Abstract
Purpose
This retrospective study aimed to evaluate the safety and efficacy of microwave ablation (MWA) for lung malignancies in patients with severe emphysema.
Materials and methods
The clinical records of 1075 consecutive patients treated for malignant lung tumors in our department were retrospectively reviewed. Emphysema was assessed based on standard-dose computed tomography (CT) and was considered severe when it occupied ≥25% of the lung. Overall, 26 patients (24 men and 2 women; mean age ± standard deviation [SD]: 71.23 ± 8.18 years, range: 59–88 years) with severe emphysema underwent CT–guided percutaneous MWA for treating 26 tumors (24: non-small cell lung cancer and 2: metastases). The mean tumor size was 3.0 cm (SD: 1.5, range: 1.2–6.5 cm). Follow-up was performed with CT at 1, 3, 6, 12 months after ablation, and every 6 months thereafter. Complications and efficacy were evaluated.
Results
The median follow-up duration in all patients was 17.5 months (range: 5–37 months, interquartile range: 15.8). The mortality rate was 0% within 30 days after ablation. Major complications including pneumonia, lung abscess and refractory pneumothorax occurred in 19.2% (5/26) patients. The technical success and efficacy rates were 88.5% (23/26) and 87.0% (20/23), respectively, and the local tumor progression rate was 30.0% (6/20).
Conclusion
MWA appears to be a safe and effective therapeutic option for treating lung malignancies in patients with severe emphysema.
Introduction
Pulmonary emphysema (PE) is a common comorbidity with lung malignancies. It is defined as an abnormal and permanent enlargement of airspaces distal to the terminal bronchioles, and the destruction of alveolar walls [Citation1], resulting in a restrictive defect in pulmonary function; the changes are particularly notable in patients with severe emphysema. The treatment of patients with coexisting severe emphysema and lung malignancies is particularly challenging for thoracic surgeons, mainly owing to the poor prognosis and considerably high rates of severe postoperative complications [Citation2–4]. Other clinicians, such as oncologists and radiologists, may also face similar difficulties in treating these patients with chemotherapy or radiotherapy, or both, as they are liable to aggravate respiratory deterioration.
Image-guided thermal ablation of lung malignancies, including radiofrequency ablation (RFA) and microwave ablation (MWA), are becoming increasingly recognized therapeutic options, and have been proven to be safe and effective alternatives for patients who are not surgical candidates [Citation5]. Ablative techniques may offer therapeutic benefits with relatively minimal complications in patients with poor pulmonary reserve, as they spare healthy lung parenchyma and minimize changes in lung function [Citation6–8]. Therefore, thermal ablation seems to be a promising therapeutic option for primary lung cancer that is either unresectable or unsuitable for radiotherapy, due to poor pulmonary reserve caused by underlying pulmonary disease or previous lung resection. And for colorectal pulmonary metastases, thermal ablation is recommended alone or in combination with surgery as long as all visible disease is eradicated, and radiotherapy is only recommended when surgery and/or ablation are not indicated or within a clinical trial [Citation9]. A prospective clinical trial of RFA on 54 patients at high-risk for any form of pulmonary resection showed good tolerance without adverse effect on pulmonary function [Citation8]. Moreover, several studies on RFA or MWA showed good tolerance for patients with a single lung [Citation10–12]. Compared with RFA, MWA generates higher temperatures with a lower heat-sink effect, requires shorter treatment time and achieves ablation of larger zones; the latter is therefore preferred for the ablation of lung cancer [Citation13].
Despite the benefits of MWA, its safety and efficacy for treating lung malignancies in cases with severe emphysema have not been fully investigated. In the present study, we retrospectively reviewed the data of patients with malignant lung tumors and coexisting severe emphysema who underwent MWA; we intended to evaluate the technical feasibility of MWA, focusing on particular risks and patient safety.
