Abstract
Objective: Thermal treatment (TT), defined as treatment using supra-physiological body temperatures (39–45 C), somewhat resembles fever in terms of temperature range, one of the first natural barriers for the body to fight exposure to external pathogens.
Methods: Whole-body thermal treatment (WBTT) consists of heating up the complete body to a temperature range of 39 to 45 C. Despite the recognized therapeutic potential of hyperthermia, the broad clinical use of WBTT has been limited by safety issues related to medical devices and procedures used to achieve WBTT, in particular adequate control of the body temperature. To circumvent this, a sophisticated medical device was developed, allowing long-term temperature controlled WBTT (41.5 C for up to 8 h). Technical feasibility and tolerability of the WBTT procedure (including complete anesthesia) were tested using female Aachen minipig. Optical fiber temperature sensors inserted in multiple organs were used and demonstrated consistent monitoring and control of different organs temperature over an extended period of time.
Results: Clinical evaluation of the animals before, during and after treatment revealed minor clinical parameter changes, but all of them were clinically acceptable. These changes were limited and reversible, and the animals remained healthy throughout the whole procedure and follow-up. In addition, histopathological analysis of selected key organs showed no thermal treatment-related changes.
Conclusion: It was concluded that WBTT (41.5 C for up to 8 h) was well tolerated and safe in female Aachen minipigs. Altogether, data supports the safe clinical use of the WBTT medical device and protocol, enabling its implementation into human patients suffering from life-threatening diseases.
Introduction
It is known and well accepted that fever represents one of the body’s first lines of defense against invading pathogens, as the higher body temperature is a hostile environment for pathogens development [Citation1]. In addition, the rise in body temperature signals white blood cells production, which will significantly contribute to fight off infections. In the last few decades, therapeutic hyperthermia (thermal treatment, TT) mimics the temperature range (38–42 °C) observed in feverish conditions, and has been successfully explored and tested in (pre)clinical studies at various temperatures [Citation2]. The scientific origin of TT as a potential therapeutic modality goes back to the 1890s, when Dr. William Coley treated about 900 inoperable cancer patients with bacterial extracts. He observed a relationship between survival rate and the achieved body temperature [Citation3,Citation4]. Although it was unclear whether the observed effects were caused by the fever itself, or by a stronger immune response due to an increase in body temperature. Coley’s observations initiated further explorations in the field of TT [Citation5]. The concept of TT covers various applications of external heat to raise a patient’s body temperature in the range of 39 to 45 °C. TT can be applied using several methods, either locally, regionally or systemically (WBTT) [Citation6].
Local TT is the most common and has been used extensively for superficial lymph nodes, prostate cancer, and cutaneous or subcutaneous melanoma metastasis [Citation7]. Regional TT has been mainly used primarily for recurrent or advanced breast cancer, advanced pelvic tumors and for sarcomas of the trunk or extremities. In contrast to local and regional TT, WBTT is often preferred for treating systemic cancers (e.g., metastases and/or circulating tumor cells) [Citation8]. In general, TT can be used with all stages of cancer. However, its current use is with advanced solid tumors that are hardly operable or inoperable, as well as with recurrent tumors and metastases [Citation9]. Also, where conventional therapy approaches (e.g., surgery, chemotherapy, radiation therapy) are not very likely to be successful, or have proven to be inadequate, TT has already been successfully used with some tumors including their metastases in different organs, both experimentally in animal models and in human [Citation10,Citation11]. More specifically, in mice models of melanoma and lung carcinoma, WBTT application (39.5–40 °C) was able to significantly reduce lung metastases and increase immune response [Citation12]. In a controlled clinical trial, patients with stage IV advanced gastric cancer submitted to WBTT combined with chemotherapy presented a significant therapeutic benefit against the primary tumors and metastases to the lymph nodes and liver, thus reinforcing the therapeutic potential of WBTT combined with standard of care [Citation13]. TT modalities vary from short-term (typically between 1 and 4 h) to long term (exceeding 4 h). However, the tolerance of liver and brain tissue limits the maximum use of WBTT to 41.8–42 °C temperature range, which may be maintained for several hours [Citation14,Citation15].
The current molecular arguments for using TT for the treatment of life-threatening diseases such as cancer are: (1) the observation that heat can induce direct tumor cell death; (2) the changes in the oxidative metabolism sensitize tumors to chemo- and radiotherapeutic strategies; and (3) it triggers an immune stimulating effect [Citation11,Citation16–20]. Proven mechanisms include inhibition of different DNA repair processes, (in)direct reduction of the hypoxic tumor cell fraction, enhanced drug uptake, increased perfusion, and oxygen levels. All mechanisms show different dose-dependent effects, and different optimal scheduling with radiotherapy and chemotherapy [Citation21]. A significant range of TT modalities are currently being used in the clinic and clinical trials are ongoing, including trials investigating the effect of fever-range temperatures (<41 °C) [Citation10]. In randomized clinical trials, the great potential of adding thermal treatment to chemotherapy was demonstrated for the treatment of high-risk soft tissue sarcoma[Citation22], and for ovarian cancer [Citation23]. Despite multiple reports of promising clinical responses in human cancer patients undergoing loco-regional WBTT [Citation18], thermal treatment does not yet belong to the standard of care. A possible explanation is a lack of clinical safety to efficacy ratio, and WBTT is still under debate due to the relatively high temperatures applied, safety issues and the lack of well-designed clinical studies [Citation24–28]. It is known that there are no universal solutions for technical realization of artificial TT, and for choosing its rational temperature range and duration. It is necessary to take into account the concrete clinical situation of each patient (personalized precision medicine) to achieve the maximal therapeutic outcome [Citation29]. In addition, another main rate-limiting challenge hampering widespread clinical adoption and deployment of TT in the treatment of life threatening diseases is the development of a suitable device that is capable of inducing, measuring, and maintaining a homogeneous body temperature in a safe way [Citation11].
