Abstract
Objective
To investigate the risk factors affecting the technical failure of artificial ascites (AA) formation and to evaluate the local control efficacy of percutaneous thermal ablation assisted by the AA for hepatic tumors.
Methods
A total of 341 patients with 362 hepatic tumors who underwent thermal ablation assisted by AA were reviewed retrospectively. The technical success of AA, the volume of liquid, and local efficacy after ablation were assessed. Predictive factors for the technical failure of AA formation and local tumor progression (LTP) were analyzed using univariate and multivariate analysis.
Results
The technical success rate of AA formation was 81.8% (296/362). The amount of fluid was higher when the tumor was located in the left lobe of the liver than when it was located in the right lobe (median 950 ml versus 700 ml, p < 0.001). Previous hepatic resection (OR: 12.63, 95% CI: 2.93–54.45, p < 0.001), ablation (OR: 6.48, 95% CI: 1.36–30.92, p = 0.019) and upper-abdomen surgery (OR: 11.34, 95% CI: 1.96–65.67, p = 0.007) were the independent risk factors of AA failure. In the AA success group, the complete ablation rate was higher and the LTP rate was lower than that in the AA failure group (98.7 versus 92.4%, p = 0.012; 8.8 versus 21.2%, p = 0.004). Multivariate analysis identified AA failure (p = 0.004), tumor size (>3.0 cm) (p = 0.002) and metastatic liver tumor (p = 0.008) as independent risk factors for LTP.
Conclusion
History of hepatic resection, ablation and upper abdomen surgery were significant predictive factors affecting the technical failure of AA formation. Successful introduction of AA before thermal ablation can achieve better local tumor control efficacy.
Introduction
Percutaneous thermal ablation has been widely accepted as an effective and safe minimally invasive treatment for malignant liver tumor [Citation1–4]. It can offer therapeutic outcomes similar to those after surgical resection in terms of overall and disease-free survival, especially for small liver tumors [Citation4–6]. However, there is a substantial risk of thermal injury to perihepatic structures such as the diaphragm and gastrointestinal tract when the index tumor is located close to these major structures. To overcome this problem, various strategies have been used to separate the liver from heat-vulnerable structures including artificial fluid [Citation7], balloon catheter interposition [Citation8] and laparoscopic or open approaches [Citation9,Citation10].
Recently, several experimental and clinical studies reported that thermal ablation assisted by artificial ascites (AA) can safely and effectively treat hepatic tumors abutting heat-vulnerable organs [Citation11–14]. This approach could enable an improved sonic view and protect adjacent vital organs from thermal injury induced by thermal ablation. However, introducing AA is not always successful, and the success rate reported ranged from 78 to 91% [Citation14–16]. To our knowledge, few clinical reports concerning the influencing factors of AA failure have been published.
Therefore, we conducted this study to elucidate the incidence of AA success and to evaluate the risk factors related to technical failure. Furthermore, we also investigated the effect of successfully introducing artificial ascites on the local control efficacy of thermal ablation.
Materials and methods
The study was approved by the institutional review board, and all patients signed an informed consent form before treatment in accordance with the clinical protocols.
Patients
In this retrospective cohort study, we reviewed the electronic medical records of consecutive patients who underwent AA assisted thermal ablation at our hospital from January 2015 to July 2018. The collection of follow-up data was terminated on 31 March 2020. All patients with HCC were confirmed according to pathology or the diagnostic algorithm of the American Association for the Study of Liver Disease [Citation1]. Patients with metastatic liver cancer (MLC) were diagnosed based on pathological diagnosis or new lesions after primary cancer resection with typical enhanced imaging manifestations of MLC and clinical follow-up. The inclusion criteria for patients who underwent liver thermal ablation in this study were (1) number of treated lesions ≤5; (2) maximum diameter of treated lesions ≤5 cm; (3) Child–Pugh class A or B; (4) platelet count >40 × 109/L; and (5) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 to tolerate the procedure. The exclusion criteria were recent upper gastrointestinal bleeding caused by portal hypertension, severe coagulation disorders, bleeding tendencies, and uncontrolled infections.
