Abstract
Introduction
The combination of hyperthermia with low LET (linear energy transfer) radiation may have similar anti-tumor effects as high LET radiation alone. This pre-clinical study determined the optimal heating temperature and time interval between radiation and heat to achieve this equivalent effect.
Methods
C3H mammary carcinomas (200 mm3 in size) growing in the right rear foot of CDF1 mice was used in all experiments. Tumors were locally irradiated with graded doses of either 240 kV ortho- or 6 MV mega-voltage X-rays to produce full dose-response curves. Heating (41.0–43.5 °C; 60 min) was achieved by immersing the tumor bearing foot in a water-bath applied at the same time, or up to 4-hours after, irradiating. The endpoint was the percentage of mice showing local tumor control at 90 days, with enhancements calculated from the ratios of the radiation doses causing 50% tumor control (± 95% confidence intervals).
Results
Previous published results in this tumor model reported that carbon ions were 1.3–1.7 times more effective than low LET radiation at inducing tumor control. Similar enhancements occurred with a temperature of only 41.0 °C with a simultaneous heat and radiation treatment. However, higher temperatures were needed with the introduction of any interval; at 42.5 °C, the enhancement was 2.5 with a simultaneous treatment, decreasing to a value within the carbon ion range with a 4-hour interval.
Conclusions
Combining hyperthermia with low LET radiation can be as effective as high LET at inducing tumor control, but the temperature needed depended on the time interval between the two modalities.
Introduction
Radiation at low LET (linear energy transfer) is an effective cancer therapy whether applied in a conventional fractionated schedule or hypofractionated [Citation1,Citation2]. However, its success is limited by the presence of regions of low oxygenation (hypoxia) within tumors [Citation3,Citation4]. The growth and development of tumors require they have an adequate supply of oxygen and nutrients [Citation5,Citation6]. This originally comes from the vascular supply of the host tissue in which the tumor arises. However, due to the rapid growth of the tumor mass the tumor outgrows this supply and must then form its own vascular supply. It has been proposed that there are three patterns of tumor derived blood vessels [Citation7]. These are vascular mimicry in which tumor cells organize themselves in three-dimensional channel-like structures [Citation8]; mosaic vessels whereby both endothelial cells and tumor cells form the luminal surface [Citation9]; and the more typical endothelial-dependent vessels [Citation10]. Regardless of the vascular pattern, the system that develops is primitive and chaotic, and is unable to meet the oxygen demands of the growing tumor mass [Citation5,Citation6] thus hypoxia develops. This is a universal finding, making hypoxia a characteristic feature of virtually all animal [Citation11] and human [Citation5] solid tumors. Although hypoxia has a negative impact on low LET radiation, it is much less of a problem with high LET radiation [Citation12,Citation13]. Unfortunately, there are currently only 13 facilities world-wide capable of treating cancer patients with high LET and almost 70% of these are located in Asia [Citation14].
Since the 1970s, numerous hypoxia targeted approaches have been combined with low LET radiation to overcome the radiation resistance [Citation3,Citation4]. One of the most effective treatments is hyperthermia using temperatures of up to 43 °C for around 60 min [Citation15,Citation16]. In 1974, it was even suggested that combining hyperthermia with low LET radiation could actually be as effective as high LET radiation alone in reducing hypoxia induced radiation resistance [Citation17]. While this effect was demonstrated in vitro, the concept was never developed [Citation17,Citation18]. Yet, now clinical trials have been initiated in which this suggestion has been adopted, albeit with hyperthermia and protons [Citation19]. What is not known, is the heating temperature or the time interval between the radiation and heat necessary for the enhancement of low LET radiation to induce an equivalent degree of local tumor control as observed with high LET radiation alone. This was the issue investigated in our well established C3H mammary carcinoma murine model, one of the few pre-clinical models that allows for clinically relevant local tumor control assessment [Citation20,Citation21]. It is also one of the tumor models in which pre-clinical studies [Citation20,Citation21] played a major role in establishing clinical trials with hypoxic modifiers [Citation22–24].