Materials and methods
Emphysema evaluation
Emphysema assessment was performed based on standard-dose CT, performed within one to three months before the treatment. All CT scans were coded and masked for identifiable information and evaluated by two radiologists, who were blinded to the clinical data. Emphysema is defined as the permanent enlargement of airspaces distal to the terminal bronchiole, with the destruction of the alveolar walls. It is manifested on CT scans as well-demarcated focal areas or regions of low attenuation, usually without visible walls; methods for quantification of emphysema have been reported [Citation14–16]. In this study, all images were transferred to a workstation (IntelliSpace Portal 7.0, Philips Healthcare) for lung density analysis. In summary, the extent of emphysema was calculated by measuring the percentage of lung volume with attenuation below −950 Hounsfield units (HU). Measurements were performed by two radiologists, and any significant inconsistencies in the same patient leading to different classifications of severity were resolved through consensus. The degree of severity was classified as follows: mild (≤5%), moderate (6–24%), and severe (≥25%).
Patients
Our institutional review board approved this retrospective single-center study and waived the requirement for informed consent. Moreover, written informed consent was obtained from all patients before lung tumor MWA. We performed a retrospective search of the electronic medical record and CT images at our center to identify patients who underwent MWA of lung tumors with coexisting severe emphysema. Between September 2016 and August 2019, a total of 1075 patients presented to our medical center with either primary lung cancer or pulmonary metastases. Among them, 1029 patients with lung neoplasms were evaluated and scored for the presence of emphysema at our center, using chest CT imaging; 46 patients were excluded owing to the unavailability of standard-dose CT data. Emphysema was designated as present or absent; if present, it was graded according to the percentage of the lung it occupied on CT, as follows: mild (≤5%), moderate (6–24%), and severe (≥25%). Patients were selected according to the following inclusion criteria:(a) pathologically confirmed primary or metastatic lung cancer; (b) percentage of emphysema ≥25% (severe emphysema); (c) patients unsuitable for surgical treatment or with operable disease refusing surgery; (d) available standard-dose CT data within one to three months before treatment at our center; and (e) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2. Patients undergoing both curative and palliative treatments were included for analysis; the patient selection process is shown in .
Figure 1. Flow chart of the eligibility process for the study. CT: computed tomography imaging; MWA: microwave ablation; ECOG PS: Eastern Cooperative Oncology group performance status.
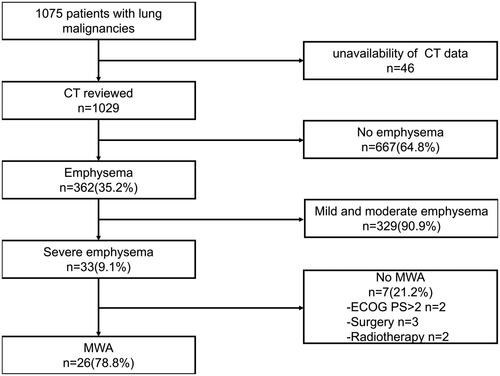
The indication, feasibility, and safety of MWA were evaluated by a multidisciplinary team consisting of thoracic surgeons, oncologists, radiation therapists, and interventional radiologists. Overall, 33 patients were diagnosed with malignant lung tumors and coexisting severe emphysema; 7 patients received other treatment (3: surgery, 2: radiotherapy, and 2: supportive treatment owing to PS > 2). Therefore, a total of 26 consecutive patients (24 men and 2 women; mean age ± SD: 71.23 ± 8.18 years; range: 59–88 years) with 26 tumors received MWA between September 2016 and August 2019; these patients met all eligibility criteria. Overall, 23 and 3 patients underwent radical and palliative ablation, respectively. The three patients underwent palliative ablation to reduce tumor burden and relieve tumor-related pain; moreover, two of the three also received targeted therapy after MWA. These patients were either inoperable (n = 19) or refused to undergo surgery (n = 7). Prior to MWA, all patients were pathologically confirmed to have malignancies by CT-guided percutaneous lung biopsy; 24 tumors were identified as primary non-small cell lung cancer (NSCLC), and 2 were confirmed to be metastases from colorectal cancer, with controlled primary disease. Except for the 2 patients with pulmonary metastases from colorectal cancer, all others were treatment-naive and had no history of surgery, radiotherapy, or chemotherapy for cancer. The average tumor size was 3.0 cm (SD: 1.5, range: 1.2–6.5 cm). Overall, 23 and 3 tumors were located in the periphery and central zones of the lung, respectively. The tumors were located in the left upper lobe (n = 8), left lower lobe (n = 1), right upper lobe (n = 8), middle lobe (n = 1), and right lower lobe (n = 8). Further details have been mentioned in .