Artificial ascites technique
The detailed method of introducing AA was the same as described in the previous study [Citation14]. Following the administration of local anesthesia with 1% lidocaine to the skin, abdominal wall and peritoneum, an 18 GPTC needle was punctured into the abdominal cavity along the edge of the liver under ultrasonography guidance. The puncture point selected was at the right midaxillary line on the lower margin of the liver for lesions in the right lobe or at the upper abdomen on the left margin of the liver for lesions in the left lobe. The needle was inserted into the predetermined site along the edge of the liver, to avoid injury to the liver and adjacent organs. When the ultrasound showed that the needle was inserted into the peritoneal spaces, the inner stylet was removed, and a 5% glucose solution kept at room temperature was infused at a rate of 50 ml/min. The location of the needle tip in the peritoneal cavity could be assured when an enlarging anechoic area could be seen surrounding the liver contour with drips of the solution. When the sonic window was improved and the distance from the adjacent organ was more than 0.5 cm, we considered the induction of AA successful and performed the ablation procedure (). The procedure was judged as unsatisfactory if sufficient separation had not been obtained with 1500 ml solution () [Citation14]. During the ablation procedure, we could continue the infusion drip into the intraperitoneal cavity until the treatment was finished if the ascites flowed away.
Figure 1. (A) 65-year-old female with hepatocellular carcinoma. A CT prior to ablation demonstrates a hypoenhancing lesion during venous phase in segment 6. The tumor is close to the colon, thus thermal injury to the adjacent colon after thermal ablation is expected. (B) Ultrasound image shows the lesion is located close to the adjacent colon wall and partly protrudes from the liver capsule. (C) After introducing artificial ascites, a small amount of loculated fluid collection is made in the perihepatic space near the index tumor. (D) Microwave antenna was inserted into the target tumor under ultrasound guided. (E) One-month post-ablation CT demonstrates the ablation zone with complete response and no damage to surrounding colon.
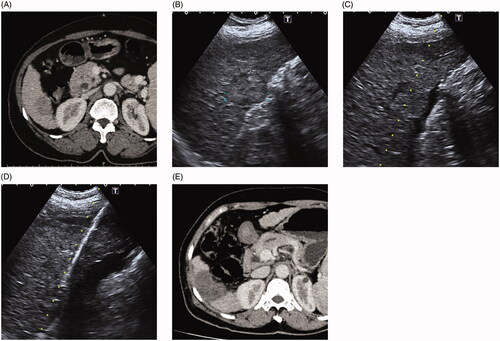
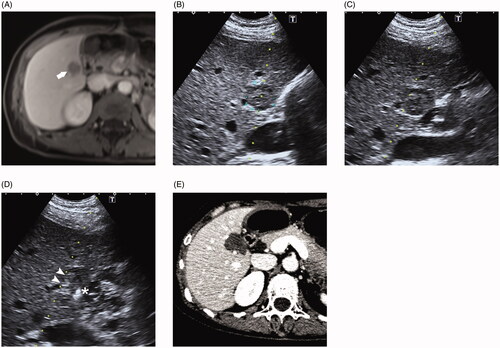
Figure 2. (A) 47-year-old female who had a history of hepatic resection for hepatocellular carcinoma shows a recurrence lesion in segment 6 demonstrated by MRI. The hepatic mass is close proximity to the duodenum. (B) Ultrasound reveals the lesion is in direct contact with adjacent duodenum. (C) After introducing artificial ascites, the duodenum is not completely separated from the contacting liver. (D) Radiofrequency ablation (RFA) combined with ethanol injection was performed to ablate this tumor. A PTC needle (asterisk) was inserted into the tumor margin proximal to the duodenum and the RFA electrode (arrowheads) was positioned 10 mm away from the PTC needle. (E) One-month post-ablation CT showed a complete ablation without duodenum injury.
Ablation procedure
A Cool-tip™ RFA system (Covidien, Mansfield, Mass) with a 17-gauge electrode and a 2 ∼ 3 cm exposed tip and a KY-2000 MWA system (Kangyou Medical, Nanjing, China) consisting of a 15-gauge needle antenna with a 1.1 cm exposed tip were used in this study. All ablation procedures were performed percutaneously with real-time ultrasonography guidance. A standard protocol for conscious analgesic sedation consisting of 0.1 mg of fentanyl, 5 mg of droperidol and 0.1 mg of tramadol hydrochloride was administered intravenously prior to ablation. Local anesthesia was applied with 1% lidocaine hydrochloride along with the planned puncture site. After successful AA was produced, the treatment was performed according to the standard ablation specification for the lesions (). In cases without the satisfactory introduction of AA, we performed thermal ablation assisted by small-dose ethanol injection. One or two 21-gauge PTC needles were placed into the tumor margin proximal to the perihepatic structures, and the RFA electrode was positioned 10 mm away from the PTC needle (). Before ablation, 99.5% sterile ethanol was slowly injected into the marginal tissue of the tumor. The therapeutic goal was to achieve an ablative margin of at least 0.5 cm in the normal liver tissue surrounding the tumor, with the exception of subcapsular and perivascular tumors. Vital signs were continuously monitored during the procedure. After ablation, we cauterized the electrode path during retraction of the electrode to minimize bleeding and track seeding. We did not aspirate or drain the infused artificial ascites after the ablation procedure. All AA and ablation procedures were performed by Xie XY, Kuang M, and Lu MD who had more than 10 years of clinical experience in performing percutaneous ablation.