To circumvent these issues, a WBTT medical device was developed. Its technical performance was tested in female (Aachen) minipigs to evaluate the tolerability and safety of the procedure [Citation30]. The Aachen minipigs were chosen due to their anatomical, physiological and genetic similarities with humans [Citation31,Citation32]. In addition, the pig is one of the most frequently used large animal models for biomedical research, especially in the field of translational research [Citation31]. In this manner, the minipig has been established in the pharmaceutical industry as a valuable non-rodent species for safety testing with convincing arguments demonstrating its closeness to human biophysiology, making them highly competitive versus the dog or non-human primate [Citation33–35]. Additionally, minipigs are able to tolerate the proposed thermal dose, and previous studies already demonstrated the safety of TT applied to minipigs [Citation36–38]. Interesting, Sebeke and collaborators very recently established a workflow for the in vivo high-intensity focused ultrasound ablation of the porcine pancreas under MRI guidance to facilitate related research and accelerate clinical translation [Citation39].
Preclinical investigation is a crucial part of the evaluation and validation of a novel WBTT procedure for the treatment of patients suffering from life threatening diseases (e.g., cancer treatment), and an essential step toward the translation of the WBTT into clinical studies and finally into clinical practice. In support to a planned clinical trial to treat advanced metastatic pancreatic cancer patients and in addition to evaluation of the efficacy/tolerability of WBTT in tumor-bearing dogs (ElmediX manuscript in preparation), we performed a safety/tolerability study in healthy female Aachen minipigs to define appropriate WBTT conditions and help to develop a suitable and safe procedure. In the present manuscript, the validation of the WBTT protocol (41.5 °C for up to 8 h), the encountered challenges and its tolerability/safety is described. Overall, findings strongly support a safe and clinically-manageable exploration of this therapeutic modality and procedure in advanced cancer patients.
Materials and methods
Animals, viability, clinical signs, body weight and food consumption
This study was performed at Medanex (Diest, Belgium) in accordance with the Ethical Standards of the Regional Committee on animal experimentation (EC MxCI 2016-073), according to the laws of animal protection for experimentation and all applicable regulations. Female Aachen minipigs [Citation40], Sus scrofa domesticus, were allowed an acclimatization period of at least 2 weeks prior to experimental procedure () [Citation34]. Animals were housed individually on a straw bedding. Ambient temperature was monitored and maintained within the range of 22–26 °C. Animals were examined daily and visually inspected for appetite, behavior, feces and urine production. Detailed observations were recorded, and minipigs were weighed upon arrival to the facility, the day before treatment, weekly and before scheduled necropsy.
Table 1. Female Aachen minipigs submitted to whole-body thermal treatment (WBTT) or normothermia session(s).
Whole-body thermal treatment procedure
Animals were subjected to either single or repeated (3; one week apart) whole-body thermal treatment (WBTT) session(s) (41.5 °C, as controlled by intrahepatic temperature via series of temperature sensors surgically inserted into a liver lobe) for a period of 6–8 h, or to normothermia (37°C) for 8 h (). WBTT was performed with a medical device (HyperTherm GEN3, which was further optimized for clinical use under the name of ElmediX HyperTherm) able to deliver WBTT to large animals by the convective exchange of heat between an electrical heating element and the animal body, through circulating air (). During the initial heating phase, the heat produced by the electrical heating element is used to slowly increase the temperature of the animal to the targeted treatment setpoint. Then the body temperature was maintained at the desired treatment temperature via liver sensor-driven temperature control (). The air temperature was controlled by adapting the electrical power delivered to the heating element. To accurately measure its body temperature, fiber optic temperature sensors (THR-NS-1084A, THR-NS-1172A, FISO Technologies Inc.) were placed above and in the animal body, at different anatomical locations (e.g., liver, rectum, esophagus, skin, bladder, subcutaneous, …). Each sensor is a fiber optic sensor providing 4 independent temperature measuring points, one at the tip of the sensors and three more respectively 1, 2, and 3 cm from the tip.
Figure 1. Schematic diagram of ElmediX HyperTherm GEN3 medical device components. Hot air is injected at high speed in a treatment chamber. Air temperature is modified, in order to reach and maintain a liver temperature of 41.5 °C. All optical fiber sensor data is logged simultaneously on a personal computer for control and data reporting.