Table 1. Patient demographics and tumor characteristics.
Pre-ablation assessment
Before ablation, all patients underwent routine assessment by an interventional radiologist, including the history of illness, physical examination, laboratory and relevant imaging studies, including thoracoabdominal CT, whole-body positron emission tomography/computed tomography (PET/CT), or both. Anticoagulant and antiplatelet medications were temporarily discontinued between 1 day and 1 week depending on the types of drugs. Prophylactic antibiotics were not routinely administered, and none of the 26 patients received prophylactic antibiotics.
Instrumentation and procedure
All lung MWA procedures were performed with CT guidance (GE Discovery 16 Slice CT, GE Healthcare) under local anesthesia with 5–10 ml of 2% lidocaine. Morphine (10 mg, subcutaneous) was administered 30 min before treatment. Continuous electrocardiograph monitoring was performed every 5 min throughout the procedure, including heart rate, blood pressure and pulse oximetry.
All patients were treated by the same interventional radiologist (Xg Li, with 20 years of experience in thermal ablation) using a water circulation cooling microwave ablation system (Vision Medical Devices R&D Center, Nanjing, China), the MWA of which had a shaft length of 100–180 mm and an outside diameter of 15–18 G. The ablation parameters, including the type of antenna and number and duration of treatments, were planned based on tumor size, shape and location. A single antenna was usually applied for tumors of ≤3.0 cm in diameter, and two were used for tumors of >3.0 cm in diameter. The probe was inserted through the skin into the target lesion avoiding vessels, bronchi, emphysema, and fissures, to minimize the risk of pulmonary bleeding and pneumothorax. In unavoidable cases, the probe was inserted directly into the target tumor through emphysematous zones (). Ablation was performed to achieve a complete ablation zone that encompassed the tumor with an optimal circumferential margin of at least 1 cm. The ablation power output was set at 40–80 W, and the duration ranged from 5 to 20 min. During the ablation, CT scans were performed every 2–5 min to monitor the ablation zone and identify any complications.
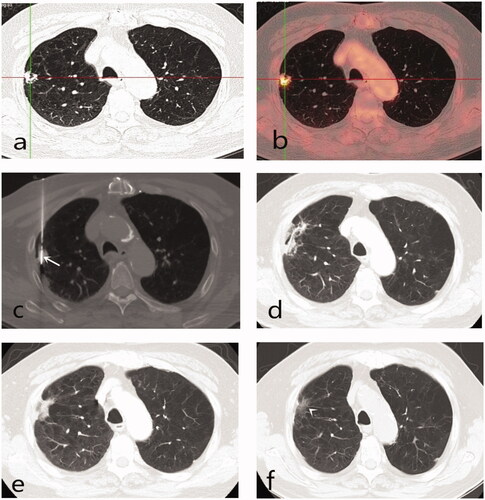
Figure 2. Eighty-year-old male with 1.4 × 1.4 cm right upper lobe T1N0M0 non-small cell lung cancer (adenocarcinoma) comorbid severe emphysema completely ablated by microwave ablation (MWA). (a, b) Tumor lesion on positron emission tomography-computed tomography prior to MWA (standardized uptake value 10.3); (c) MWA, the antenna punctured the central position of the lesion through emphysematous zones (arrow); (d–f) Axial CT scans between 1 month and 18 month post-ablation showed gradual shrinkage of the ablated lesion; and became a fiber scar 18 months after ablation (arrowhead).
