on CMCs at the time point of usual maximum systemic toxicity of TNFα [Citation3].
Quantitative RT-PCR
The following criteria were used to assess whether the HILP procedure affects the number of CMCs in peripheral blood samples and any association between the changes in the number of CMCs and the course of the systemic disease: PCR-based amplification of at least one mRNA marker in a peripheral blood specimen was considered a signal of the presence of CMCs [Citation18]. The biomarkers used for the detection of CMCs were: Microphthalmia associated Transcription Factor (MITF), Tyrosinase mRNA (TYR), Melan-A/MART-1 (Melan-A) and S100b [Citation19–22].
Peripheral blood mononuclear cells (PBMC) were obtained by gradient density centrifugation, and RNA extraction was performed [Citation13]. RNA concentration was measured using the NanoDrop ND-1000 v3.3 (Thermo Fisher Scientific, Wilmington, DE, USA) equipment. Amplification of the β-actin as an internal reference gene was done to verify the RNA integrity. RNA prepared from the A375 and ARH-77 CRC, and leukemic cell lines were used as positive and negative controls. The SuperScript III PlatinumTranscriptase (Invitrogen, Carlsbad, CA, USA) was used to prepare cDNA from 5 μg of total RNA, according to the manufacturer protocol. The RT-qPCR reaction for β-actin and MITF, S100b, Melan-A, and Tyrosinase was carried out using 2.5 μL of cDNA template and 6.25 μL of TaqMan Universal Master Mix (A.B.; Applied Biosystems), 1.25 μL– of each specific primer (3 μmol/L) (), and 0.5 μL of hydrolysis probe (2.5 μmol/L) for each gene and adjusted with DPEC water to a final volume of 12.5 μL/reaction. Quantification of gene expression was performed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Grand Island, NY, USA). All experiments were performed in triplicates. Only triplicates with a standard deviation of the quantification cycle (Cq) value less than 0.25 were accepted.
Quantification was based on an external calibration curve that was obtained by using external standard cDNAs. Total RNA was prepared from 1 × 106 A375 melanoma cells. cDNA synthesis of serial dilutions of this RNA, corresponding to 1 × 105 A375 cells, was also analyzed in each run. This calibration curve was created by plotting the number of A375 cells corresponding to each standard external cDNA versus its Cq value. The number of MITF, S100b, Melan-A, and Tyrosinase-positive cells for all the tested samples was expressed as A375 cell equivalents/5 μg of total RNA according to the external standard calibration curve. Besides, to determine the number of A375 cells that could be recovered, we added a decreasing number of A375 cells (from 103 to 1) in 106 negative PBMCs in 5 different experiments. The SDS 2.3 software was used for the analysis of the results [Citation23]. According to the external standard calibration curve, the measurement units refer to A375 melanoma cell equivalents/5 μg of total RNA.
Statistical analysis
Repeated measures were used to compare the distribution of variables at different time points. Parametric tests (repeated measures ANOVA) were used for normally distributed variables. Non-parametric tests (Friedman’s) were used for variables not following a normal distribution. Multivariate logistic regression analysis was used to adjust the effects of multiple variables. Survival time was analyzed with Kaplan Meier curves and the log-rank test. The effect of continuous variables and multiple variables on survival time was analyzed with Cox-regression analysis. Spearman test was used to assess the correlation between continuous variables. p Values lower than .05 were considered statistically significant. All analyses were performed with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA).
Results
20 patients, 16 females and 4 males, with lower extremity melanoma stage IIIB-IIID, were submitted to TM-HILP with the indication of recurrent unresectable satellite and/or in transit metastases (). The median age was 61 years (range 25–78). All patients had histological confirmation of diagnosis. All patients had been initially treated elsewhere and were referred to us after recurrence or after failure to control recurrence by systemic therapy or surgery.
Table 1. Baseline clinical variables and postoperative outcomes of patients undergoing TM-HILP treatment for advanced melanoma.
