Abstract
Purpose
Modulated electro-hyperthermia (mEHT) may enhance the tumor response, although the effectiveness of combined neoadjuvant therapy remains unclear. Therefore, we investigated the role of mEHT with neoadjuvant therapy for locally advanced rectal cancer.
Materials and methods
Clinical data were analyzed for 120 patients who received neoadjuvant treatment for locally advanced rectal cancer (T3/4 or N+, M0) from May 2012 to December 2017. Capecitabine or 5-fluorouracil was administered along with radiotherapy. Patients were categorized into mEHT group (62 patients) and non-mEHT group (58 patients) depending on whether mEHT was added. Surgery was performed 6–8 weeks after the end of radiotherapy.
Results
The median age was 59 years (range, 33–83). The median radiation dose was significantly less for mEHT group (40 Gy) than for non-mEHT group (50.4 Gy). In mEHT group, 80.7% showed down-staging compared with 67.2% in non-mEHT group. For large tumors of more than 65 cm³ (mean), improved tumor regression was observed in 31.6% of mEHT group compared with 0% of non-mEHT group (p = .024). The gastrointestinal toxicity rate of mEHT group was 64.5%, which was found to be statistically significantly less than 87.9% of non-mEHT group (p = .010). The 2-year disease-free survival was 96% for mEHT group and 79% for non-mEHT group (p = .054).
Conclusion
The overall mEHT group had a comparable response and survival using less radiation dosing compared with standard care; the subgroup with large tumors showed improved efficacy for tumor regression after mEHT.
Introduction
Neoadjuvant radiotherapy is required for the treatment of rectal cancer with clinical T3-4 stage, positive lymph node status, or location adjacent to the anal verge. Current standard course neoadjuvant radiotherapy schedule (45–50 Gy with conventional fractionation) entails extended overall treatment time and may result in a considerable number of complications [Citation1]. Modulated electro-hyperthermia (mEHT) can be considered as a useful tool for effective tumor control overcoming radiotoxicity problems.
mEHT can induce a moderate temperature increase in the target. The major antitumor effects are supposed to be a result of non-thermal mechanisms of the radiofrequency and amplitude modulation, which are still under research [Citation2]. This treatment can be administered to most solid tumors including gynecologic malignancies, hepatocellular carcinoma, pancreatic cancer, brain tumors, and others [Citation3–7]. Combined with other treatment modalities including radiotherapy, mEHT is expected to have a synergistic effect in terms of tumor response. However, few studies of the mEHT have been conducted on neoadjuvant treatment in locally advanced rectal cancer [Citation8].
A prospective single-arm study has been underway in our institution since 2014 to assess the role of mEHT during neoadjuvant radiochemotherapy for locally advanced rectal cancer [Citation9]. We compared this cohort to consecutive cases that received standard neoadjuvant treatment without mEHT about the same period (a combined retrospective-prospective analysis). The purpose of the current study was to evaluate the benefit of mEHT on neoadjuvant treatment platform in terms of clinical outcome.
Materials and methods
This study was approved by the Yonsei University Wonju Severance Christian Hospital Institutional Review Board (IRB No. CR317107).
Patient characteristics
Between May 2012 and December 2017, 160 patients were referred for neoadjuvant radiotherapy for locally advanced rectal cancer. Among them, cases in which proper treatment schedule and follow-up were not performed, exceptional pathologic diagnosis (signet ring cell carcinoma), distant metastasis, and short course radiotherapy, etc. were excluded. Finally, clinical data of 120 patients were analyzed. The staging was performed using computed tomography (CT), magnetic resonance imaging (MRI), according to the 7th edition of the American Joint Committee on Cancer manual [Citation10]. 18 F-fluorodeoxyglucose positron emission tomography was scanned in 113 patients (94.2%) for the purpose of distant workup.
Histological diagnoses were all adenocarcinoma. The primary tumor was located within 15 cm from the anal verge. The Eastern Cooperative Oncology Group performance status score was 2 or less. Patients were categorized into the mEHT group (62 patients, 51.7%) and the non-mEHT group (58 patients, 48.3%) according to whether or not mEHT was added. In most cases of mEHT, a slightly reduced radiation dose (40 Gy) was applied according to the prospective single arm protocol with informed consent for each patient [Citation9]. For the other mEHT cases, conventional radiation dose (50.4 Gy) was intended and the consistency of the number of mEHT sessions was relatively low (). The distributions of age, sex, pathologic diagnosis, and initial clinical T and N stages were not different between the two groups. The initial primary tumor volume was measured as 62.3 ± 54.8 mL. Most of the patients were clinically node-positive, while the non-mEHT group included 4 patients with clinically node-negative status ().