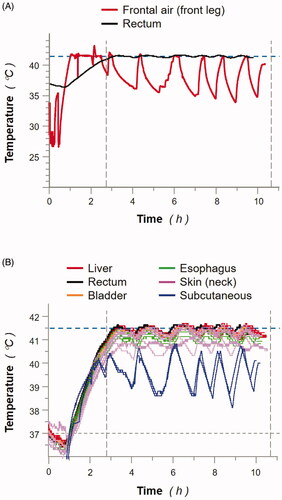
Figure 2. Treatment chamber and animal (MP3) body temperatures monitored during whole-body thermal treatment (WBTT). (A): frontal air (red line; front leg) and rectum (black line) temperatures were recorded throughout WBTT procedure of MP3 (heating phase, and 8 h at 41.5 °C). (B): animal body temperatures were recorded throughout WBTT procedure of MP3 (heating phase, and 8 h at 41.5 °C) via individual temperature sensors positioned in the indicated tissues: liver (red lines; 8 sensors), rectum (black line; 1 sensor), bladder (orange line; 1 sensor), esophagus (green lines; 3 sensors), skin (neck; pink lines; 8 sensors); and subcutaneous (blue lines; 2 sensors). Results presented for MP3 are representative of all tested animals (data not shown).
All WBTT sessions were conducted under full anesthesia of the animals (similar to future clinical applications in human patients) which was induced by intramuscular (IM) infusion of tiletamine (2.75 mg/kg) and zolazepam (2.75 mg/kg) at a rate of 0.055 ml/kg, along with IM infusion of xylazine (2.4 mg/kg) at a rate of 0.12 ml/kg. Animals were then intubated with a cuffed endotracheal tube and placed inside the HyperTherm GEN3 device. Mechanical ventilation with isoflurane in O2-air mixture was started and maintained during anesthesia. In addition to the requirement dictated by the need for intubation/ventilation of the animal/patient throughout the ElmediX WBTT procedure, by affecting the central system-driven thermoregulation, complete anesthesia may facilitate the body temperature increase during WBTT sessions, and therefore is currently preferred over sedation [Citation41]. Once the anesthesia was well established, animals were positioned in the device and all monitoring equipment (pulse oximeter, electrocardiogram (ECG), permanent catheter for venous blood sampling) and sensors were introduced. Adapted fluid therapy, supportive medication, and pain management were provided to each specific animal. Heart rate, blood oxygen saturation and ECG were monitored non-invasively. Blood pressure was monitored through a carotid arterial catheter. After completion of the WBTT session, animals were awakened and kept under close clinical observation in the veterinary facility for 2–3 weeks.
Hematology, biochemistry, coagulation, urinalysis, and organ functions
Hematology, blood chemistry, coagulation and urine tests were performed at a specialized veterinary laboratory (MedVet, Antwerp, Belgium). Blood for hematology, biochemistry and coagulation was collected via the jugular vein before initiation of each WBTT session, and during and after treatment. The evaluation of organ functions was performed by testing specific parameters within the blood obtained before, during and after WBTT. For the liver, pancreas, muscle and kidney function, levels of alanine aminotransferase (AST), aspartate aminotransferase (ALT); amylase and lipase; creatinine kinase (CK), lactate and troponin; and creatinine were analyzed, respectively.
Necropsy and tissue handling
Two to three weeks after the (last) WBTT session, animals were euthanized, and a necropsy was performed to evaluate any gross anatomical and/or histological changes induced by the WBTT procedure that can be biologically relevant. Animals were deeply sedated using a combination of tiletamine-zolazepam, maintained with isoflurane, followed by slow intravenous injection of T-61 (0.1 ml/kg). All tested animals were subjected to a detailed necropsy: kidney, muscle, heart, brain and liver were examined for grossly visible lesions. All relevant organs were weighed, and tissue samples were taken and fixed. For histology, tissue samples were dehydrated, embedded in paraffin wax, and sectioned at a nominal 4- to 5-µM thickness. Sections were stained with hematoxylin and eosin.
Results
Whole-body thermal treatment device (HyperTherm GEN3) performance
The accurate recordings from the fiber optic sensors and the thermal controller, enabled a precise and responsive regulation of the animal body temperature. As indicated in , a good correlation between the liver-inserted fiber optic sensors and rectal temperature was observed in all animals with low temperature variations. Also, a lack of significant variations above the desired treatment temperature (41.5 °C) was observed. As expected, temperatures measured in the liver were higher (or equal) than all other tested organs preventing overheating of key organs and confirming the safety of liver temperature controlled WBTT.
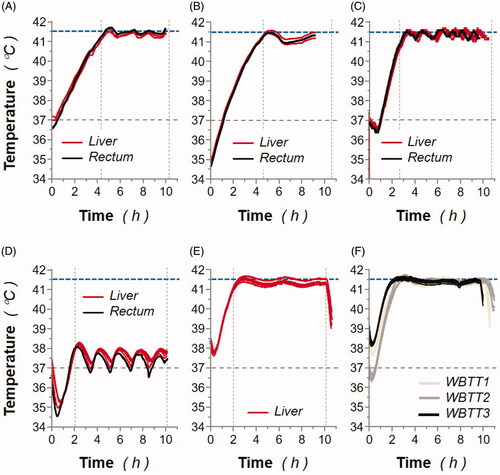
Figure 3. Animal body temperatures monitored during whole-body thermal treatment (WBTT) or normothermia procedures. (A) MP1: individual liver (red lines) and rectal (black line) sensor-monitored animal temperatures were recorded during the WBTT procedure (41.5 °C for 6 h). (B) MP2: individual liver (red lines) and rectal (black line) sensor-monitored animal temperatures were recorded during the WBTT procedure (41.5 °C for 6 h). (C) MP3: individual liver (red lines) and rectal (black line) sensor-monitored animal temperatures were recorded during the WBTT procedure (41.5 °C for 8 h). (D) MP4: individual liver (red lines) and rectal (black line) sensor-monitored animal temperatures were recorded during the whole-body normothermia procedure (37 °C for 8 h). (E) MP5: individual liver (red lines) sensor-monitored animal temperatures were recorded during the first WBTT session (41.5 °C for 8 h). (F) MP5: individual liver sensor-monitored animal temperatures were recorded during either the first (light gray lines), second (dark gray lines), or third (black lines) WBTT session (41.5 °C for 8 h).
