Abstract
Purpose
To assess the feasibility, efficacy, and safety of ultrasound (US)-guided microwave ablation (MWA) for the treatment of papillary thyroid cancer (PTC) located in the thyroid isthmus.
Materials and methods
Thirty-four patients (mean age, 43 ± 11 years; 26 women) with isthmic PTC treated with MWA between June 2014 and September 2020 were included in this retrospective study. The follow-up time after MWA was 17 ± 9 months (range, 8–50 months). Changes in thyroid function, parathyroid function, and tumor size were evaluated, along with the rates of tumor disappearance and complications.
Results
The treatment was technically feasible and successfully completed in all 34 patients (100%). Measures of thyroid function (i.e. serum triiodothyronine, free thyroxine, and thyrotropin) and parathyroid function (i.e. serum calcium and intact parathyroid hormone) showed no changes from pretreatment levels at 1, 3, and 6 months after MWA (p > 0.05 for all). Tumor size was found to be increased at 1 and 3 months after MWA compared with before MWA (p < 0.05). However, the tumor sizes measured at 6, 9, 12, and 18 months after MWA were smaller than the pretreatment sizes (p < 0.05 for all). In 24 cases (70.6%), the tumors completely disappeared on US examination. Five cases (2.9%) experienced side effects from MWA treatment, but no major or minor complications were recorded.
Conclusion
The results of this study demonstrate that US-guided MWA is a feasible, effective, and safe treatment option for selected patients with PTC located in the thyroid isthmus.
Introduction
The thyroid is an endocrine gland composed of right and left lateral lobes connected by a strip of thyroid tissue called the isthmus. The thyroid isthmus is located anterior to the trachea and is covered by the strap muscles, fascia, and skin in the middle of the neck. Isthmic papillary thyroid cancer (PTC) is defined as thyroid cancer in which the tumor center is located between two lines perpendicular to the surface of the skin from the most lateral points of the trachea [Citation1]. Previous studies have reported incidence rates for PTC arising in the thyroid isthmus of 1–12.3% [Citation2–4]. To date, the American Thyroid Association, National Comprehensive Cancer Network, European Thyroid Association, and British Thyroid Association have not provided specific guidelines for managing isthmic PTC yet. According to previous reports, total thyroidectomy is the mainstream management for isthmic PTC [Citation2,Citation5–7]. However, this treatment is associated with some complications, such as hypothyroidism, hypoparathyroidism, and recurrent laryngeal nerve (RLN) injury, all of which can be detrimental to the patient’s quality of life.
Ultrasound (US)-guided microwave ablation (MWA) is a promising minimally invasive technique for treating a variety of tumors, including thyroid nodules and parathyroid nodules [Citation8,Citation9]. Moreover, it has been shown to be an effective and safe treatment modality for PTC [Citation10–13]. However, to our knowledge, none of the previous studies have investigated the use of MWA specifically for isthmic PTC. The aim of the present retrospective study was to assess the feasibility, efficacy, and safety of US-guided MWA therapy for the treatment of PTC located in the thyroid isthmus, in order to determine its potential as a treatment option for these patients.
Methods
Patients
This retrospective study was approved by the Ethics Committees of China-Japan Friendship Hospital and was carried out according to the Declaration of Helsinki. The need to obtain informed consent to publish the data was waived for this retrospective investigation. Written informed consent was obtained from each patient before MWA treatment.
The inclusion criteria for the present study were: (1) PTC diagnosis pathologically confirmed by fine needle aspiration (FNA); (2) unifocal PTC located in the isthmus on US examination; (3) maximum tumor diameter ≤1 cm; (4) no extrathyroidal extension; (5) no lymph node metastasis (LNM) or distant metastasis on imaging examination; and (6) follow-up of >6 months after MWA.
Pretreatment assessments
Before ablation, all patients underwent neck US examination, neck and chest computed tomography (CT) examination, FNA, and laboratory tests. The US examination and FNA guidance were performed using either a GE LOGIQ E9 (GE Healthcare, Pittsburgh, PA, USA) or Aplio 500 (Toshiba, Tokyo, Japan) US unit. Vocal cord function was assessed by US before ablation [Citation14]. All tumors were evaluated by US examination, and the size, volume and location of the tumors were recorded. Tumor volume was calculated using the ellipsoid volume formula: V = 0.524abc, where V is the volume; a, the maximum diameter, and b and c are the other two perpendicular diameters [Citation15]. CT was performed to detect LNM or distant metastasis. Specimens from FNA were sent for cytological pathology analysis and BRAF V600E mutation testing. Laboratory examinations included measurements of thyroid function (serum triiodothyronine [T3], serum free thyroxine [fT4], serum thyrotropin [TSH]) and parathyroid function (serum calcium and serum intact parathyroid hormone [iPTH]).