Figure 1. CONSORT diagram (RT: radiotherapy; CRT: chemoradiotherapy; mEHT: modulated electro-hyperthermia; fx: fractions).
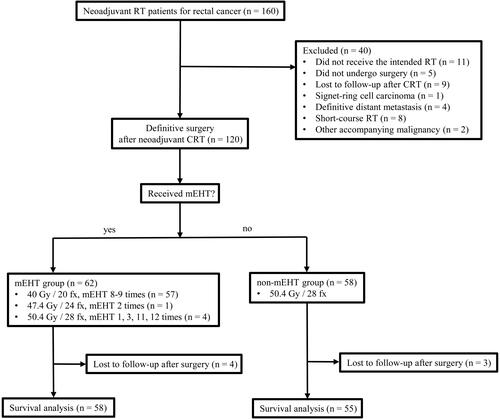
Table 1. Patient characteristics before neoadjuvant treatment.
Neoadjuvant treatment
During the radiotherapy period of approximately 4-6 weeks, 5-fluorouracil (5-FU) or capecitabine was administered concurrently as a chemotherapeutic agent [Citation11]. 5-FU with leucovorin was injected as an intravenous bolus for 3 days in the first and last weeks of radiotherapy. Capecitabine was administered orally twice daily during radiotherapy. The mEHT was added during the radiotherapy period twice a week. Most patients (59 patients, 95.2%) in the mEHT group had more than 8 sessions and the mean mEHT total energy was 3769.9 KJ. Approximately 6-8 weeks after the completion of radiotherapy, surgery was performed. The choice of the surgical procedure was at the surgeon’s discretion and the type of resection was not significantly different between the two groups. The details of the treatment are summarized in .
Table 2. Neoadjuvant treatment summary.
Radiotherapy
Patients received linear accelerator-based external beam radiotherapy in the supine or prone position after planning CT (Aquillion LB, TSX-201A, Toshiba Medical Systems Corporation, Otawara, Japan) scan. The three- or four-field technique was used reflecting the disease extent and location. The clinical target volume included the tumor, mesorectum, internal iliac nodes, and presacral nodes up to the sacral promontory level. Radiation was delivered with a 6/15 MV linear accelerator (Elekta Synergy, Elekta, Stockholm, Sweden). During radiotherapy, all patients underwent weekly medical examinations. The initial primary tumor volume was measured during the contouring process to evaluate the tumor response to neoadjuvant treatment. The Pinnacle system (ADAC Laboratories, Milpitas, CA, USA) was used for treatment planning and tumor volume measurement.
Modulated electro-hyperthermia
mEHT was performed using a 13.56-MHz capacitive-coupled device (EHY-2000 Plus, Oncotherm GmbH, Troisdorf, Germany). A 30 cm diameter-sized electrode was attached to the front of the body with a supine position adjusted to be located in the middle of the clinical target volume. Each mEHT session time was 60 min and the time interval with radiotherapy was within one hour. On the first day of mEHT, the power of mEHT was set at 100 W for the first 20 min, 120 W for the next 20 min, and 140 W for the last 20 min, if no toxicities were observed. From the subsequent treatment session, 140 W was used. These power values were partially modified when side effects such as patients’ heat sense occurred. We applied mEHT in conjunction with an amplitude modulation (pink noise with lowest frequency of 0.5 kHz), which is the default status of the system.
Evaluation of pathologic outcome and toxicity
Treatment response was assessed by the pathologic stage and tumor regression status after surgery. A pathologic complete response (pCR) was defined as no evidence of residual tumors in the surgical specimen (ypT0N0). T- and N-downstaging were defined as reduced pathologic stage compared to the initial clinical stage, as icT3 becomes ypT2 or ypT1. Also, downstaging means the entire stage change, not each T or N, as stage III becomes stage II or I. Tumor regression was scored using the standardized 5-point grading system 0–4 described by Dworak et al. [Citation12]. We defined as a good tumor regression grade (TRG) score the highest grades 3 or 4 [Citation13]. Treatment-related acute toxicities were assessed by National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (NCI, Bethesda, MD, USA). The mEHT-related toxicities were evaluated separately according to the Berlin scoring system [Citation14].