Post-ablation management and follow-up
All patients were hospitalized for MWA and observation. A non-enhanced chest CT scan was routinely performed immediately after ablation, to assess whether the tumor was completely ablated and to check for possible complications. If the ablation was deemed complete and no urgent management of complications was needed, the patient was transferred to the inpatient ward and observed. All patients were advised absolute bed rest without special positioning maneuvers, and vital signs were monitored with electrocardiography in 8 h after ablation, including heart rate, blood pressure, and pulse oximetry. Prophylactic antibiotics were not routinely administered after MWA; only three patients were administered antibiotics after ablation owing to a diagnosis of pulmonary infection, which was established from the clinical symptoms, including high fever and radiological findings. A non-enhanced chest CT scan was then individually performed 24–48 h after the procedure to assess for changes and check for subtle complications. If no complications were requiring further treatment, the patients were usually discharged 2–3 days after the ablation procedure. After discharge from the hospital, contrast-enhanced CT follow-up imaging was performed at 1, 3, 6 and 12 months post-ablation and every 6 months thereafter. The images obtained 1-month post-ablation were considered the baseline scans, with which subsequent images were compared. Data pertaining to complications and efficacy were recorded.
Post-ablation complications
Morbidity and mortality data were recorded according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0) [Citation17]. The primary outcomes of interest were the rates of post-procedure morbidity and mortality. Periprocedural and 30-day mortality rates after ablation were calculated. Any patient death within 30 days was defined as a grade 5 adverse event. The grade 3 or 4 and grade 1 or 2 adverse events were regarded as major and minor complications, respectively.
Efficacy
Technical success was defined as complete coverage of the tumor by the ablation zone, with an adequate ablative margin on the unenhanced CT scan performed immediately after the procedure. Technique efficacy referred to complete ablation without evidence of residual tumor on the 1-month contrast-enhanced CT image after ablation. Irregular or nodular enhancement (>15 HU) in the ablation zone signified residual tumor. Local tumor progression (LTP) was identified by enlargement of the ablation zone or nodular enhancement inside or at the edge of the ablation zone during imaging follow-up, provided that the primary technique efficacy was achieved. Measurement of the minimal ablative margin (MM) was performed for tumors that achieved technique efficacy. MM was defined as the shortest distance between the ablated lesion and the edge of the ablation zone and was measured on the CT image taken immediately after ablation.
Statistical analysis
All data were analyzed using SPSS for Windows Version 19.0 (IBM, USA). All categorical variables were compared using the χ2 test, and all continuous variables were compared using the t-test. p-values <0.05 were considered to be statistically significant.
Results
Efficacy
Procedures were completed following the protocol in all cases. In three patients, the ablation antenna displaced from the lesion during the procedure due to severe pneumothorax. The ablations were successfully completed by aspirating the maximum possible volume of free air from the pleural cavity, after placing a 6–10 F drainage catheter. The rate of technical success was 88.5% (23/26); all 23 radical ablations were technically successful. Incomplete ablation was observed in all three patients who underwent palliative-therapy, whose tumors were ≥6.0 cm. Technique efficacy was achieved in 20 (87.0%) of the 23 patients. Measurement of the MM was possible for all 20 tumors that achieved technique efficacy. The median MM was 7 mm (range 0–14 mm). MM of >5 mm and ≤5 mm was achieved in 13/20 (65%) and 7/20 (35%) tumors, respectively. Residual tumor was present in the remaining 3 patients at the 1-month CT follow-up. Three of the six patients with residual tumors received targeted therapy based on the results of genetic testing, while the remaining did not receive any anticancer treatment.