The medical records of patients, including the results from US examinations, CT images, and laboratory tests, were reviewed by two doctors who had more than 3 years of experience in thyroid ablation.
Ablation procedure
The MWA systems used for the procedures in this study (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China; or KY-2000, Kangyou Medical, Nanjing, China) consisted of a MWA generator and a 16-gauge, internally cooled 10- or 15-cm shaft antenna (3 or 5 mm in length). Before MWA, contrast-enhanced US (CEUS) examination was performed to evaluate the extent of the tumor and its enhancement mode. After that, the relationship between the tumor and cervical critical structures (i.e. vessels, trachea, esophagus) was carefully evaluated with high-resolution US by the operator to determine the best puncture site. Topical 1% lidocaine was applied for local anesthesia. The hydrodissection technique was employed at the designated ablation site before insertion of the ablation antenna. The hydrodissection technique was used to achieve a distance of at least 2 mm and to prevent thermal injury to the trachea, anterior portion muscularis, and skin. The output MWA power was 30 W for the ablation. The moving-shot or fixed-applicator technique was used for the procedure. The mean ablation time was 110 ± 45 s (range, 32–180 s). If the transient hyperechoic echotexture covered the entire tumor, the ablation was terminated. After MWA, CEUS was performed to confirm whether the ablation was complete. If an unenhanced ablation zone on CEUS was found covering the tumor and extending beyond the margin of the tumor by at least 2 mm, the ablation was considered complete. If an enhanced area was detected within the tumor or the extension beyond the tumor margin was not sufficient, an additional ablation was immediately carried out. Vocal cord function was evaluated again by US after the procedure [Citation14]. After ablation, the patients were carefully observed to evaluate complications according to the standard for image-guided thyroid ablation [Citation15]. The typical procedure is outlined in .
Post-MWA assessments
In the first year after MWA, patients were followed up approximately every 3 months. In the second year post-MWA, the patients were followed up every 6 months approximately, and after that, follow-up examinations were conducted approximately every 12 months. US, CT, and clinical assessments were performed for all patients after MWA. Laboratory tests and US examinations were conducted at each follow-up, and neck and chest CT examinations were performed every 12 months. If tumor recurrence or LNM was suspected, CEUS and FNA were carried out. Technical feasibility was defined as the ability to target the tumor and carry out the ablation as preoperatively planned. Technical success was defined as complete ablation at the end of every procedure.
Statistical analysis
Statistical analysis was performed using the statistical software SPSS, version 26.0 (SPSS Inc., Chicago, IL, USA). Statistical tests were two-sided, and P values less than 0.05 were considered to indicate statistical significance. Continuous data are presented as the mean ± standard deviation, and categorical data are summarized as frequency (percentage). The changes in tumor size (maximum diameter and volume) from before to after MWA were compared by the Wilcoxon signed rank test. The changes in thyroid function and parathyroid function from before to after MWA were compared by paired t-test. Technical feasibility, technical success, tumor disappearance, local recurrence, new tumors, LNM, and complications rate were evaluated.
Results
Demographic and clinical characteristics of isthmic PTC patients
From June 2014 to September 2020, a total of 393 consecutive patients with PTC were treated with MWA in our hospital, and after exclusion of 359 patients according to the inclusion criteria, 34 patients with isthmic PTC were finally included in this study (). The demographic characteristics of the included patients are summarized in . The mean patient age was 43 ± 11 years (range, 27–74 years). The 34 patients included 8 (23.5%) men and 26 (76.5%) women. Five (14.7%) patients had Hashimoto’s thyroiditis. Testing for the BRAF V600E mutation was positive in 28 (82.4%) cases and negative in 6 (17.6%) cases. The mean follow-up time after MWA was around 17 ± 9 months (range, 8–50 months), and 34, 31, 22, 13, and 4 patients were followed up for more than 6, 9, 12, 18, and 24 months, respectively. The mean volume for hydrodissection was 51 ± 10 ml (range, 35–67 ml).
Figure 2. A 31-year-old woman with papillary thyroid cancer in the isthmus of the thyroid was treated with microwave ablation (MWA). (A) Before MWA, ultrasonography (US) showed a hypoechoic target tumor (arrows); (B) before MWA, contrast-enhanced US (CEUS) showed a hypo-enhancement pattern in the artery phase (arrows); (C) the hydrodissection technique (arrowheads) was used to protect the trachea surrounding the tumor (arrows); (D) US showed a hyperechoic pattern in the tumor (arrows) during ablation; (E), after MWA, CEUS showed no enhancement (arrows) in the tumor area; and (F) on day 1 after MWA, US showed a hypoechoic ablation zone (arrows).