Follow-up
Postoperative chemotherapy continued after surgery for all patients except pCR cases, whose regimen differed depending on the outcome of the surgical pathology. Regular follow-up was performed every 3 months for 3–4 years after surgery, and every 6 months for 1–2 years thereafter. At each follow-up examination carcinoembryonic antigen, carbohydrate antigen 19-9, complete blood count, and blood chemistry were checked. CT was scanned every 6 months until 3–4 years after surgery, and then once a year for 1–2 years.
Statistical analysis
Chi-square test and Fisher’s exact test were used for comparing the mEHT group and the non-mEHT group. Independent t-test and Wilcoxon rank-sum test (Mann Whitney U test) were used for comparing continuous variables between the two groups. For determining the relationship between the initial primary tumor volume and its regression grade, the Kruskal-Wallis test was employed. Post-hoc analysis was performed by the Dwass-Steel-Critchlow-Fligner method. Overall survival (OS) was defined as the time from the day of radiotherapy to the day of the death for any reason or last follow-up. Disease-free survival (DFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS) were measured from the day of surgery to the day of the recurrence, death, or last follow-up. Cox proportional hazard regression analysis presented the univariate and multivariate-adjusted hazard ratios (HR) and 95% CI for OS, DFS, LRRFS, and DMFS. A p-value of less than .05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Pathologic outcomes
Surgical pathology results are displayed in . The ypN positive rate was 19.3% (12 patients) in the mEHT group and 29.3% (17 patients) of the non-mEHT group. Downstaging was observed in 80.7% of patients in the mEHT group and in 67.2% of patients in the non-mEHT group (p = .094). The TRG was not significantly different between both groups (p = .146). There was no difference in the incidence of the good TRG score between the two groups (p = .637). A good TRG score was significantly more frequent in the mEHT group (6 patients, 31.6%) than in the non-mEHT group (0 patients) for primary tumor volumes larger than 65 mL (p = .024). No correlation between tumor volume and TRG was found in the mEHT-group (, p = .912), but an inverse correlation in the non-mEHT group (, p = .009).
Figure 2. Box plot with scatter plot between initial primary tumor volume and tumor regression grade (TRG) in mEHT group (A) and non-mEHT group (B). In mEHT group, TRG distribution according to the initial primary tumor volume was relatively uniform. In non-mEHT group, the larger the tumor volume, the lower the TRG as expected, which was found to have a relatively higher distribution of the tumor volume in TRG1 compared to TRG3 or TRG4.
Table 3. Surgical pathology results.
Toxicity
There were 2 patients with the grade 3 mEHT-related toxicity, one with hot spots and the other with suspected fat necrosis, whose mEHT was discontinued. One patient had the mEHT only 3 sessions due to personal economic reasons. The incidence of leukopenia, neutropenia, and genitourinary toxicities were similar between the two groups (). The proportion of gastrointestinal toxicity occurrence was 64.5% (40 patients) of the mEHT group, and 87.9% (51 patients) in the non-mEHT group (p = .010).
Table 4. Neoadjuvant treatment-related toxicities.
Survival
Survival analysis was performed on 113 of 120 patients, except those who were lost to follow-up after surgery. The median follow-up period was 45 months (range, 7–71 months) in the mEHT group and 58 months (range, 6–95 months) in the non-mEHT group. The 2-year OS was 100% in the mEHT group, not significantly different from 96% in the non-mEHT group. The 2-year DFS showed a difference between 96% in the mEHT group and 76% in the non-mEHT group (p = .054). The 2-year LRRFS was 98% and 94%, and the 2-year DMFS was 94% and 79%, respectively (). In univariate analysis, resection margin status was significant in DFS, LRRFS, and DMFS. ypN was related to DFS, DMFS. In multivariate analysis, resection margin status was found to be a dominant factor in DFS, LRRFS, and DMFS as well as in OS. ypT and ypN was associated with OS and DFS, respectively. mEHT presented the potential to improve DFS and DMFS ().
Figure 3. Survival analysis between the mEHT group and the non-mEHT group. (A) Overall survival, (B) Disease-free survival, (C) Locoregional recurrence-free survival, and (D) Distant metastasis-free survival.
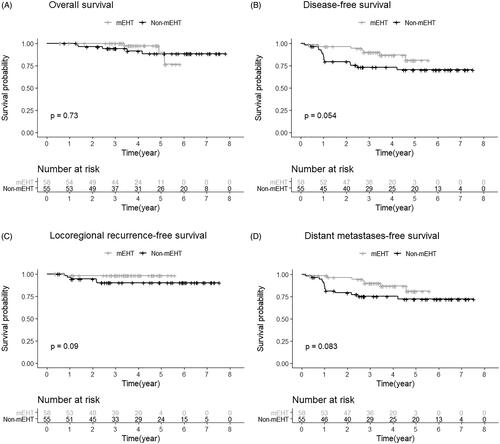
Table 5. Univariate and multivariate Cox analysis of OS, DFS, LRRFS and DMFS for patients.