Associations of CMCs with baseline patient characteristics
There was no difference in the baseline numbers of CMCs according to the number of lesions (> <10 lesions) (p = .759 for MITF and p = .428 for S100b marker). There was no difference in the baseline numbers of CMCs in patients with or without histologically proven lymph node metastasis (p = .416 for MITF and p = .285 for S100b marker). There was no correlation of baseline CMCs with the patients’ age (p = .524 for MITF and p = .752 for S100b).
Alterations of CMCs after HILP
No baseline values and no changes in CMCs values in any patient were proven according to the Tyrosinase and Melan-A assays. Thus, these biomarkers were not used in any of the subsequent statistical analyses. All patients had preoperative values of CMCs according to MITF and S100b biomarkers. In , the fold increase of S100b biomarker at different time points versus preoperative values is presented, showing an increase at postoperative day 1 and 7 in 8 out of 20 and 13 out of 20 patients, respectively. The values of the S100b biomarker are presented in . A non-parametric Friedman test of differences among repeated measures was conducted and rendered a Chi-square value of 18.256, which was significant (p = .001) (). For pairwise comparisons between time points, Bonferroni adjusted pairwise tests showed a statistically significant difference between intraoperative sample 2 and postoperative day 7 (p < .0001). There was no statistically significant increase of mean S100b values at postoperative day 1 or 7 (p > .05) ().
Figure 2. Fold increase of CMCs according to S-100 at different time points using as reference preoperative values.
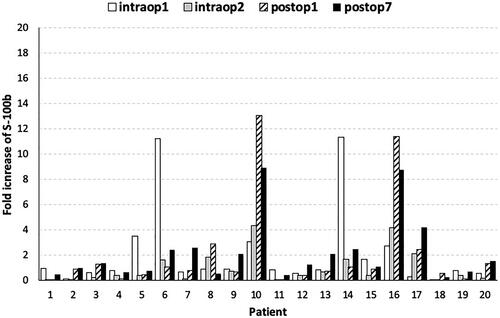
Figure 3. Fold increase of CMCs according to MITF at different time points using as reference preoperative values.
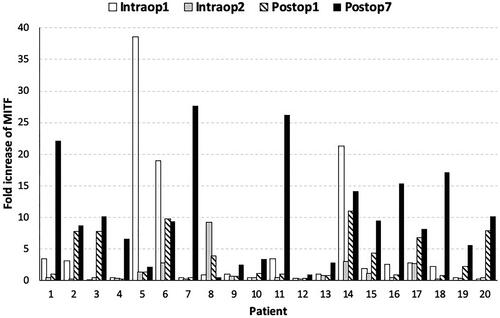
Figure 4. Plot of means of MITF (A) and S100b (B) at different time points. Error bars represent 95% confidence intervals. p Values are according to the Friedman test. Stars represent a statistically significant difference at the .05 level using the Bonferroni adjusted pairwise test.
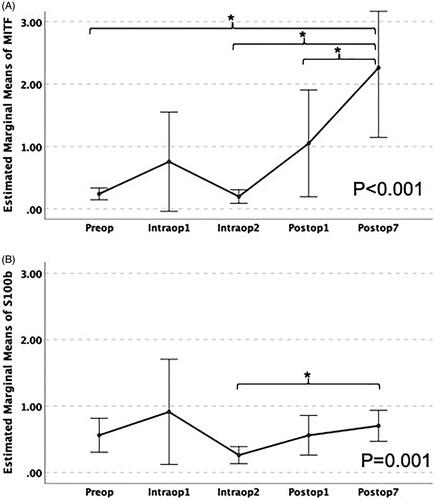
Table 2. Circulating melanoma cells at various time points before and after HILP in 20 patients.
In , the fold increase of MITF biomarker at different time points versus preoperative values is presented, showing an increase at postoperative day 1 and 7 in 11 out of 20 and in 18 out of 20 patients, respectively. The values of MITF-biomarker are presented in . A non-parametric Friedman test of differences among repeated measures was conducted and rendered a Chi-square value of 34.616, which was significant (p < .0001) (). For pairwise comparisons between time points, Bonferroni adjusted pairwise tests showed a statistically significant increase of MITF levels at postoperative day 7 compared with postoperative day 1, intraoperative sample 2, and preoperative levels (p < .05) (). These results suggest that the HILP procedure is associated with an increase in CMCs. The increase is higher for the MITF marker on the 7th postoperative day.