Materials and methods
Animal and tumor model
C3H mammary carcinomas, subcutaneously implanted in the right rear foot of 10–14 week-old male or female CDF1 mice were used for all experiments. Details of the derivation and maintenance of this model has been described previously [Citation25]. Basically, every three months tumor material stored in liquid nitrogen was thawed and implanted on the flanks of mice. When large, these tumors were excised and minced with a pair of scissors under sterile conditions. Tumor material could then either be implanted on the flanks of additional mice for continued passage or 5–10 µl of this material injected into the right rear foot of mice used for experimental studies. Experiments were initiated when tumors had reached approximately 200 mm3 in size. This volume was achieved about three weeks after inoculation and was calculated from the formula D1 × D2 × D3 × π/6, where the D values represent the three orthogonal diameters. Attempts were made to randomize the tumor bearing mice into the different radiation-dose groups. However, since the tumors grew at different rates they did not achieve the 200 mm3 starting volume at the same time. Consequently, some selection was necessary to ensure that tumors starting treatment on the same day were distributed among the different treatment groups. All animal studies were conducted according to the animal welfare policy of Aarhus University (http://dyrefaciliteter.au.dk), and with the Danish Animal Experiments Inspectorate’s approval.
Tumor irradiation
Single dose tumor irradiations were given as described previously [Citation20,Citation21]. Initially the radiation source was a conventional therapeutic Philips X-ray machine (240 kV ortho-voltage X-rays, 10 mA, dose rate of 2.3 Gy/min). With this apparatus, the radiation dose was determined using an integrating chamber. This involved using a modified mouse restraining jig in which an ionizing chamber could be held in exactly the same position as the mouse foot tumor as described later in this section. However, during this study, this old X-ray tube broke and since it could not be replaced, additional studies were performed using a clinical Linear Accelerator (Varian Clinac iX) with 6 MV mage-voltage X-rays (dose rate of 6 Gy/min). The Linac dose output was isocentrically calibrated in 5 cm depth according to IAEA TRS398 (Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry based on Standards of Absorbed Dose to Water, IAEA TRS-398) and is stable to within ± 1%. Doses to the mouse legs were determined by use of Varian Eclipse Treatment Planning System (AAA-algorithm) from CT-scan of the mouse/water tank. Dose variation caused by uncertainty in mouse leg position laterally and in-depth was approx. ± 3%. All irradiations to the tumor-bearing feet were given locally to the tumors of non-anesthetized mice, which were restrained in specially constructed Lucite jigs; the tumor-bearing legs being exposed and loosely attached to the jig with tape, without impairing the blood supply to the foot [Citation21]. To secure homogeneity of the radiation dose, the tumors were immersed in a circulating water bath (type TE 623; Heto, Birkerød, Denmark) set at 25 °C with about 5 cm of water between the X-ray source and the tumor. The water-bath was covered with a Lucite plate with holes allowing immersion of the foot approximately 1 cm below the water surface. In order to irradiate only the tumors, the remainder of the mouse was shielded by 1 cm of lead.
Hyperthermia treatments
Non-anesthetised mice, restrained in Lucite jigs, had their tumor bearing legs fixed as described above. Hyperthermia treatments (41.0–43.5 °C for 60 min) were achieved by immersing the tumor bearing leg in the circulating water-bath as described for the radiation treatments. Previous measurements of intra-tumor temperature have shown stabilization within 1 to 2 min to approximately 0.2 °C below the water-bath temperature [Citation26]. The temperature of the water-bath was therefore, adjusted to 0.2 °C above the desired tumor temperature. All temperature measurements were calibrated against a certified mercury thermometer. Tumors were either treated simultaneously with heat and radiation, the radiation being applied in the middle of the 1-h heating period, or the heating initiated up to 4-h after irradiating.
Response endpoint
Following irradiation, the animals were returned to their cages, observed on a weekly basis, and the percentage of animals in each treatment group showing local tumor control at 90 days, determined. For radiation alone, and each radiation and heat combination, full radiation dose-response curves were produced. From these curves, logit analysis allowed us to calculate the TCD50 dose (the radiation dose necessary to induce a response in 50% of animals), with 95% confidence intervals. The ratio of these values obtained for radiation alone and radiation with hyperthermia were used to calculate enhancement ratios (± 95% confidence intervals). Statistical comparison of the different treatments was achieved by converting the 95% confidence intervals into standard error values and then performing a t-test, with a significance level of p < .05.