Discussion
The main objective of this study was to assess the effect of mEHT in preoperative treatment for rectal cancer. mEHT might improve pathohistologic tumor response. Furthermore, we found indications that mEHT might improve DFS, LRFS and DMFS, though mean observation time of the mEHT arm was limited to <4 years. On the other hand, the toxicity profile of mEHT is pleasantly low and can even contribute to lower the acute toxicity by reducing the radiation dose.
Despite lower radiation doses in the mEHT group, the pathologic outcomes such as downstaging, pCR, and TRG ratio were comparable to those in the non-mEHT group. Although not significant, the proportion of downstaging (80.7% versus 67.2%) and pCR (17.7% versus 8.6%) was relatively high in the mEHT group compared to the non-mEHT group. Schroeder et al. used the annular-phased-array technology, which was more complicated, expensive and burdening because of distinctly higher total power (>600 W for APA-systems versus 150 W for mEHT). Nevertheless the pCR rates were comparable [Citation15].
Although not present in all cases, our TRG results were notable in terms of the therapeutic role of mEHT. According to Yoon et al., in cases of typical neoadjuvant chemoradiation for rectal cancer, TRG showed favorable features for small pretreatment tumor volume, which was the same pattern in the non-mEHT group of our study [Citation16]. However, this correlation disappeared when mEHT was used in parallel. Furthermore, it has been found that mEHT can be a useful tool in overcoming hypoxia-related resistance, which is consistent with the role of conventional hyperthermia for large-sized tumors [Citation17]. To the best of our knowledge, our study was the first showing favorable TRG results by mEHT in a subgroup characterized by large primary tumor volume.
Larger-sized tumors are more likely to be associated with hypoxic or necrotic histological properties, where hypoxic cells become resistant to chemoradiotherapy [Citation18,Citation19]. Currently, one of the major roles of hyperthermia is to improve susceptibility to radiation by increasing perfusion to the tumor [Citation20]. Although the detailed mechanism is not clear, this basic hyperthermia principle appears to have resulted in comparable clinical outcomes in the mEHT group even with relatively low radiation dose. In addition, modulation-related non-thermal effects, which are characteristics of mEHT, may have played a role although the mechanisms are still unresolved [Citation21,Citation22].
The mEHT group showed improved survival results, especially in DFS, LRRFS, and DMFS, though statistical significance was not clear. It is noteworthy that mEHT presented a potential impact on survival rate compared to previous hyperthermia studies. In the recently published long-term results, the combination of hyperthermia with conventional neoadjuvant chemoradiotherapy tended to improve OS and local control [Citation23]. According to one systematic review, which studied the effect of hyperthermia on rectal cancer neoadjuvant chemoradiotherapy, OS was significantly better in the group with hyperthermia after 2 years, but this difference disappeared after a longer period (3, 4, and 5 years) [Citation24]. The relative survival superiority of mEHT may represent the possibility of the systemic effect of mEHT. Experimental studies confirmed an abscopal effect of mEHT [Citation25] as well as a few clinical observations [Citation26,Citation27], but this issue needs further clinical investigations.
Resection margin status was confirmed as the most noticeable factor in most survival outcomes including OS, DFS, LRRFS, and DMFS (). It should be noted that a positive resection margin is related to the size of the primary tumor, while its impact is significant in itself. According to tumor data in our study, the initial primary tumor volume was differentiated by the margin status (92.2 ± 80.8 mL for positive margin and 59.2 ± 51.3 mL for negative margin). Thus, more active application of mEHT is needed considering the effectiveness of mEHT for large-sized tumors.
Another finding of the current study was that the mEHT group had a low ypN positive rate, considering that the non-mEHT group included four icN0 patients. The ypN stage after neoadjuvant chemoradiotherapy is known to be an important prognostic factor for locally advanced rectal cancer. Gani et al. reported that the ypN stage was the only prognostic factor for OS, DFS, and DMFS on multivariate analysis [Citation23]. In the current study, ypN was found to be important in the prognosis of DFS.
This study has some limitations due to retrospective approaches with small sample size, lack of data on late toxicities, and relatively short term follow-up results. Even though both groups are properly matched according to , a selection bias cannot be excluded. The other limitation included the non-uniform chemotherapy regimen between the two groups though the efficacy of each regimen is known to be almost the same for colorectal cancer [Citation11,Citation28]. These points should be improved with a more homogeneous clinical protocol design in future studies.