The last follow-up was on 31 March 2020, and the median contrast-enhanced CT follow-up duration in all patients was 17.5 months (range: 5–37 months, interquartile range: 15.8 months), without any loss during follow-up. Despite primary technical efficacy, 6 tumors among 6 patients including those with lung adenocarcinoma (n = 2), squamous carcinoma (n = 3), and large cell carcinoma (n = 1) progressed at the ablation zone on the follow-up CT scans; this resulted in an LTP rate of 30.0% (6/20). All six progressed tumors were located at the periphery of the lung; three were adjacent to the pleura (distance from pleura: <10 mm). Four of the six LTPs were observed in tumors with MM ≤ 5 mm. MM ≤ 5 mm was also associated with LTP rate after MWA (p < 0.001) (). The mean tumor size was 3.3 ± 0.9 cm (range, 2.2–4.3 cm). The tumor size differed significantly between completely ablated (n = 20) and locally progressed (n = 6) tumors; (p < 0.001) (). LTP was more commonly found in tumors with MM ≤ 5 mm (n = 4) and tumors exceeding 3.0 cm (n = 4), and it occurred at 5.9–14.7 months after initial MWA. The median time to LTP was not reached.
Table 2. Characteristics of six local progressed tumors after MWA.
Safety
No death occurred both during and within 30 days after tumor ablation. The complications resulting from ablation have been listed in . Among the 26 microwave ablation sessions, complications were observed in 20 (76.9%); these were mostly minor.
Table 3. Procedural complications of 26 MWA sessions.
Major complications (CTCAE Grade 3) occurred in five of the 26 patients (19.2%); 2, 1, and 2 cases had pneumonia, lung abscess and refractory pneumothorax, respectively. And CTCAE Grade 4 adverse events were not observed. Patients with pneumonia and lung abscess showed a good response to sputum culture-specific antibiotics and were discharged within 2 weeks. The two refractory pneumothorax patients showed no evidence of bronchopleural fistula, and were effectively managed by combined intratumoral injection of hypertonic glucose and continuous catheter drainage, which has been described in our previous publication [Citation18]. The two patients were discharged after 20- and 30-day hospital stays, respectively, without recurrence of pneumothorax during follow-up.
Pneumothorax was the most common complication, occurring in 17 of 26 patients (65.4%). However, except for the two patients above, the pneumothorax in most cases (n = 15) were either CTCAE Grade 1 or CTCAE Grade 2 severity. The proportion of pneumothorax cases requiring chest tubes was 42.3% (11 of 26 patients). Among these 11 patients, three had coexisting subcutaneous emphysema. Pleural effusion was another common complication, and the incidence rate of requiring drainage was 15.4%.
Pneumothorax was significantly higher in MWA sessions where the antenna traversed emphysematous lung (p = 0.006) (). All eleven patients in whom the antenna traversed emphysematous lung developed pneumothorax. There was no significant association between tumor size, location and pathology, and complications, including pneumothorax, pleural effusion, hemoptysis, and pulmonary infection.
Table 4. Pneumothorax and risk factors analysis after MWA.
Most patients were discharged after hospitalization for 1 week (median: 6 days, range: 2–30 days). Five patients were hospitalized for more than 7 days (), including 3 and 2 who developed pulmonary infections and refractory pneumothorax, respectively. None of the patients had any clinical worsening of respiratory insufficiency, and most resumed their daily routine activities within several days after ablation; they did therefore not systematically undergo lung function testing after MWA.
Discussion
In this retrospective cohort, the rate of technical success of MWA was 88.5% (23/26); all 23 radical ablations were technically successful, and no deaths occurred both during and within 30 days after tumor ablation. Although the median time to LTP was not reached, the LTP rate was found to be higher in patients with tumors exceeding 3.0 cm in size.
Emphysema is not only an independent risk factor for lung cancer but also a significant predictor of death from lung cancer [Citation19,Citation20]. The treatment of lung malignancies with concurrent severe emphysema remains a challenge. The scope of surgery and radiotherapy is limited in cases of severe emphysema. Previous studies have shown that severe emphysema is a poor prognostic predictor of long-term survival in lung cancer patients undergoing surgery. Moreover, coexisting emphysema increases the incidence of perioperative complications requiring treatment and mortality [Citation2,Citation3]. A study reported a high in-hospital mortality rate of 14% following anatomical lobectomy in patients with NSCLC and severe emphysema [Citation21]. MWA can be an alternative treatment for lung malignancies in patients who are medically inoperable due to high-risk conditions or refuse to accept surgery. For patients with high-risk conditions, such as patients with a single lung after prior pneumonectomy, thermal ablation appeared to be a safe and effective option as demonstrated in several studies [Citation10–12]. However, the safety and efficacy of MWA for primary lung cancer or metastases with coexisting severe emphysema have not been fully determined.