Associations of CMCs with the response to HILP
12 patients (60%) had a complete locoregional response, five patients (25%) had a partial response, and three patients (15%) had stable disease. The mean values of MITF of patients who experienced a complete response (CR) versus the others (non-CR) are displayed in . Within the CR group, a non-parametric Friedman test of differences among repeated measures was conducted and rendered a Chi-square value of 15.511, which was significant (p = .004). Within the non-CR group, a non-parametric Friedman test of differences among repeated measures was conducted and rendered a Chi-square value of 24.179, which was significant (p < .0001). Between CR and non-CR groups comparisons, MITF mean values of CR patients were higher at all time points and this difference reached statistical significance at intraoperative sample 2 (p = .014) and on postoperative day 1 (p = .046). Patients with regional lymph node metastasis had significantly lower complete response rates than patients without regional lymph node metastasis (16.7% vs. 62.5%, p = .035). This association remained in the multivariable logistic regression analysis adjusted for MITF levels at postoperative day 7. (OR[95%CI]: 9.91 [1.07–91.47], p = .043 for negative versus positive lymph node metastasis and OR[95%CI]: 1.10[0.92–1.22], p = .430 for the increase of MITF versus preoperative levels). These results show that both CR and non-CR patients experience a statistically significant increase in postoperative MITF levels. Moreover, the levels of MITF are statistically higher for CR vs. non-CR patients at intraop2 sample and postop1 sample.
Table 3. MITF circulating melanoma cells values according to response to hyperthermic isolated limb perfusion in 20 patients.
Associations of CMCs with disease-specific survival
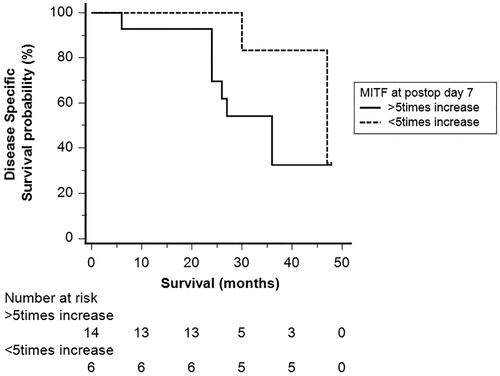
2 years after the HILP procedure, nine patients (45%) were alive with no clinical evidence of disease, eight patients (40%) were alive but with locoregional relapse of disease, and three patients (15%) were dead of disease. 4 years after the HILP procedure, five patients (25%) were alive and 12 patients (60%) were dead. Patients who were alive at 4 years after TM-HILP had lower levels of MITF at postoperative day 7 than those who died of melanoma, but this difference was not statistically significant (mean ± SD, 8.5 ± 5.5 vs. 11.2 ± 9.5, p = .480). In , Kaplan Meier survival curves are displayed for patients who had a higher and lower than 5fold increase of MITF at postop day 7. Patients with lower MITF levers at postoperative day 7 had longer disease-specific survival, but the difference was not statistically significant (log-rank test, p = .340). When the fold increase of MITF levels at postoperative day 7 was analyzed as a continuous variable in a Cox regression model, there was no statistically significant association with disease specific survival (HR[95%CI]: 1.07[0.99–1.16], p = .07).
Discussion
The recent molecular and cell biological studies suggest that cancer cell metastasis occurs through two major phases: the first enables the physical dissemination of cancer cells from the core of the primary tumor to the parenchyma of a distant tissue, while in the second phase, colonization depends on the adaptation of disseminated cancer cells to the microenvironment of this tissue [Citation24,Citation25]. In HILP procedure, the effect of TNFα over the tumor is a disruption of its vasculature that facilitates the penetration of the chemotherapeutic agent (Melphalan) and destruction of the tumor. Alongside this therapeutic effect, another act occurs through the lack of stable links between the endothelial cells: the exfoliation and liberation of tumor/melanoma cells into the systemic circulation (the process of intravasation). In our study, the HILP procedure was associated with an increase in circulating melanoma cells in the patient’s peripheral blood.