Conclusion
A lower radiation dose combined with mEHT can be associated with improved tumor responses especially for large-sized tumors. A certain level of survival gain and toxicity reduction may be available with a well-designed combination protocol focusing on active use of mEHT.
Acknowledgment
We would like to thank Editage (www.editage.co.kr) for English language editing.
Disclosure statement
The authors have no financial conflicts of interest.
References
- Swellengrebel H, Marijnen C, Verwaal V, et al. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg. 2011;98(3):418–426.
- Wust P, Kortüm B, Strauss U, et al. Non-thermal effects of radiofrequency electromagnetic fields. Sci Rep. 2020;10(1):1–8.
- Minnaar CA, Kotzen JA, Ayeni OA, et al. The effect of modulated electro-hyperthermia on local disease control in HIV-positive and -negative cervical cancer women in South Africa: early results from a phase III randomised controlled trial. PLoS One. 2019;14(6):e0217894.
- Gadaleta-Caldarola G, Infusino S, Galise I, et al. Sorafenib and locoregional deep electro-hyperthermia in advanced hepatocellular carcinoma: a phase II study. Oncol Lett. 2014;8(4):1783–1787.
- Ferrari V, De Ponti S, Valcamonico F, et al. Deep electro-hyperthermia (EHY) with or without thermo-active agents in patients with advanced hepatic cell carcinoma: phase II study. J Clin Oncol. 2007;25(18_suppl):15168–15168.
- Hager D. Survival and quality of life of patients with advanced pancreatic cancer. Proc Am Soc Clin Oncol. 2002;21:2359.
- Hager ED, Sahinbas H, Groenemeyer DH, et al. Prospective phase II trial for recurrent high-grade gliomas with capacitive coupled low radiofrequency (LRF) hyperthermia. J Clin Oncol. 2008;26(15_suppl):2047–2047.
- Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 2009;28(2):148–165.
- You S, Kim S. Feasibility of modulated electro-hyperthermia in preoperative treatment for locally advanced rectal cancer: early phase 2 clinical results. Neoplasma. 2020;67(3):677–683.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474.
- Kim DY, Jung KH, Kim TH, et al. Comparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67(2):378–384.
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23.
- Park SH, Kim JC. Preoperative chemoradiation for locally advanced rectal cancer: comparison of three radiation dose and fractionation schedules. Radiat Oncol J. 2016;34(2):96–105.
- Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg. 1998;227(3):380–389.
- Schroeder C, Gani C, Lamprecht U, et al. Pathological complete response and sphincter-sparing surgery after neoadjuvant radiochemotherapy with regional hyperthermia for locally advanced rectal cancer compared with radiochemotherapy alone. Int J Hyperthermia. 2012;28(8):707–714.
- Yoon SM, Kim DY, Kim TH, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69(4):1167–1172.
- Elming PB, Sørensen BS, Oei AL, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers. 2019;11(1):60.
- Chaplin D, Durand R, Olive P. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986;12(8):1279–1282.
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(S5):4–9.
- Mallory M, Gogineni E, Jones GC, et al. Therapeutic hyperthermia: the old, the new, and the upcoming. Crit Rev Oncol Hematol. 2016;97:56–64.
- Andocs G, Renner H, Balogh L, et al. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009;185(2):120–126.
- Szasz A, Vincze G, Szasz O, et al. An energy analysis of extracellular hyperthermia. Electromagn Biol Med. 2003;22(2–3):103–115.
- Gani C, Schroeder C, Heinrich V, et al. Long-term local control and survival after preoperative radiochemotherapy in combination with deep regional hyperthermia in locally advanced rectal cancer. Int J Hyperthermia. 2016;32(2):187–192.
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Sys Rev. 2009;(3):CD006269.
- Qin W, Akutsu Y, Andocs G, et al. Modulated electro-hyperthermia enhances dendritic cell therapy through an abscopal effect in mice. Oncol Rep. 2014;32(6):2373–2379.
- Minnaar CA, Kotzen JA, Ayeni OA, et al. Potentiation of the abscopal effect by modulated electro-hyperthermia in locally advanced cervical cancer patients. Front Oncol. 2020;10:376.
- Chi M-S, Mehta MP, Yang K-L, et al. Putative abscopal effect in three patients treated by combined radiotherapy and modulated electrohyperthermia. Front Oncol. 2020;10:254.
- Van Cutsem E, Hoff P, Harper P, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90(6):1190–1197.