Survival, clinical observations, and urinalysis
All animals remained overall in good health throughout the study, and there was no treatment-related death. Bodyweight gain was constant between animals. The animal details and treatment schemes are depicted in . Evolution of the respiratory parameters (summary of respiratory rate, tidal volume, O2) was followed continuously, while blood gases were determined in arterial blood during treatment every 2.5 h. In all animals (including normothermia at 37 °C: MP4), a pronounced increase in pO2 was observed during treatments, due to the oxygenation associated with the anesthesia procedure (duration of anesthesia up to 15 h) (). During WBTT procedures, an increase in cell metabolism was observed, causing a higher production of CO2 and an increase of pCO2 in the blood. When this process started, the accumulation of CO2 and acidosis of the body was prevented by increasing the respiratory rate in order to evacuate the CO2 and maintain homeostasis. In addition, the only change observed in the urinalysis was the detection of bilirubin in the first 350 ml of MP3 (beginning of WBTT treatment), and also in the first cycle of MP4 (last hours of treatment), which is probably related to urinary catheterization during the procedure (data not shown).
Table 2. Summary of arterial blood gas analysis in female Aachen minipigs submitted to whole-body thermal treatment or normothermia sessions (s).
Hematology parameters
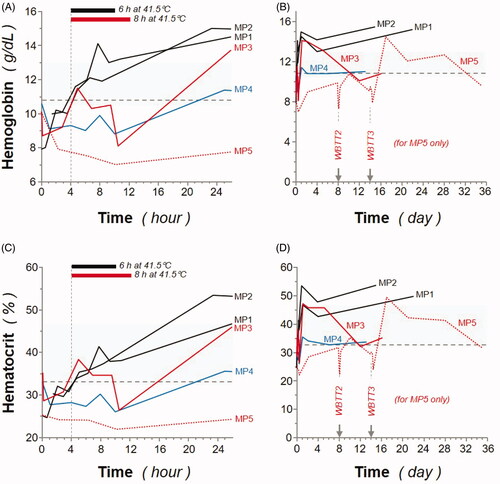
Hemoglobin concentration and hematocrit remained within, or near, the normal range during and after WBTT procedures, and no significant difference was observed between normothermia (37 °C) and TT (41.5 °C), and between 1 or 3 WBTT session(s) (). The observed increase of both hemoglobin and hematocrit could be attributed to blood thickening due to WBTT procedure. All blood cells were evaluated and remained within normal range (data summarized in ). Slight decreases during WBTT procedures in lymphocytes and monocytes were observed (data not shown), but not considered clinically relevant.
Figure 4. Effect of whole-body thermal treatment (WBTT) procedure(s) on animal hemoglobin levels and hematocrit. Hemoglobin levels (A, B; expressed as g/dL) and hematocrit (C, D; expressed as %) were determined during the first 26 h (A, C) and throughout the entire study, up to 36 days (B, D), for MP1 and MP2 (black lines), MP3 (red line), MP4 (blue line), and MP5 (dash red line). Basal values for MP4 are indicated (blue dash line), and light gray areas represent reference values for female Aachen minipigs31.
Coagulation properties
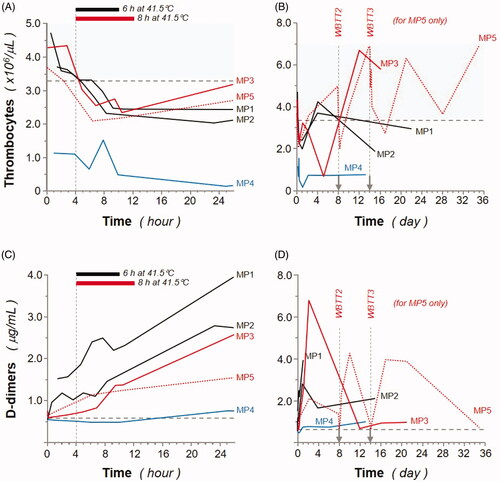
As illustrated in , levels of thrombocytes dropped after WBTT procedures but remained stable and within the normal range after some days, for most of the minipigs. Together with the observed parallel rise of D-dimer (also known as fragment D-dimer or fibrin degradation fragment) concentration, this could be the result of disseminated coagulation. D-dimer is one of the protein fragments produced when a blood clot gets dissolved in the body. Therefore, D-dimer testing is classically performed to help rule out clotting (thrombotic) episodes and to help diagnose conditions related to thrombosis [Citation42,Citation43]. However, there was no clear sign of clotting in any histologically investigated organ, and all functions and parameters (including thrombocyte levels) recovered quickly after the experiment. In MP3 and more pronounced in MP2, the drop-in thrombocyte counts already started during the warmup phase. The mirror image is consistently observed in the D-dimer concentration changes, which increase from the start of the experiment, and then back to baseline after ∼1 week. Potentially the thrombocyte count and D-dimer concentration changes are the results of intravascular physical intervention (i.e. animal handling operations and surgical placement of catheters and temperature sensors) potentially enhanced by thermal treatment, as the changes observed in MP4 (normothermia, 37 °C for 8 h) are less pronounced on these parameters.