Results
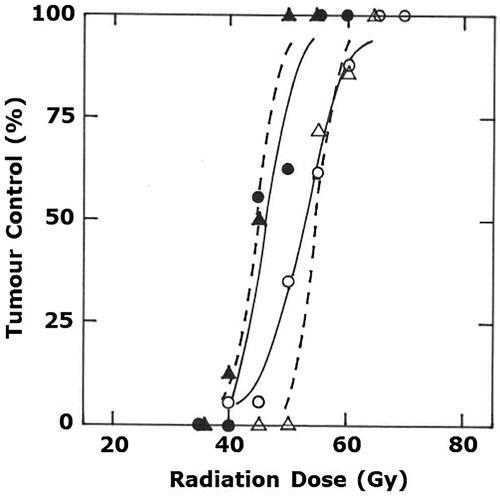
Logit analysis of the radiation dose-response curve obtained when irradiating C3H mammary carcinomas with low LET 240 kV ortho-voltage X-rays resulted in a TCD50 value (± 95% confidence intervals) 53 Gy (51–55) as shown in . When using 6 MV mage-voltage X-rays, the TCD50 value was slightly, yet non-significantly, higher at 55 Gy (52–58). There was a suggestion that the slope for radiation alone curve obtained with the Philips X-ray source was different from that found with the clinical Linac machine. However, this might have been an artifact due to the small number of animals in each group, since our previous studies where we had more comprehensive data [Citation27,Citation28] showed that the Philips X-ray radiation only curve actually lay exactly in the same position as the current Linac radiation curve. For both approaches the combination of radiation followed 1-h later by a mild heat temperature of 41.5 °C for 60 min resulted in a significant reduction of the TCD50 values to 46 Gy (44–49) and 44 Gy (40–49) for 240 kV and 6 MV X-rays, respectively. Both these new TCD50 values were again not significantly different ().
Figure 1. Radiation dose-response curves for C3H mammary carcinomas treated with either radiation alone (○, Δ) or radiation followed one hour later by heating at 41.5 °C for 60 min (●, ▲). The radiation treatment was administered using either an experimental Philips X-ray tube (○, ●) or a clinical Varian Clinac iX Linear Accelerator (Δ, ▲). Results show percentage of animals with local tumor control at 90 days after treating and are based on a minimum of 7 mice/group. Lines through the data were fitted following logit analysis.
Similar full radiation-dose response curves were produced for a range of temperatures between 41.0 °C and 43.5 °C, with the radiation administered in the middle of the heating period, or the heating applied at various times up to 4-h after irradiating. From the estimated TCD50 values we were able to calculate enhancement ratios (with 95% confidence intervals) by comparing the TCD50 values for radiation alone to those for radiation with hyperthermia and the results are summarized in and . Also shown in those figures is the 95% confidence intervals for the enhancement ratio obtained with high LET carbon ions, when compared to X-rays. When heat and radiation are applied simultaneously, the 1.3–1.7 enhancement ratio found for carbon ions is achieved even with a low temperature of 41.0 °C (). As the temperature increases, so too does the enhancement ratio, reaching a value of around 5 with the highest temperature of 43.5 °C. A temperature dependent increase in the enhancement ratio is also seen when the radiation and heat are separated by a 4-h interval (). However, the curve is less steep and the 1.3–1.7 enhancement ratio for carbon ions is only achieved when a temperature of 42.5 °C is reached.
Figure 2. The effect of different heat temperatures on the radiation response of C3H mammary carcinomas. Data similar to that shown in was produced for all temperatures shown and from the resulting radiation dose-response curves the TCD50 (radiation dose that causes 50% tumor control) values were calculated. The symbols represent the enhancement ratios (ratio of the TCD50 value for radiation alone compared to radiation and heat) with 95% confidence intervals and are for radiation applied in the middle of the heating period (●) or when heat was given 4-h after irradiating (○) and includes both previously published data [Citation21, Citation25, Citation27, Citation28] and more recent unpublished results. Shaded area shows the 95% confidence interval for carbon ion irradiated tumors, taken from [Citation29]. *Indicates the heat and radiation values that were significantly different from the carbon ion value (t-test; p < .05).