Since 1960 when Romsdahl [Citation26] began his first attempts at studying circulating melanoma cells, several scientific announcements of research results concerning the effect of circulating tumor cells over metastasis, disease progression, and survival have been made. Although the investigations are heterogeneous and difficult to compare for drawing conclusions, a general view is outlined: circulating melanoma cells are a suitable biomarker for disease status. Their levels in limited studies may convey prognostic and predictive information in melanoma [Citation27,Citation28]. CMCs can be detected in any melanoma stage, but their presence indicates melanoma disease progression as dissemination or locally recurrent disease [Citation29]. Stage III melanoma patients with positive PCR test for >1 marker at any point showed worse disease-free survival and a significantly higher risk of developing distant metastasis [Citation30]. However, isolation of CMCs from patients is challenging because of their low concentration in peripheral blood and the fact that common markers used in epithelial cancers are not commonly expressed in CMCs because they originate from the neural crest and not from the epithelium. Additional factors making challenging their isolation are the significant heterogeneity among circulating tumor cells subpopulations in melanoma and the differential response of these subpopulations to drug therapy [Citation28,Citation31]. This is perhaps the reason why no standard set of CMCs has been utilized so far [Citation28]. By the time we commenced our study in 2012, MITF, S100b, Melan-A, and Tyrosinase were the most commonly used markers for detecting circulating melanoma cells, and the kit containing these four biomarkers was commercially available and accessible by our laboratory.
In our study, most of the patients had a statistically significant postoperative increase of CMCs according to the qRT-PCR analysis for the biomarker MITF. We showed that patients who experience a complete response to TM-HILP have significantly higher mean levels of MITF immediately after the operation and postoperative day 1. However, this difference was not significant on postoperative day 7, and this might suggest that the alteration is transient and explain why the increase of MITF is not associated with worse long-term survival. We noted an increase in MITF and S100b at intraoperative sample 1 compared with preoperative levels, which was not statistically significant. This sample is obtained after lymph node dissection and before the cannulation of the vessels of the limb. Unless this increase is due to sampling variation, it can be explained partly by surgical manipulation of regional lymph nodes containing melanoma metastasis in 35% of our patients. However, we did not find a significant difference in the levels of CMCs according to regional lymph node status at any time point (data not presented). Intraoperative sample 2 is taken 20 min after limb washout and reintroduction of the limb into the systemic circulation. Thus, it might represent immediate cytotoxicity of TM-HILP, and it is not surprising that the numbers of CMCs might decrease. Moreover, the complete washout of the limb with normal saline at the end of the operation, as well as the dilutional effect of fluids administered throughout a lengthy procedure, might contribute to any observed decrease at that time point.
We note that the clone of CMCs, which was isolated during our measurements, expresses MITF in most patients. MITF is recognized as a key conductor of melanoma progression. It appears to act as a ‘rheostat,’ integrating various signals regulating its expression and activity to yield a variable output that translates into differential biological phenotypes [Citation32]. Melanoma cells expressing MITF at a high level can either differentiate or proliferate. Low activity of MITF is related to stem cell-like or invasive potential. MITF expression and activity in melanoma cells are determined by genetic alterations, epigenetics, changes in upstream signaling pathways, and microenvironment [Citation33]. The quantitative measure of expression is mostly heterogeneous, and immunological staining identifies ‘MITF -positive’ cells expressing higher and lower levels of MITF, but also cells that lack MITF expression entirely. Melanoma cells labeled as MITF positive populations can have low levels of MITF expression [Citation34]. Finally, there may be cells with high expression of MITF mRNA, which does not lead to protein production, so in reality, there is a MITFlow melanoma [Citation35].