Figure 5. Effect of whole-body thermal treatment (WBTT) procedure(s) on animal thrombocyte and D-dimer levels. Thrombocyte (A, B; expressed as million/μL) and D-dimer (C, D; expressed as μg/mL) levels were determined during the first 26 h (A, C) and throughout the entire study, up to 36 days (B, D), for MP1 and MP2 (black lines), MP3 (red line), MP4 (blue line), and MP5 (dash red line). Basal values for MP4 are indicated (blue dash line), and light gray areas represent reference values for female Aachen minipigs31.
Organ-specific functions
Liver function tests, also known as liver chemistry, help determine the health of the liver by measuring the blood levels of liver enzymes (amongst others). Detection of liver enzymes in the blood is often part of an initial screening for liver disease[Citation44], in addition the surgical transient implantation of liver temperature sensors pushed us to carefully monitor liver functions. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are both normally found inside liver cells. However, when the liver is damaged or inflamed, ALT/AST can be released into the bloodstream, causing serum levels to rise. The liver function test demonstrated a delayed and limited increase (up to 3-fold) in both AST and ALT serum levels suggesting transient and mild stress liver response to WBTT ().
Figure 6. Effect of whole-body thermal treatment (WBTT) procedure(s) on animal liver functions. Aspartate aminotransferase (A; AST) and alanine aminotransferase (B; ALT) levels, expressed as U/L, were determined at the indicated times for MP1 and MP2 (black lines), MP3 (red line), MP4 (blue line), and MP5 (dash red line). Basal values for MP4 are indicated (blue dash line), and light gray areas represent reference values for female Aachen minipigs46. The thick dotted black lines illustrate the 5-fold basal levels threshold representing the ‘clinically relevant’ levels above which a sustained observed liver enzyme levels for both enzymes would require medical intervention.
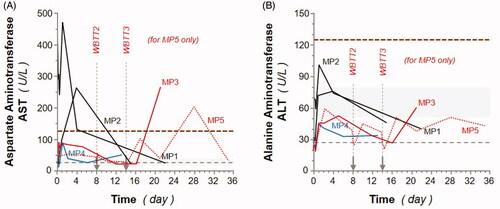
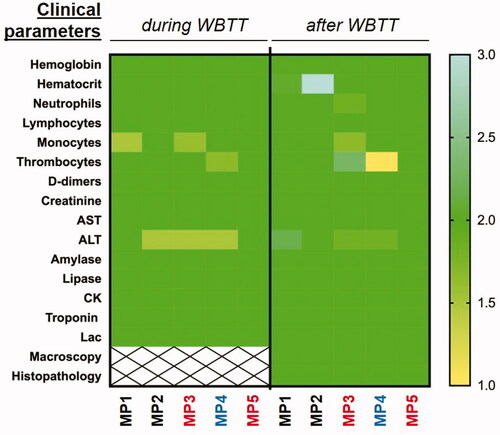
All the other organ-specific function analysis is summarized in . Muscle damage was evaluated by detection of blood creatinine kinase (CK), troponin and lactic acid levels. More specifically, kidney function was assessed by detection of blood creatinine concentration. Creatinine is considered a waste product, produced by muscles from the breakdown of a compound called creatine. Creatinine is removed from the body by the functional kidneys, which filter almost all of it from the blood and release it into the urine. Measuring blood creatinine levels then represent a relevant clinical indicator of kidney function [Citation45]. Creatinine blood levels increased during the WBTT session(s) but returned to baseline values shortly after and remained stable afterwards. The rise of the creatinine could be the result of transient blood thickening due to the WBTT procedure. Transient elevation of troponin and lactic acid was observed in minipigs in response to TT (as measured 24 h post-treatment) before rapidly dropping back to baseline levels. Otherwise, all levels remained within normal range (). The pancreas function test showed that the amylase and lipase blood levels remained normal during and after WBTT procedure.
Figure 7. Summary of clinical safety monitoring. All clinical safety observations are summarized for all animals in a heatmap-like format. Briefly, values within normal range (reference values46) were defined as 2, above normal range were defined as how many times the parameters were out of the normal range, and the media established between 2-3, and below normal range was also established between 2-1. AST: aspartate aminotransferase; ALT: alanine aminotransferase; CK: creatin kinase; Lac: lactic acid.
Macroscopic and microscopic observations
Macroscopic and microscopic analysis were performed on the kidney, muscle, heart, brain and liver. No significant changes were observed in all 5 minipigs () (data not shown). No clear significant histopathological changes were present which could be related to the TT (data not shown).