Assessment of treatment response
Complications caused by the procedure were determined at the 1-month follow-up after ablation and were detected using ultrasound, CT or MRI and laboratory tests. Complications were assigned to major and minor categories in accordance with the Society of Interventional Radiology guidelines [Citation17].
Contrast-enhanced ultrasound (CEUS) was performed the following morning, providing an initial evaluation of the treatment effect. An additional treatment would be performed if any residue tumor was found. For an evaluation of the completeness of the ablation, contrast-enhanced computed tomography (CECT) was performed 1 month after the ablation. Complete ablation was defined as a lack of enhancement of the entire tumor with or without a peripheral enhancing rim. When the tumor was not ablated completely (when enhancement was still observed in part of the index tumor on CT images), ablation was repeated, as described above. If the tumor was still viable after additional ablation, the combined ablation was considered a failure, and the patient was referred for other therapies. In patients in whom complete ablation was achieved, each underwent follow-up every 3 months after treatment during the first 2 years, and every 6 months thereafter, with subsequent blood tests for liver function and tumor markers. In addition, either contrast-enhanced ultrasound (CEUS), CECT or MRI examinations were part of the follow-up protocol to assess local tumor progression (LTP). LTP was defined as the reappearance of tumor enhancement within or adjacent to the ablation zone [Citation17].
Statistical analysis
Demographic and clinical variables were compared between the AA success and failure groups. Categorical variables were compared by using the Chi-squared test or Fisher’s exact test, and continuous variables were compared by using the Mann–Whitney U test or Student’s t-test according to whether the normality assumption was met. Univariate and multivariate logistic regression analyses were performed to determine the influencing factors for predicting the technical failure of AA. The potential influencing factors considered were treatment history, tumor location and perihepatic structures based on previous studies. Prognostic factors for LTP were assessed by using univariate and multivariate Cox proportional hazards model. Potential prognostic factors for LTP included age, sex, tumor diagnosis, Child–Pugh class, ALBI grade, size of the index tumor, tumor marker level, perihepatic structures, tumor location, AA success and ablation technique. Survival curves were generated by the Kaplan–Meier method and compared by the log-rank test. A p-value less than 0.05 was considered to indicate a significant difference. Statistical analyses were performed by using MedCalc statistical software (version 10.2.0.0; MedCalc Software, Mariakerke, Belgium).
Results
Baseline characteristics
shows the flow diagram of the study population. A total of 341 patients with 520 tumors underwent thermal ablation assisted by AA during the study period. Among the 341 patients, 212 patients had HCC, 61 patients had MLC and 8 patients had intrahepatic cholangiocarcinoma (ICC). The primary sites of the MLC are described in the Supplemental Materials. Among these 520 tumors, 362 tumors in high-risk locations were ablated assisted by AA. There were 296 tumors in the AA success group and 66 tumors in the AA failure group.
The demographic and clinical data of patients in the AA success and failure groups are compared in . The two groups did not differ significantly for any variable except tumor location, treatment history, abdominal surgery site and perihepatic structures ().
Table 1. Baseline characteristics of the artificial ascites success group and failure group.