![Figure 2. The effect of different heat temperatures on the radiation response of C3H mammary carcinomas. Data similar to that shown in Figure 1 was produced for all temperatures shown and from the resulting radiation dose-response curves the TCD50 (radiation dose that causes 50% tumor control) values were calculated. The symbols represent the enhancement ratios (ratio of the TCD50 value for radiation alone compared to radiation and heat) with 95% confidence intervals and are for radiation applied in the middle of the heating period (●) or when heat was given 4-h after irradiating (○) and includes both previously published data [Citation21, Citation25, Citation27, Citation28] and more recent unpublished results. Shaded area shows the 95% confidence interval for carbon ion irradiated tumors, taken from [Citation29]. *Indicates the heat and radiation values that were significantly different from the carbon ion value (t-test; p < .05).](/cms/asset/61313cd3-0e69-45b4-94c5-cce189eb5178/ihyt_a_1876929_f0002_b.jpg)
Figure 3. The effect of varying the time interval between radiation and heat on the radiation response of C3H mammary carcinomas. Data similar to that shown in was produced for all temperatures shown and from the resulting radiation dose-response curves the TCD50 (radiation dose that causes 50% tumor control) values were calculated. The symbols represent the enhancement ratios (ratio of the TCD50 value for radiation alone compared to radiation and heat) with 95% confidence intervals and are for radiation combined with 42.5 °C (●) or 41.5 °C (○) and includes both previously published data [Citation25, Citation27, Citation28] and more recent unpublished results. Shaded area shows the 95% confidence interval for carbon ion irradiated tumors, taken from [Citation29]. *Indicates the heat and radiation values that were significantly different from the carbon ion value (t-test; p < .05).
![Figure 3. The effect of varying the time interval between radiation and heat on the radiation response of C3H mammary carcinomas. Data similar to that shown in Figure 1 was produced for all temperatures shown and from the resulting radiation dose-response curves the TCD50 (radiation dose that causes 50% tumor control) values were calculated. The symbols represent the enhancement ratios (ratio of the TCD50 value for radiation alone compared to radiation and heat) with 95% confidence intervals and are for radiation combined with 42.5 °C (●) or 41.5 °C (○) and includes both previously published data [Citation25, Citation27, Citation28] and more recent unpublished results. Shaded area shows the 95% confidence interval for carbon ion irradiated tumors, taken from [Citation29]. *Indicates the heat and radiation values that were significantly different from the carbon ion value (t-test; p < .05).](/cms/asset/0c65b3ff-a1f5-4c79-b313-3e592136cb20/ihyt_a_1876929_f0003_b.jpg)
summarizes the effect of varying the time interval between the two extremes shown in for 41.5 °C and 42.5 °C. With the lower temperature the 1.5 enhancement factor seen with the simultaneous radiation and heat treatment drops down very quickly with the introduction of an interval of only 30 min; with this time interval, and longer intervals up to 4-h, the enhancement ratios are significantly below that for carbon ions. For the higher 42.5 °C temperature, the simultaneous radiation and heat treatment resulted in an enhancement ratio of around 2.5. Again, this drops off rapidly with the introduction of a time interval, but even with a 4-h interval, it is not below that seen for carbon ions.