Discussion
In the development process for a WBTT treatment device, the 93/42/EEC medical device directive prescribes safety tests as a first mandatory step in the development of a medical device.Citation39 Herein, we introduced and validated an experimental setup and procedure which enables long-term WBTT in large animals and human beings; including a full anesthesia protocol also to be implemented in future clinical applications in patients. The vital organs, body fluids, vital signs and the well-being of the animal during and after treatment were analyzed. To accomplish a clinically feasible treatment, different schemes of WBTT were tested in a swine model. In total, 4 minipigs were subjected to WBTT (41.5 °C) procedures, and 1 minipig (MP4) was subjected to a ‘sham’ treatment (normothermia at 37 °C, but with all other procedures identical, including full anesthesia).
Current clinically used hyperthermic treatment protocols involve short term (2–4 h), locally applied, moderately to more pronounced elevated temperatures (40–43 °C) or WBTT at moderate temperature (≤40 °C). Artificial heating of the entire body above 41.8 °C is linked to the risk of development of serious adverse events such as thermal shock, brain edema, hepato-renal syndrome, and disseminated intravascular coagulation [Citation29]. Despite this, a large body of evidence in the literature documents the lethal effect of heat on cancer cells [Citation46], at temperatures above 41 °C [Citation47–49]. In addition to the results in cell-based assays, and linked to the likelihood for enhanced anti-tumor specific activation of immune cells, and other prolonged metabolic changes in the tumor (e.g., enhanced oxygenation and perfusion, acidosis, acidosis and nutrient depletion) [Citation50–53], and postulate that high temperatures can damage and kill cancer cells, usually with minimal injury to normal tissues [Citation17,Citation54,Citation55], clinical effectiveness of WBTT in eradicating tumors have been reported [Citation56]. These results strengthened the rationale to establish a long term WBTT protocol up to 8 h of duration and at 41.5 °C, proven to be the most lethal operating temperature without significantly altering the conditions of the healthy cell population.
Regarding the ElmediX device performance (HyperTherm GEN3), optical fiber temperature catheters were employed, and demonstrated consistent monitoring of different body structure temperatures over an extended period of time. Optical fiber sensors, as a result of their unique properties (small dimensions, capability of multiplexing, chemical inertness, and immunity to electromagnetic fields) have found a wide application, ranging from structural health monitoring to biomedical and point-of-care instrumentation. These sensors usually have good linearity, rapid response for real-time monitoring, and high sensitivity to small temperature variations [Citation57]. Herein, the temperature signals recorded displayed similar trends as standard veterinary equipment (such as the temperature sensors commonly used by Medanex), while providing higher accuracy.
However, different sensors displayed different time constants depending on their anatomical locations. Muscle, blood and esophageal sensors displayed the largest temperature swings (), whereas liver and rectal probes were the most stable during treatment (), and it was clearly demonstrated that the rectal temperature better predicts body temperatures than sensors inserted in the esophagus, or in large muscles. However, the direct measurement of liver temperature was still preferred over rectal measurement for body temperature control, because of the high dependence of the latter on probe positioning. Superficial sensors inserted e.g., subcutaneously or attached to the skin of the animal were primarily influenced when the temperature of the treatment chamber is lower than the body. This observation suggests that the methodology used in this study may have some limitations in the treatment of diseases affecting superficial organs (e.g., superficial tumor lesions), which most likely will then require special local heating patches ensuring accurate desired treatment temperature in these targeted areas. In conclusion, the uniform achieved temperatures obtained with the device demonstrated the capability of maintaining well-controlled temperatures during WBTT procedure, preventing any disturbing levels which could result in serious adverse events.
To evaluate the clinical safety of the WBTT procedure, different parameters were analyzed. The choice of the anesthetic breathing system is critically important, which will allow tested animals to breathe spontaneously throughout a prolonged period of time. Controlled ventilation is also necessary to prevent the animals from developing respiratory acidosis. When pCO2 in the blood increases, the respiratory rate should be increased to evacuate the CO2 and prevent acidosis [Citation58,Citation59]. Herein, although an increase in pO2 was observed, due to the oxygenation associated with the anesthesia procedure (), no acidosis occurred.
Regarding the hemoglobin and hematocrit testing, levels remained within, or near, the normal range during and after treatment, and no significant difference was observed between normothermia (37 °C) and treated animals (). Only a slight increase was observed of both hemoglobin and hematocrit can be attributed to blood thickening due to WBTT procedure. Therefore, adequate fluid balance and hyperhydration (+70% up to 150% of calculated basal need) are essential. It has been already established that fluid balance is important in prolonged anesthesia [Citation60].
Coagulation properties were assessed by thrombocyte count and measurement of D-dimer concentration (). Thrombocyte levels during treatment dropped slowly to acceptable levels, and together with the rise of D-dimer observed, this could be a result of mild disseminated intravascular coagulation (DIC) without clinical repercussions. DIC is a syndrome characterized by a systemic activation of coagulation [Citation42]. To confirm the setting of this condition, the activation of hemostasis can be determined by different parameters, such as D-dimers and thrombocyte count, as well as organ dysfunction, platelet exhaustion and tissue hemorrhage. None of the last parameter changes were observed during the follow up, as well as no tissue hemorrhage in histological analysis was seen (data not shown).
Liver function was analyzed by determination of AST and ALT levels (). There was a delayed limited increase in both ALT and AST serum levels suggesting a mild and transient stress liver response to WBTT. Considering the low extent of increase of the liver enzyme, these observations were not considered clinically relevant [Citation43]. No specific reason could be found to explain the abnormal higher levels of both ALT and AST in the blood of MP1 (including before WBTT), and the decrease of these blood levels during WBTT.