There are 3 possible states in CMCs populations: A) Moderate to high levels of MITF, proliferating rapidly but poorly invasive, B) Mixed gene expression, C) low MITF expression with slower growth but more invasive. Research data shows that gene expression patterns belonging to states A and C could switch phenotypes. The result of such plasticity is a revised rheostat model incorporating six different phenotypic states of melanoma according to MITF activity: (1) Hyper-differentiated with high MITF activity and mainly proliferative character, (2) Melanocytic with a high MITF state and a proliferative character also, (3) Intermediate and (4) Starved cells which can express either a proliferative or invasive character (they have intermittent MITF expression levels). (5) Neural crest stem cell-like cells and (6) Undifferentiated cells with low MITF expression and have invasive character [Citation35]. Notably, all the above states are generated by microenvironmental influences, including hypoxia, nutrient limitations, and inflammatory signaling, as well as stresses caused by therapies [Citation36], as we analyze below.
Melanoma plasticity underlies key traits such as metastasis, and it is linked to phenotype switching, where the microenvironment induces switches between invasive/MITFlow versus proliferative/MITFhigh states [Citation35]. The HILP procedure is an aggressive therapy that functions as an exogenous factor affecting the microenvironment at the molecular level both locoregional and systematically. All factors related to HILP (hyperthermia, hypoxia, acidosis, inflammation, Melphalan, TNFα and gene expression changes) cause changes in MITF expression levels and therefore, could cause corresponding changes in the course of the disease. True hyperthermia at 45 °C can kill melanoma cells, but mild hyperthermia used in HILP (up to 40 °C), which makes the tumor more sensitive to chemotherapeutics, does not affect in any way the expression of MITF [Citation37]. Hypoxia observed during HILP in the extremity decreases MITF expression, favoring metastasis development [Citation38]. Acidosis observed after HILP locally and systematically is a critical microenvironmental factor that triggers phenotypic plasticity and promotes tumor progression [Citation39]. Acute exposure to proinflammatory cytokines like TNFα can induce melanoma cell growth through increasing MITF expression [Citation40]. Prolonged exposure to TNFα and IL-6, on the other hand, suppresses MITF and cause ‘invasive’ phenotype switching [Citation41]. Melphalan results in decreased expression of MITF mRNA [Citation42]. A five-gene set (ATF3, CYR61, IER5, IL6, and PTGS2) of stress-induced genes, is upregulated after HILP treatment [Citation41]. ATF3 causes a decrease in MITF expression through the hypoxia reaction pathway mentioned above [Citation38]. CYR61/CCN1 is a gene that normally controls melanogenesis in melanocytes. As a stress gene causes upregulation of MITF [Citation43]. IER5, a DNA damage response gene, inhibits MITF expression in melanoma [Citation41]. PTGS2 (Cyclooxygenase 2), a gene regulating the prostaglandin pathway in inflammation, causes an increase of MITF expression [Citation44].
All the above data suggest that CMCs are probably a heterogeneous population of cells with different properties. There is a suggestion that heterogenous tumor subpopulations cooperate to drive invasion and thus survival of cancer cells, even without switching [Citation45]. Other data suggest that single melanoma cells with no specifically identifiable gene signature can reestablish melanoma tumors [Citation46].
A few limitations of our study have to be mentioned. Small sample bias is the main limitation of this study, as well as selection bias. Our hospital is a reference center for this treatment, and referrals represent a highly selected sample of patients who have a locally advanced disease without distant metastasis and are fit to undergo a major operation. Another limitation is the heterogeineity of our sample with different tumor burden, different prior surgical and medical treatments, and different adjuvant therapies. Finally, we assessed CMCs at five time points up to postoperative day 7 because of logistics as patients referred from distant hospitals could not be followed up in our center. Assessment of CMCs at a later time could reveal how long the observed alterations persist after the operation and possible implication of long-term alterations of CMCs after TM-HILP.
Conclusions
TM-HILP is associated with a postoperative increase of CMCs, according to MITF which is higher on the 7th postoperative day. Patients who experienced a complete response to treatment had higher levels of CMCs according to MITF biomarker immediately after the operation and on postoperative day 1. In this sample of 20 patients with irresectable in-transit melanoma, the increase of CMCs according to MITF biomarker after TM-HILP was not associated significantly with 4-year disease-specific survival.