Discussion
Our study clearly showed that combining hyperthermia with low LET radiation significantly enhanced the local control of C3H mammary carcinomas compared to that seen for radiation alone. The enhancement obtained was dependent on both the heating temperature and the time interval between the radiation and heat treatments; the higher the heating temperature and the shorter the interval between the radiation and heat, the greater the degree of enhancement. This is not a novel finding and has been well documented in pre-clinical studies [Citation15,Citation16]. In fact, it was shown previously in our C3H mammary carcinoma model and some of that data were included in the current study [Citation21,Citation25,Citation27,Citation28]. The enhancement observed with a simultaneous application of radiation and heat is generally considered to result from a heat-induced radiosensitization. Two different mechanisms have been suggested to account for this radiosensitization. One involves an improved oxygen delivery to tumors. Pre-clinical studies have clearly shown that during the heating period tumor oxygenation increases [Citation30–34], most likely as a result of a heat-induced enhancement of tumor blood flow [Citation32–35]. This would decrease tumor hypoxia and thus enhance radiation response [Citation3,Citation4]. However, rapid-mix studies have demonstrated that improved levels of oxygenation are only beneficial if present at the time of irradiation or up to a few milliseconds after irradiating [Citation36,Citation37]. Yet significant radiosensitization is seen in our C3H mammary carcinoma model when heat is applied 1–2 h after irradiating [Citation15,Citation16], although the tumor oxygenation status at this time has returned to pretreatment levels [Citation31]. This suggests an alternative radiosensitizing mechanism must be operating in this tumor model. The most likely explanation involves hyperthermia inhibiting the repair of radiation-induced DNA damage as proposed and demonstrated by others [Citation38–40]. This radiosensitization decreases with the introduction of an interval between the two modalities, and when the interval is sufficient (i.e., around 4-h, depending on the temperature) there is no radiosensitizing effect [Citation15,Citation16]. The enhanced response then is simply due to heat killing the radioresistant hypoxic cells [Citation15,Citation16]. In other tumor models, the situation may be more complex. Apart from heat-induced direct cell killing and an inhibition of radiation-induced DNA damage repair [Citation41], the effect on tumor oxygenation is not as transient as shown for our C3H mammary carcinoma. Other studies, using rodent [Citation42,Citation43], canine [Citation44], and even human [Citation45–47] cancers, report that tumor oxygenation can still be elevated 24-h after heating. This would clearly enhance radiation response when heat is applied prior to radiation in single treatment schedules [Citation43], but probably plays a more significant role when hyperthermia is part of a fractionated schedule as used in the clinical situation [Citation45]. Clinically, the importance of the time interval between radiation and heat has been less easy to demonstrate. Studies in both malignant melanoma [Citation48] and cervix cancer [Citation49] clearly confirmed the pre-clinical findings that the time interval influenced outcome, with a shorter interval being superior. However, another study in cervix cancer suggested that the time interval had no effect on clinical outcome [Citation50].
The most significant aspect of our current study was that we were able to directly compare the effects of combining radiation and hyperthermia under different conditions with those seen with carbon ions using the same experimental set-up and the same tumor model. This has not previously been done in vivo and certainly not with the most clinically relevant endpoint of local tumor control. We found that with a truly simultaneous radiation and heat treatment a response equivalent to that seen with carbon ions was possible with only a temperature of 41.0 °C. However, with the introduction of an interval between the radiation and heat treatment then higher temperatures of at least 42.5 °C were required. A clinical study in sarcoma cancer patients was recently started (HYPROSAR), in which proton irradiation and hyperthermia were combined [Citation19]. It was based on the concept that this approach should have anti-tumor effects equivalent to those expected following treatment with carbon ions. The plan was to heat tumors to temperatures of 41.5–42.5 °C for 60 min, 90–150 min following the first radiation treatment each week in a fractionated schedule [Citation19]. Our pre-clinical results support this study, but emphasize the need to go for the higher temperature rather than the lower limit. Of course, our conclusions are based on results obtained in only one tumor model and only when using single dose irradiations rather than more clinically relevant fractionated schedules. However, this C3H mammary carcinoma model is one of the few available models in which the most clinically relevant endpoint of local tumor control for radiation-based studies is possible and the model we previously used to help establish certain clinical treatments. Specifically, it was the only model used to establish the potential of the radiosensitizer nimorazole to overcome hypoxia and enhance radiation response [Citation20]; nimorazole is now standard therapy for head & neck cancer patients in Denmark [Citation22]. It was also the first model in which we demonstrated that nicotinamide could be used to prevent acute hypoxia and how that drug should be combined with agents that eliminated chronic hypoxia to improve tumor response to radiation [Citation21]; that concept, albeit combining nicotinamide with carbogen breathing, eventually underwent successful clinical evaluation in bladder [Citation23] and head & neck [Citation24] cancer. Furthermore, our experiments did not use protons as proposed for the clinical study. This may make a difference since protons share similar physical dose profiles as carbon ions and have a higher RBE (Relative Biological Effect) than x-rays [Citation51], which could lead to an increased effect at all temperatures. However, higher radiation doses are required with fractionated radiation schedules to induce the same tumor control as found with single treatments [Citation52] and this may result in a reduced effect of heat, thus compensating for any increase due to the higher RBE.