Optimal resection of lung cancer depends on cardiopulmonary function assessment and many other factors. Percutaneous image-guided MWA is minimally invasive compared with surgery and is therefore theoretically preferable in cases of underlying pulmonary diseases and other comorbidities. Recent studies demonstrated no significant changes in lung function tests after RFA for stage IA NSCLCs; however, pulmonary function tests are strongly recommended in patients with a history of pulmonary disease [Citation8,Citation22,Citation23]. Unfortunately, due to the retrospective nature of this study, all patients did not undergo lung function testing before and after ablation, quantitative analyses of lung function changes were therefore not possible. However, the results of the current study demonstrated that the procedure was relatively safe and did not worsen respiratory insufficiency. This was consistent with the findings from a study by Hess et al. who reported that none of their patients with a single lung had worsening respiratory insufficiency clinically after RFA [Citation10]. However, Sofocleous et al. suggested it was critical to diagnose and manage complications promptly for this high-risk population because they not only had diminished pulmonary reserve but were also more susceptible to the serious complications of thermal ablation [Citation11].
No peri-procedural death occurred in the 26 sessions of MWA. To a certain extent, our study showed that the presence of severe emphysema did not negatively influence procedure-related mortality. However, the incidence of overall complications(82.0%) and major complications(19.2%) was higher than that of other thermal ablation studies [Citation22,Citation23]. However, no complications of Grade ≥4 were observed.
Reports suggest that patients with emphysema need particular care in case of severe complications [Citation24]. This may be attributed to the fact that emphysema significantly increases the risk of pneumothorax and lung abscess [Citation25]. In our study, the incidence of pneumothorax reached up to 65.4% and was much higher than that of recent studies on lung MWA and RFA (8.5–32%) [Citation26–28]. However, most cases of pneumothorax were of either CTCAE grade 1 or 2 severity. The present study showed that traversing emphysematous zones was a risk factor for developing a pneumothorax. Lung abscess occurred in 3.8% of patients, this proportion was slightly higher than that reported by Zheng et al. for pulmonary MWA (0.5%) [Citation24] and by Kashima et al. for pulmonary RFA (1.6%) [Citation25].
A relatively short hospital stay also indicated good tolerance of the MWA procedure in our cohort; however, the median hospital stay of 6 days was slightly longer than that of previous reports on MWA series [Citation26–28]. The prolonged hospital stay may have been related to the higher rate of complications. However, most patients were discharged after hospitalization for one week.
The LTP rate of the present study (30.0%) was slightly higher than that of previous lung MWA studies, such as those performed by Kurilova et al. [Citation29] (10%), Zheng et al. [Citation30] (19.1%), and Wolf et al. [Citation31] (26.0%). This may be related to two reasons; first, this could have been related to bias due to the small study sample, and second, the existence of severe emphysema may have limited the selection of the optimal puncture approach and necessary adjustments, thereby increasing the risk of LTP. Our study showed that MM and tumor size were associated with LTP. We found that MM ≤ 5 mm was associated with higher LTP (p<0.001). This was consistent with the findings of the study by Kurilova et al. [Citation29], where they showed that tumors ≥1 cm and with MM ≤ 5 mm were associated with a higher rate of LTP. In the studies by Zheng et al. and Wolf et al., tumors larger than 3.0 cm were found to be significantly more likely to progress at the ablation zone [Citation30,Citation31]. However, in the study by Egashira et al., there was no correlation between LTP and tumor size [Citation32]. Our study showed that tumor size was associated with LTP, especially in tumors exceeding 3.0 cm (p<0.001).