Acknowledgments
We are immensely grateful for the expert assistance kindly offered by Mrs. Ruth Monkman in language editing the final manuscript.
Disclosure statement
The authors report no conflicts of interest.
References
- Stucky CC, Gray RJ, Dueck AC, et al. Risk factors associated with local and in-transit recurrence of cutaneous melanoma. Am J Surg. 2010;200(6):770–774; discussion 4-5.
- Deroose JP, Eggermont AM, van Geel AN, et al. 20 years experience of TNF-based isolated limb perfusion for in-transit melanoma metastases: TNF dose matters. Ann Surg Oncol. 2012;19(2):627–635.
- Lejeune FJ, Lienard D, Matter M, et al. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6.
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408.
- Paulsen IF, Chakera AH, Drejoe JB, et al. Tumour response after hyperthermic isolated limb perfusion for locally advanced melanoma. Dan Med J. 2014;61(1):A4741.
- Kiyohara E, Hata K, Lam S, et al. Circulating tumour cells as prognostic biomarkers in cutaneous melanoma patients. Methods Mol Biol (Clifton, NJ). 2014;1102:513–522.
- Karakousis G, Yang R, Xu X. Circulating melanoma cells as a predictive biomarker. J Investig Dermatol. 2013;133(6):1460–1462.
- Mumford BS, Robertson GP. Circulating melanoma cells in the diagnosis and monitoring of melanoma: an appraisal of clinical potential. Mol Diagn Ther. 2014;18(2):175–183.
- Reid AL, Millward M, Pearce R, et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br J Dermatol. 2013;168(1):85–92.
- Ireland A, Millward M, Pearce R, et al. Genetic factors in metastatic progression of cutaneous melanoma: the future role of circulating melanoma cells in prognosis and management. Clin Exp Metastasis. 2011;28(4):327–336.
- Hoshimoto S, Faries MB, Morton DL, et al. Assessment of prognostic circulating tumour cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann Surg. 2012;255(2):357–362.
- Rao C, Bui T, Connelly M, et al. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol. 2011;38(3):755–760.
- Rodic S, Mihalcioiu C, Saleh RR. Detection methods of circulating tumor cells in cutaneous melanoma: a systematic review. Crit Rev Oncol Hematol. 2014;91(1):74–92.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990). 2009;45(2):228–247.
- Lasithiotakis K, Economou G, Gogas H, et al. Hyperthermic isolated limb perfusion for recurrent melanomas and soft tissue sarcomas: feasibility and reproducibility in a multi-institutional Hellenic collaborative study. Oncol Rep. 2010;23(4):1077–1083.
- Stamatiou D, Ioannou CV, Kontopodis N, et al. Hyperthermic isolated limb perfusion. The switch from Steinmann pins to Omni-tract assisted isolation. J Surg Res. 2017;213:147–157.
- Zoras O, editor. Regional oncotherapies. Athens: Paschalidis Medical Publications LTD; 2006.
- Palmieri G, Satriano S, Budroni M, et al. Serial detection of circulating tumour cells by reverse transcriptase-polymerase chain reaction assays is a marker for poor outcome in patients with malignant melanoma. BMC Cancer. 2006; 6:266.
- Koyanagi K, O'Day SJ, Gonzalez R, et al. Microphthalmia transcription factor as a molecular marker for circulating tumour cell detection in blood of melanoma patients. Clin Cancer Res. 2006;12(4):1137–1143.
- Curry BJ, Myers K, Hersey P. MART-1 is expressed less frequently on circulating melanoma cells in patients who develop distant compared with locoregional metastases. J Clin Oncol. 1999;17(8):2562–2571.
- Samija I, Lukac J, Maric-Brozic J, et al. Prognostic value of microphthalmia-associated transcription factor and Tyrosinase as markers for circulating tumor cells detection in patients with melanoma. Melanoma Res. 2010;20(4):293–302.
- Gogas H, Eggermont AM, Hauschild A, et al. Biomarkers in melanoma. Ann Oncol. 2009;20(Suppl 6):vi8–13.
- Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumour cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009;27(13):2177–2184.
- Weinberg Robert A. The invasion-metastasis cascade. In: Weinberg RA, editor. The biology of cancer. 2nd ed. New York: Garland Science; 2014. p. 644.
- Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–1840.
- Romsdahl MM, Potter JF, Malmgren RA, et al. clinical study of circulating tumour cells in malignant melanoma. Surg Gynecol Obstet. 1960;111:675–681.
- Koyanagi K, O'Day SJ, Boasberg P, et al. Serial monitoring of circulating tumour cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16(8):2402–2408.
- Marsavela G, Aya-Bonilla CA, Warkiani ME, et al. Melanoma circulating tumor cells: benefits and challenges required for clinical application. Cancer Lett. 2018;424:1–8.
- Hida T, Yoneta A, Wakamatsu K, et al. Circulating melanoma cells as a potential biomarker to detect metastasis and evaluate prognosis. Australas J Dermatol. 2016;57(2):145–149.
- Khoja L, Lorigan P, Dive C, et al. Circulating tumour cells as tumour biomarkers in melanoma: detection methods and clinical relevance. Ann Oncol. 2015;26(1):33–39.
- Gray ES, Reid AL, Bowyer S, et al. Circulating melanoma cell subpopulations: their heterogeneity and differential responses to treatment. J Invest Dermatol. 2015;135(8):2040–2048.
- Carreira S, Goodall J, Denat L, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20(24):3426–3439.
- Hartman ML, Czyz M. MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci. 2015;72(7):1249–1260.
- Wellbrock C, Arozarena I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015;28(4):390–406.
- Rambow F, Marine JC, Goding CR. Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Genes Dev. 2019;33(19-20):1295–1318.
- Kim IS, Heilmann S, Kansler ER, et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat Commun. 2017;8:14343.
- Garcia MP, Cavalheiro JR, Fernandes MH. Acute and long-term effects of hyperthermia in B16-F10 melanoma cells. PloS One. 2012;7(4):e35489.
- Cheli Y, Giuliano S, Fenouille N, et al. Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene. 2012;31(19):2461–2470.
- Bohme I, Bosserhoff A. Extracellular acidosis triggers a senescence-like phenotype in human melanoma cells. Pigment Cell Melanoma Res. 2020;33(1):41–51.
- Smith MP, Sanchez-Laorden B, O’Brien K, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 2014;4(10):1214–1229.
- Wouters J, Stas M, Govaere O, et al. Gene expression changes in melanoma metastases in response to high-dose chemotherapy during isolated limb perfusion. Pigment Cell Melanoma Res. 2012;25(4):454–465.
- Comparative Toxicogenomics Database. [cited 2021 Jan 7]. Available from: http://ctdbase.org/query.go?type=ixn&chemqt=equals&chem=name%3AMelphalan&actionDegreeTypes=increases&actionDegreeTypes=decreases&actionDegreeTypes=affects&actionTypes=ANY&geneqt=equals&gene=name%3AMITF&pathwayqt=equals&pathway=&taxonqt=equals&taxon=&goqt=equals&go=&sort=chemNmSort&perPage=50&action=Search.
- Xu Z, Chen L, Jiang M, et al. CCN1/Cyr61 stimulates melanogenesis through integrin α6β1, p38 MAPK, and ERK1/2 signaling pathways in human epidermal melanocytes. J Invest Dermatol. 2018;138(8):1825–1833.
- Kim JY, Shin JY, Kim MR, et al. siRNA-mediated knock-down of COX-2 in melanocytes suppresses melanogenesis. Exp Dermatol. 2012;21(6):420–425.
- Chapman A, Fernandez del Ama L, Ferguson J, et al. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Rep. 2014;8(3):688–695.
- Ziman M. Circulating melanoma cells. In: Tanaka Y, editor. Breakthroughs in melanoma research. Rijeka (Croatia): IntechOpen; 2011. p. 1–16. Available from: https://www.intechopen.com/books/breakthroughs-in-melanoma-research/circulating-melanoma-cells.