Clearly, there is a need to undertake additional pre-clinical studies combining heat with proton radiation, and in fractionated schedules, to determine the heating temperature and time interval between the radiation and heat for the optimal anti-tumor response. Compared to X-rays, protons have a better depth-dose distribution and thus the dose to critical normal tissues can be reduced without compromising the dose to the tumor [Citation53]. This is important because it could result in less normal tissue damage when applying the heat, especially when using shorter time intervals between the radiation and heating [Citation15,Citation16]. The clinical application of radiation and heat will also be in fractionated schedules and here thermotolerance and step-up/step-down heating become relevant issues, especially where tumors are heterogeneously heated [Citation26,Citation54,Citation55]. Without information on these aspects, we cannot be sure whether the criteria selected for the heat application in future clinical trials with protons and heat is sufficient. Nevertheless, our current data certainly argues for aiming for higher temperatures rather than lower ones. Alternatively, one could apply other treatments that enhance the combination of mild temperature hyperthermia and radiation. Our previous studies demonstrated that systemic administration of the vascular disrupting agent (VDA) OXi4503 not only enhanced the response of our C3H mammary carcinoma to a combined radiation and mild hyperthermia treatment, but also actually prevented the decrease seen when the time interval between radiation and heat was extended [Citation27]. Several other VDAs are in clinical development [Citation56] and may be just as effective as OXi4503 in this context.
Acknowledgments
The authors thank Ms. Dorthe Grand, Ms. Maria Lynnerup Bech, Ms. Marianne Kristensen, and Ms. Inger Marie Horsman for excellent technical help with the experiments and animal care. We also thank Dr. Bo Martin Bibby, Department of Biostatistics, Aarhus University, for help with the statistical analysis.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Hall EJ, Giaccia AJ, editors. Radiobiology for the radiologist. 7th ed. Philadelphia (USA): Lippincott, Williams & Wilkins; 2012.
- van der Kogel AJ, Joiner M, editors. Basic clinical radiobiology for radiation oncologists. 5th ed. London (UK): Hodder Arnold; 2019.
- Horsman MR, Mortensen LS, Petersen JB, et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9(12):674–687.
- Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–124.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465.
- Horsman MR, Vaupel P. Pathophysiological basis for the formation of the tumor microenvironment. Front Oncol. 2016;6:66.
- Zhang S, Guo H, Zhang D, et al. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15(1):15–20.
- López-Camarillo C, Ruiz-Garcia E, Starling N, et al. Editorial: neovascularization, angiogenesis and vasculogenic mimicry in cancer. Front Oncol. 2020;10:1140.
- Chang YS, di Tomaso E, McDonaold DM, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97(26):14608–14613.
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? Cancer Res. 1986;46:467–473.
- Moulder JE, Rockwell S. Hypoxic fractions of solid tumors. Int J Radiat Oncol Biol Phys. 1984;10(5):695–712.
- Barendsen GW. Responses of cultured cells, tumours and normal tissues to radiations of different linear energy transfer. Curr Topics Radiat Res Q. 1968;4:293–356.
- Wenzl T, Wilkens JJ. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys Med Biol. 2011;56(11):3251–3268.
- https://www.ptcog.ch/index.php/facilities-in-operation.
- Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007;19(6):418–426.
- Elming PB, Sørensen BS, Oei AL, Franken NAP, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. 2019;11(1):60.
- Robinson JE, Wizenberg MJ, McCready WA. Combined hyperthermia and radiation suggest and alternative to heavy particle therapy for reduced oxygen enhancement ratios. Nature. 1974;251(5475):521–522.
- Gerner EW, Leith JT. Enteraction of hyperthermia with radiations of different linerar energy transfer. Int J Radiat Biol Relat Stud Phys Chem Med. 1977;31(3):283–288.
- Datta NR, Puric E, Schneider R, et al. Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia. 2014;30(7):524–530.
- Overgaard J, Overgaard M, Nielsen OS, et al. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br J Cancer. 1982;46(6):904–911.
- Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumour cells. Cancer Res. 1990;50(23):7430–7436.
- Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46(2):135–146.