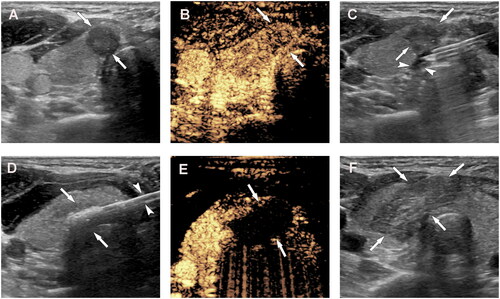
Table 1. Demographic characteristics of the isthmic PTC patients included in this study (n = 34).
Technical feasibility and success of US-guided MWA for treatment of isthmic PTC
Technical feasibility and treatment success were achieved in all 34 patients (100%). All tumors were correctly targeted and successfully ablated with US-guided MWA according to the preoperative plan. At the end of all MWA procedures, all of the tumors had been completely ablated.
Changes in thyroid and parathyroid function after US-guided MWA for isthmic PTC
The measurements for all indicators of thyroid function and parathyroid function before and after MWA are presented in and . Before MWA, mean values within the normal range were observed for T3 (1.11 ± 0.18 ng/ml; normal range, 0.8–2.0 ng/ml), fT4 (1.28 ± 0.15 ng/dL; normal range, 0.93–1.7 ng/dL), TSH (1.79 ± 1.29 uIU/ml; normal range, 0.27–4.2 uIU/ml), calcium (2.23 ± 0.16 mmol/L; normal range, 2.00–2.75 mmol/L), and iPTH (45 ± 18 pg/ml; normal range, 12–88 pg/ml). The measured values for these indicators of thyroid function (T3, fT4, and TSH) and parathyroid function (calcium and iPTH) at 1, 3, and 6 months after MWA did not differ significantly different from the pre-MWA values (p > 0.05 for all).
Table 2. Indicators of thyroid function before MWA and at each follow-up time-point after MWA.
Table 3. Indicators of parathyroid function before MWA and at each follow-up time-point after MWA.
Changes in tumor size after US-guided MWA for isthmic PTC
The mean maximum tumor diameter in the isthmic PTC patients before MWA was 6.0 ± 1.9 mm (range, 3–10 mm), and the mean tumor volume was 87.4 ± 74.9 mm3 (range, 6–367 mm3). The changes in tumor size (maximum diameter and volume) from before to after MWA are presented in . Due to expanding ablation, the sizes of the ablation zone at the 1- and 3-month follow-ups after MWA were larger than those of the original tumor recorded before MWA (p < 0.05 for all). However, at 6, 9, 12, and 18 months after MWA, the sizes of the ablation zone were smaller than that of the original tumor recorded before MWA (p < 0.05 for all).
Table 4. Tumor size (maximum diameter and volume) before MWA and at each follow-up time-point after MWA.
At the last follow-up, the tumors in 24 (70.6%) patients had completely disappeared on US examination. Three tumors (8.8%) disappeared within 6 months, 9 (26.5%) within 9 months, 10 (29.4%) within 12 months, and 2 (5.9%) within 18 months. Ten (29.4%) tumors did not completely disappear, but no tumor displayed any regrowth of the residual ablated lesion during the follow-up period.
None of the patients showed local tumor recurrence, new PTC lesions, distant metastasis, or mortality from PTC during the follow-up time. No patients needed additional surgery for the primary PTC.
Complications of US-guided MWA for isthmic PTC
In this study, no major or minor complications developed. Five (14.7%) patients had side effects after MWA, including mild pain (n = 1) and subcutaneous edema (n = 4). All of the side effects resolved within 1 week without any specific therapy. No patient experienced a life-threatening or delayed complication during the follow-up period.
Discussion
Controversy regarding the optimal therapeutic strategy for PTC located in the isthmus has existed due to this unique anatomical location in close proximity to the trachea. However, to date, there has been no study focusing on the management of thyroid cancers originating in the isthmus of the thyroid gland with MWA. The present study was undertaken to assess the feasibility, efficacy, and safety of US-guided MWA therapy for patients with PTC located in the thyroid isthmus.