In summary, during the WBTT sessions and the follow-up monitoring, minor clinical parameter changes were detected, but none of them were clinically relevant (). These changes were limited and reversible, and the animals were perfectly healthy throughout the whole procedures and follow-up until euthanasia. In addition, the tissue histopathological analysis performed on several key organs showed no WBTT-related changes. Therefore, it is concluded that under the described experimental conditions and study design, WBTT (at 41.5 °C) using the HyperTherm GEN3 is well tolerated and safe in female Aachen minipigs. The uniform temperature distribution obtained in this procedure is probably due to the combination of specific heating techniques, the cautious heating slope, and the anesthesia protocol. Additional support for the safety, tolerability, and early clinical signs of therapeutic efficacy of ElmediX WBTT have been already successfully documented in cancer-bearing dogs (ElmediX manuscript in preparation).
Taken together, these data strongly contribute and support the clinical use of the ElmediX WBTT protocol and device as a safe personalized precision medicine, enabling its translation into human beings suffering from life-threatening diseases (e.g., advanced pancreatic cancer patients).
Acknowledgements
The authors sincerely thank Bram Nachtergaele and Hadewych Van Hauwermeiren from Medanex clinic (Diest, Belgium); and Norbert Stockhofe and Sandra Vreman from Wageningen University for their valuable expertise and assistance throughout the experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat HHS Public Access. Nat Rev Immunol. 2015;15(6):335–349.
- Hurwitz MD. Hyperthermia and immunotherapy: clinical opportunities. Int J Hyperthermia. 2019;36(sup1):4–9.
- Nauts HC. Bacterial vaccine therapy of cancer. Dev Biol Stand [Internet]. 1977;38:487–494.
- Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–564.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperth. 2014;30(8):531–539.
- Bettaieb A, Wrzal PK, Averill-Bates DA. Hyperthermia: cancer treatment and beyond. cancer treat - Conv Innov approaches. InTech; 2013. DOI:https://doi.org/10.5772/55795
- Behrouzkia Z, Joveini Z, Keshavarzi B, et al. Hyperthermia: how can it be used? Oman Med J. 2016;31(2):89–97.
- Atmaca A, Al-Batran SE, Neumann A, et al. Whole-body hyperthermia (WBH) in combination with carboplatin in patients with recurrent ovarian cancer - a phase II study. Gynecol Oncol. 2009;112(2):384–388.
- Atanackovic D, Pollok K, Faltz C, et al. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol - Regul Integr Comp Physiol. 2006;290:585–594.
- Cihoric N, Tsikkinis A, Van Rhoon G, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia. 2015;31(6):609–614.
- Mallory M, Gogineni E, Jones GC, et al. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit Rev Oncol Hematol. 2016;97:56–64.
- Shen RN, Hornback NB, Shidnia H, et al. Whole-body hyperthermia decreases lung metastases in lung tumor-bearing mice, possibly via a mechanism involving natural killer cells. J Clin Immunol. 1987;7(3):246–253.
- Zhao C, Dai C, Chen X. Whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy for the treatment of stage IV advanced gastric cancer. Int J Hyperth. 2012;28(8):735–741.
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56.
- Hildebrandt B, Hegewisch-Becker S, Kerner T, et al.; German Interdisciplinary Working Group on Hyperthermia. Current status of radiant whole-body hyperthermia at temperatures >41.5 degrees C and practical guidelines for the treatment of adults. The German 'Interdisciplinary Working Group on Hyperthermia'. Int J Hyperthermia. 2005;21(2):169–183.
- Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41(6):714–729.
- van der Zee J. Heating the patient: A promising approach? Ann Oncol. 2002;13(8):1173–1184.
- Datta NR, Kok HP, Crezee H, et al. Integrating loco-regional hyperthermia into the current oncology practice: SWOT and TOWS analyses. Front Oncol. 2020;10:819. Available from: www.frontiersin.org.
- Werthmöller N, Frey B, Rückert M, et al. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperthermia. 2016;32(1):23–30.
- Multhoff G, Habl G, Combs SE. Rationale of hyperthermia for radio(chemo)therapy and immune responses in patients with bladder cancer: Biological concepts, clinical data, interdisciplinary treatment decisions and biological tumour imaging. Int J Hyperthermia. 2016;32(4):455–463.
- Oei AL, Kok HP, Oei SB, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020;163–164:84–97.
- Issels RD, Lindner LH, Verweij J, et al .; European Organization for the Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group and the European Society for Hyperthermic Oncology. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial . JAMA Oncol. 2018;4(4):483–492.
- Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240.
- Roussakow S. The history of hyperthermia rise and decline. Int Clin Hyperth. 2013;2013:428027. Available from: https://www.hindawi.com/journals/cpis/2013/428027/.
- van der Zee J, Vujaskovic Z, Kondo M, et al. The Kadota Fund International Forum 2004-clinical group consensus. Int J Hyperthermia. 2008;24(2):111–122.
- Hegewisch-Becker S, Gruber Y, Corovic A, et al. Whole-body hyperthermia (41.8 degrees C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: a phase II study . Ann Oncol. 2002;13(8):1197–1204.