- Hoskin PJ, Rojas AM, Saunders MI, BCON investigators, et al. BCON Carbogen and nicotinamide in locally advanced bladder cancer: early results of a phase-III randomized trial. Radiother Oncol. 2009;91(1):120–125.
- Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30(15):1777–1783.
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980;6(11):1507–1515.
- Lindegaard JC, Overgaard J. Factors of importance for the development of the step-down heating effect in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1987;3(1):79–91.
- Horsman MR. The therapeutic potential of using the vascular disrupting agent OXi4503 to enhance mild temperature thermoradiation. Int J Hyperth. 2015;31(5):453–459.
- Horsman MR. Realistic biological approaches for improving thermoradiotherapy. Int J Hyperthermia. 2016;32(1):14–22.
- Sørensen BS, Horsman MR, Alsner J, et al. Relative biological effectiveness of carbon ions for tumor control, acute skin damage and late radiation-induced fibrosis in a mouse model. Acta Oncol. 2015;54(9):1623–1630.
- Iwata K, Shakil A, Hur W, et al. Tumour pO2 can be increased markedly by mild hyperthermia. Br J Cancer. 1996;74(Suppl. XXVII):S217–S221.
- Horsman MR, Overgaard J. Can mild hyperthermia improve tumour oxygenation? Int J Hyperthermia. 1997;13(2):141–147.
- Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia. 2010;26(3):211–223.
- Sen A, Capitano ML, Spernyak JA, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011;71(11):3872–3880.
- Winslow TB, Eranki A, Ullas S, et al. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia. 2015;31(6):693–701.
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res. 1984;44:4721–4730.
- Howard-Flanders P, Moore D. The time interval after pulsed irradiation within which injury in bacteria can be modified by dissolved oxygen. I. A search for an effect of oxygen 0.002 seconds after pulsed irradiation. Radiat Res. 1958;9(4):422–437.
- Michael B, Adams G, Hewitt H, et al. A post-effect of oxygen in irradiated bacteria: a submillisecond fast mixing study. Radiat Res. 1973;54(2):239–251.
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77(4):399–408.
- Roti Roti JL. Introduction: radiosensitization by hyperthermia. Int J Hyperthermia. 2004;20(2):109–114.
- Ihara M, Takeshita S, Okaichi K, et al. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperth. 2014;30:102–109.
- Dewhirst MW, Vujaskovic Z, Jones E, et al. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21(8):779–790.
- Vujaskovic Z, Song CW. Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia. 2004;20(2):163–174.
- Song CW, Park HJ, Lee CK, et al. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 2005;21(8):761–767.
- Vujaskovic Z, Poulson JM, Gaskin AA, et al. Temperature-dependent changes in physiological parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys. 2000;46(1):179–185.
- Brizel DM, Scully SP, Harrelson JM, et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56:5347–5350.
- Vujaskovic Z, Rosen EL, Blackwell KL, et al. Ultrasound guided pO2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int J Hyperth. 2003;19(5):498–506.
- Jones EL, Prosnitz LR, Dewhirst MW, et al. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res. 2004;10(13):4287–4293.
- Overgaard J, Overgaard M. Hyperthermia as an adjuvant to radiotherapy in the treatment of malignant melanoma. Int J Hyperthermia. 1987;3(6):483–501.
- van Leeuwen CM, Oei AL, Chin KWTK, et al. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat Oncol. 2017;12(1):75.
- Kroesen M, Mulder HT, van Holthe JML, et al. The effect of the time interval between radiation and hyperthermia on clinical outcome in 400 locally advanced cervical carcinoma patients. Front Oncol. 2019;9:134.
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59(22):R419–R472.
- Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol. 2013;52(7):1320–1326.
- Grau C, Durante M, Georg D, et al. Particle therapy in Europe. Mol Oncol. 2020;14(7):1492–1499.
- Overgaard J. Some problems related to the clinical use of thermal isoeffect doses. Int J Hyperthermia. 1987;3(4):329–336.
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 1989;16(3):535–549.
- Siemann DW, Chaplin DJ, Horsman MR. Realizing the potential of vascular targeted therapy: the rationale for combining vascular disrupting agents and anti-angiogenic agents to treat cancer. Cancer Invest. 2017;35(8):519–534.