Most previous studies have supported total thyroidectomy as the standard surgical protocol for PTC tumors located in the isthmus [Citation2,Citation5–7,Citation16]. It is known that total thyroidectomy carries the risk of several temporary or permanent complications, including hypothyroidism, hypoparathyroidism, RLN injury, hemorrhage, and wound infection. Indeed, all patients who undergo total thyroidectomy require life-long thyroid hormone replacement after surgery, and an estimated 22% of patients treated by lobectomy develop biochemical hypothyroidism and require thyroid hormone therapy [Citation17]. Importantly, in the present study, no significant changes in thyroid function were observed from before to after MWA (p > 0.05), and thus, thyroid hormone replacement therapy was not needed in our patients. Permanent hypoparathyroidism is a serious common complication post thyroidectomy that can reduce patients’ quality of life and even be life-threatening in severe cases. A previous study reported that the incidence of permanent hypoparathyroidism post-thyroidectomy was 3% [Citation18]. In the present study, the iPTH level was not significantly different after MWA compared with before MWA (p > 0.05), indicating that our patients did not experience hypoparathyroidism after MWA. RLN injury sustained during thyroid surgery can lead to serious conditions such as voice disorders, respiratory distress, and aspiration. In previous studies, transient RLN injury rates have varied from nearly 0 to 15.4% and permanent injury rates have ranged from 0 to 5.2% [Citation19]. With MWA, no case of transient RLN injury occurred in the present study. A previous review of thyroid surgeries reported that the incidence of tracheal injury during thyroid surgery was 0.06% [Citation20], but no case of tracheal injury occurred in the present study. The rates of hematoma and wound infection reported in the literature have also varied, from 0.2–2.7% for hematoma and 0–1.1% for wound infection [Citation21–23], but no cases of hematoma and wound infection occurred in the present study. Therefore, the outcomes of the present study demonstrated that US-guided MWA is a safe treatment option for patients with isthmic PTC.
Thyroid isthmusectomy in the treatment of PTC is still controversial. Isthmusectomy respresents a more conservative approach compared with total thyroidectomy and usually avoids the classic complications of total thyroidectomy. Some previous studies reported that thyroid isthmusectomy might be effective in the surgical treatment of selected cases of isthmic PTC [Citation3,Citation24–26]. With thyroid isthmusectomy, the incidence of hypothyroidism is reduced, as normal thyroid tissue is preserved, and there is a lower risk of nerve and parathyroid injury than with total thyroidectomy. Hee et al. [Citation25] reported that 9.1% (1/11) patients experienced hypothyroidism and transient hypoparathyroidism after isthmusectomy, but no cases of hypothyroidism and transient hypoparathyroidism occurred in the present study. In addition, isthmusectomy is performed under general anesthesia, and local scarring may occur postoperatively, which may affect the patient's esthetics. In contrast, US-guided MWA is performed under local anesthesia and causes no scarring. Therefore, the outcomes of the present study demonstrated that US-guided MWA may have some advantages in the treatment of isthmic PTC compared with isthmusectomy.
The reported incidence of PTC located in the thyroid isthmus among all PTC cases has varied from 1 to 12.3% [Citation2–4], and consistently, the percentage of patients with isthmic PTC was 8.7% among all PTC cases in our present study. The technical feasibility and success rate of MWA in our cohort were both 100% (34/34). Furthermore, the efficacy of US-guided MWA for isthmic PTC is supported by the tumor disappearance rate of 70.6% and the absence of local recurrence, new tumor formation, and local LNM in all of our patients. A previous meta-analysis including 12 studies of 1187 patients with PTC at any location reported the tumor disappearance rate varied from 34 to 91% after MWA [Citation27], and the tumor disappearance rate of 70.6% (24/34) observed in this study is within that range. The same meta-analysis found that the rate of LNM varied from 0.6 to 2.0% after thermal ablation [Citation27]. In the present study, there were no cases of LNM after MWA, nor were there any cases with local recurrence or new tumor formation during the follow-up. Therefore, the outcomes of the present study demonstrate that US-guided MWA is an effective treatment option for patients with isthmic PTC.
MWA offers several advantages for treating isthmic PTC, which may account for the high technique success rate and low rate of complications observed in this study. First, MWA only destroys the tumor and the surrounding safety boundary, allowing for preservation of most of the thyroid tissue. This explains why thyroid function was not affected in our patients after ablation. Second, the microwave antenna is guided by US to accurately puncture and destroy the tumor without damaging the parathyroid tissue on the dorsal side of the thyroid gland. Thus, it will not affect the function of the parathyroid gland. Third, isolation fluid is injected continuously and slowly during ablation, providing effective isolation to prevent heat injury to surrounding structures. This minimizes thermal damage to the RLN, trachea, and local skin. Fourth, the use of color Doppler US helps the surgeon to avoid puncturing the thyroid and its surrounding blood vessels, thereby reducing the occurrence of hematoma. Finally, MWA is a minimally invasive surgery, with only a needle track-sized incision. Therefore, the risk of wound infection is low, and no scar formation occurs after MWA.