Our study has several limitations. First, the retrospective nature, small sample size and considerable heterogeneity in tumor characteristics could have led to selection bias. Second, we could not report on pulmonary function before and after the ablation as these tests were not conducted on the patients enrolled in our study. Therefore, we could not assess the effect of ablation on lung function in patients with severe emphysema. Finally, the follow-up period was relatively short, definitive conclusions could not therefore be drawn regarding the long-term outcomes. Clinical trials should therefore be performed with large samples and long-term follow-up to obtain more convincing evidence.
Conclusion
Pulmonary MWA can be performed safely and effectively in patients with severe emphysema. The results of the present study will provide important information to physicians for offering MWA as an alternative treatment for patients with malignant lung tumors and severe emphysema.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rennard SI. COPD: Overview of definitions, epidemiology, and factors influencing its development. Chest. 1998;113(4):235S–241S.
- Takahashi Y, Matsuda M, Aoki S, et al. Qualitative analysis of preoperative high-resolution computed tomography: risk factors for pulmonary complications after major lung resection. Ann Thorac Surg. 2016;101(3):1068–1074.
- Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145(2):346–353.
- Kawaguchi T, Sawabata N, Miura S, et al. Prognostic impact of underlying lung disease in pulmonary wedge resection for lung cancer. Int J Clin Oncol. 2019;24(4):366–374.
- Abtin F, De Baere T, Dupuy DE, et al. Updates on current role and practice of lung ablation. J Thorac Imaging. 2019;34(4):266–277.
- de Baère T, Palussière J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240(2):587–596.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9(7):621–628.
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491–3498.
- Benson AB, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370–398.
- Hess A, Palussière J, Goyers J-F, et al. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology. 2011;258(2):635–642.
- Sofocleous CT, May B, Petre EN, et al. Pulmonary thermal ablation in patients with prior pneumonectomy. AJR Am J Roentgenol. 2011;196(5):W606–W612.
- Yang X, Ye X, Zhang L, et al. Microwave ablation for lung cancer patients with a single lung: clinical evaluation of 11 cases. Thorac Cancer. 2018;9(5):548–554.
- Sidoff L, Dupuy Damian E. Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia. 2017;33(1):25–33.
- Hohberger LA, Schroeder DR, Bartholmai BJ, et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol. 2014;9(5):639–645.
- Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J. 2009;34(4):858–865.
- Orting SN, Petersen J, Thomsen LH, et al. Learning to quantify emphysema extent: what labels do we need? IEEE J Biomed Health Inform. 2020;24(4):1149–1159.
- National Institute of Health. National Cancer Institute. Common terminology criteria for adverse events (CTCAE version 5) [Internet]. Washington (DC): US Department of Health and Human Services; 2017. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html
- Wang D, Li X, Yu W. Intratumoral injection of hypertonic glucose in treating refractory pneumothorax caused by microwave ablation: a preliminary study. Cardiovasc Intervent Radiol. 2019;42(6):915–919.
- Yong PC, Sigel K, de-Torres JP, et al. The effect of radiographic emphysema in assessing lung cancer risk. Thorax. 2019;74(9):858–864.
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–1223.
- Edwards JG, Duthie DJ, Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax. 2001;56(10):791–795.
- Venturini M, Cariati M, Marra P, et al. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc Intervent Radiol. 2020;43(5):667–683.
- Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018;13(1):91.
- Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98(1):243–248.
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. AJR Am J Roentgenol. 2011;197(4):W576–W580.
- Vogl TJ, Naguib NNN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261(2):643–651.
- Healey TT, March BT, Baird G, et al. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28(2):206–211.
- Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82(1):177–181.
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave ablation in the management of colorectal cancer pulmonary metastases. Cardiovasc Intervent Radiol. 2018;41(10):1530–1544.
- Zheng A, Ye X, Yang X, et al. Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol. 2016;27(12):1806–1814.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879.
- Egashira Y, Singh S, Bandula S, et al. Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: a retrospective single-center experience. J Vasc Interv Radiol. 2016;27(4):474–479.