- Hegewisch-Becker S, Braun K, Otte M, et al. Effects of whole body hyperthermia (41.8 °C) on the frequency of tumor cells in the peripheral blood of patients with advanced malignancies. Clin Cancer Res. 2003;9:2079–2084.
- Cao YL, Hu XH, Lu YK, et al. Clinical study of chemotherapy combined with whole body microwave hyperthermia in treatment of advanced non-small cell lung cancer. Chinese J. cancer Prev. Treat. 2008;15:927–929.
- Suvernev A, Ivanov G, Efremov A. et al. whole body hyperthermia at 43. 5-44° C : dreams or reality? necessity of high-level whole body hyperthermia what are possible ways to suppress the activity of trypsin during hyperthermia? Madame Curie Biosci. Database. 2013:6310. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6310/.
- Colleton C, Brewster D, Chester A, et al. The use of minipigs for preclinical safety assessment by the pharmaceutical industry: results of an IQ DruSafe minipig survey. Toxicol Pathol. 2016;44(3):458–466.
- Pawlowsky K, Ernst L, Steitz J, et al. The aachen minipig: phenotype, genotype, hematological and biochemical characterization, and comparison to the göttingen minipig. Eur Surg Res. 2017;58(5-6):193–203.
- Watson AL, Carlson DF, Largaespada DA, et al. Engineered swine models of cancer. Front Genet 2016;7:78. Available from: www.frontiersin.org.
- Bode G, Clausing P, Gervais F, et al.; Steering Group of the RETHINK Project. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62(3):196–220.
- Heining P, Ruysschaert T. The use of minipig in drug discovery and development. Toxicol Pathol. 2016;44(3):467–473.
- Van Der Laan JW, Brightwell J, Mcanulty P, et al. Regulatory acceptability of the minipig in the development of pharmaceuticals, chemicals and other products. 2010. [cited 2020 Jul 24]; Available from: http://www.ema.europa.eu.
- Gesson-Paute A, Ferron G, Thomas F, et al. Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): An experimental study. Ann Surg Oncol. 2008;15(1):339–344.
- Facy O, Al Samman S, Magnin G, et al. High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: An experimental study. Ann Surg. 2012;256(6):1084–1088.
- Ferron G, Gesson-Paute A, Classe JM, et al. Feasibility of laparoscopic peritonectomy followed by intra-peritoneal chemohyperthermia: An experimental study. Gynecol Oncol. 2005;99(2):358–361.
- Sebeke LC, Rademann P, Maul AC, et al. Feasibility study of MR-guided pancreas ablation using high-intensity focused ultrasound in a healthy swine model. Int J Hyperthermia. 2020;37(1):786–798.
- Plotzki E, Heinrichs G, Kubícková B, et al. Microbiological characterization of a newly established pig breed, Aachen Minipigs. Xenotransplantation. 2016;23(2):159–167.
- Díaz M, Becker DE. Thermoregulation: physiological and clinical considerations during sedation and general anesthesia. Anesth Prog. 2010;57(1):25–33.
- Taylor F, Toh C-H, Hoots K, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(11):1327–1330.
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood Am Soc Hematol. 2009;113:2878–2887.
- Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–379.
- Gounden V, Bhatt H, Jialal I. Renal Function Tests [Internet]. StatPearls. StatPearls Publishing; 2020. [cited 2020 Jul 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29939598.
- Kalamida D, Karagounis IV, Mitrakas A, et al. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLoS One. 2015;10(1):e0116021–12.
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. Lancet Publishing Group. 2002;3(8):487–497.
- Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells. Biochem Clin Stud. 1967;20:1351–1381.
- Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia. 2011;27(4):320–343.
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1(4):210–216.
- Hegyi G, Szigeti GP, Szász A. Hyperthermia versus oncothermia: Cellular effects in complementary cancer therapy. Evidence-Based Complement. Altern. Med. 2013;2013:1–12.
- van den Tempel N, Horsman MR, Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. Taylor and Francis Ltd. 2016;32(4):446–454.
- Oei AL, Vriend LEM, Krawczyk PM, et al. Targeting therapy-resistant cancer stem cells by hyperthermia. Int. J. Hyperth. Taylor and Francis. 2017;33(4):419–427.
- Storm FK, Harrison WH, Elliott RS, et al. Normal tissue and solid tumor effects of hyperthermia in animal models and clinical trials. Cancer Res. 1979;39(6 Pt 2):2245–2251.
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperth. 2006;22(3):197–203.
- van Rhoon GC, Franckena M, ten Hagen TLM. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv Drug Deliv Rev. 2020;163-164:145–156.
- Madrahimov N, Natanov R, Khalikov A, et al. Warming and cooling device using thermoelectric Peltier elements tested on male mice. Lab Anim. 2020;54(5):443–451.
- Ragan HA, Gillis MF. Restraint, venipuncture, endotracheal intubation, and anesthesia of miniature swine. Lab Anim Sci. 1975;25(4):409–419.
- Becker M. Anesthesia in Göttingen miniature swine used for experimental surgery - PubMed. Lab Anim Sci. 1986;36:417–419.
- Clutton RE, Reed F, Eddleston M, et al. Prolonged anaesthesia in minipigs. Prolong Anaesth Minipigs. 2013:11–15.