The present study has a few limitations. First, the study was nonrandomized and retrospective, which may have caused selection bias. Second, the number of patients enrolled was small. Third, a few patients had a relatively short clinical follow-up time because primary PTC is a slow-growing disease. Longer observation for local recurrence, new tumor formation, LNM, and distant metastasis is still necessary.
In conclusion, the results of the present study indicate that US-guided MWA is a feasible, effective, and safe treatment option for selected patients with PTC located in the thyroid isthmus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Hahn SY, Han BK, Ko EY, et al. Ultrasound findings of papillary thyroid carcinoma originating in the isthmus: comparison with lobe-originating papillary thyroid carcinoma. AJR Am J Roentgenol. 2014;203(3):637–642.
- Lee YS, Jeong JJ, Nam KH, et al. Papillary carcinoma located in the thyroid isthmus. World J Surg. 2010;34(1):36–39.
- Nixon IJ, Palmer FL, Whitcher MM, et al. Thyroid isthmusectomy for well-differentiated thyroid cancer. Ann Surg Oncol. 2011;18(3):767–770.
- Lim ST, Jeon YW, Suh YJ. Correlation between surgical extent and prognosis in node-negative, early-stage papillary thyroid carcinoma originating in the isthmus. World J Surg. 2016;40(2):344–349.
- Karatzas T, Charitoudis G, Vasileiadis D, et al. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: a case control study. Int J Surg. 2015;18:64–68.
- Lei J, Zhu J, Li Z, et al. Surgical procedures for papillary thyroid carcinoma located in the thyroid isthmus: an intention-to-treat analysis. OTT. 2016;9:5209–5216.
- Vasileiadis I, Boutzios G, Karalaki M, et al. Papillary thyroid carcinoma of the isthmus: total thyroidectomy or isthmusectomy? Am J Surg. 2018;216(1):135–139.
- Cui T, Jin C, Jiao D, et al. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: a systematic review and meta-analysis. Eur J Radiol. 2019;118:58–64.
- Zhuo L, Peng LL, Zhang YM, et al. US-guided microwave ablation of hyperplastic parathyroid glands: safety and efficacy in patients with end-stage renal disease-a pilot study. Radiology. 2017;282(2):576–584.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab. 2020;105(6):1.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34(5):653–659.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Kumar A, Sinha C, Kumar A, et al. Assessment of functionality of vocal cords using ultrasound before and after thyroid surgery: An observational study. Indian J Anaesth. 2018;62(8):599–602.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Li G, Lei J, Peng Q, et al. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine. 2017;96(24):e7143
- Verloop H, Louwerens M, Schoones JW, et al. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. 2012;97(7):2243–2255.
- Păduraru DN, Ion D, Carsote M, et al. Post-thyroidectomy hypocalcemia - risk factors and management. Chirurgia. 2019;114(5):564–570.
- Rulli F, Ambrogi V, Dionigi G, et al. Meta-analysis of recurrent laryngeal nerve injury in thyroid surgery with or without intraoperative nerve monitoring. Acta Otorhinolaryngol Ital. 2014;34(4):223–229.
- Gosnell JE, Campbell P, Sidhu S, et al. Inadvertent tracheal perforation during thyroidectomy. Br J Surg. 2006;93(1):55–56.
- Rocke DJ, Mulder H, Cyr D, et al. The effect of lateral neck dissection on complication rate for total thyroidectomy. Am J Otolaryngol. 2020;41(3):102421.
- Efremidou EI, Papageorgiou MS, Liratzopoulos N, et al. The efficacy and safety of total thyroidectomy in the management of benign thyroid disease: a review of 932 cases. Can J Surg. 2009;52(1):39–44.
- Thomusch O, Machens A, Sekulla C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg. 2000;24(11):1335–1341.
- Skilbeck C, Leslie A, Simo R. Thyroid isthmusectomy: a critical appraisal. J Laryngol Otol. 2007;121(10):986–989.
- Seo HW, Song CM, Ji YB, et al. Surgical outcomes and efficacy of isthmusectomy in single isthmic papillary thyroid carcinoma: a preliminary retrospective study. J Invest Surg. 2020;13:1–6.
- Park H, Harries V, McGill MR, et al. Isthmusectomy in selected patients with well-differentiated thyroid carcinoma. Head Neck. 2020;42(1):43–49.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.