Technical success of inducing artificial ascites
The index tumor was successfully separated from the adjacent structure after introducing AA in 296 of 362 tumors (81.8%), as confirmed by ultrasound. Forty-five (28.6%) of 157 patients with technical failure of AA formation had a history of hepatic resection, and 5 (22.7%) of 22 patients with technical failure of AA formation had a history of Upper-Abdomen surgery (). In the AA success group, the median volume of the introduced solution was much lower when the tumor was located in the right lobe than in the left lobe of the liver (700 ml versus 950 ml, p < 0.001) (). Among the potential predictive risk factors for the technical failure of AA formation, a previous history of hepatic resection (OR: 12.63, 95% CI: 2.93–54.45, p < 0.001), liver ablation (OR: 6.48, 95% CI: 1.36–30.92, p = 0.019) or upper abdomen surgery (OR: 11.34, 95% CI: 1.96–65.67, p = 0.007) were significant influencing factors after multivariable logistic regression (). However, the previous history of lower abdomen surgery was not a risk factor for AA failure (OR: 3.20, 95% CI: 0.50–20.59, p = 0.221).
Table 2. Success rate of artificial ascites creation and volume of ascites in different hepatic locations.
Table 3. Independent risk factors associated with artificial ascites failure.
Local tumor control
Ablation success, based on 1-month follow-up imaging after ablation, was achieved in 353 of 362 nodules (97.5%). Five nodules that had treatment failure after ablation were in the AA failure group and 4 nodules in AA success group, with a significant difference between the two groups (92.4 versus 98.7%, p = 0.012) (). Among these 9 nodules, 3 underwent hepatic resection, and the remaining 6 nodules were treated with percutaneous ethanol injection. All nine tumors achieved a complete response after additional treatment.
Table 5. Subgroup analysis for local tumor progression rate and complete response rate.
The median follow-up period was 21.0 months (range, 1.0–59.0 months) in the AA success group and 22.0 months (range, 2.0–60.0 months) in the AA failure group. shows the LTP curves of the entire sample. LTP occurred in 26 of 296 nodules (8.8%) in the AA success group and in 14 of 66 patients (21.2%) in the AA failure group (p = 0.004). The 1-, 2-, and 3-year cumulative LTP rates were 7.4, 8.5 and 8.8%, respectively, in the AA success group and 18.2, 19.7 and 21.2%, respectively, in the failure group; there was a significant difference between the two groups (p = 0.004, , ). In the univariable analysis, patient age (p = 0.034), MLC (p = 0.011), tumor size (p = 0.001) and AA failure (p = 0.006) were significant factors in terms of LTP. However, multivariable Cox proportional hazard analysis revealed MLC (p = 0.008), tumor size (p = 0.002) and AA failure (p = 0.004) as significant prognostic factors for LTP among the studied variables (). In the subgroup analysis, the LTP rates at 1, 2, and 3 years were 7.2, 7.8, and 8.5%, respectively for tumor sizes ≤3.0 cm and 25.6, 30.2, and 30.2%, respectively for tumor sizes between 3.1 and 5.0 cm; the difference was statistically significant (p < 0.001, , ). The LTP rates at 1, 2, and 3 years were 7.4, 8.9, and 9.2%, respectively, for HCC and 16.9, 16.9, and 18.1%, respectively, for MLC (), with a significant difference (p = 0.004, ).
Figure 4. (A) Cumulative incidence of local tumor progression of full cohort and stratified as per (B) artificial ascites status (success and failure) (C) tumor size (≤3 cm and >3 cm), and (D) tumor type (HCC and MLC).
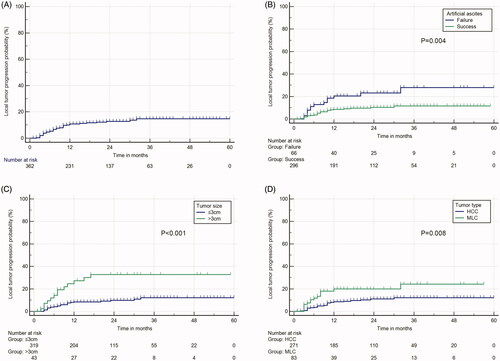
Table 4. Independent risk factors associated with local tumor progression.
Complications
There were no severe electrolyte derangements, peritonitis, peritoneal bleeding or gastrointestinal tract injury events directly associated with the AA technique and there were no cardiopulmonary complications due to volume overload. The AA was partially shifted into the right pleural space in 15 (4.4%) of 341 patients as depicted on ultrasound images one day after ablation, and 6 patients underwent drainage. All patients showed complete absorption of AA and a shifted pleural effusion, as confirmed on 1-month follow-up images. The rate of major complications was 1.1% (three of 281 patients) in the AA success group and 1.7% (one of 60 patients) in the AA failure group. The major complication rates were not significantly different between the two groups (p = 0.541). In the AA success group, three major complications were observed in different patients. One patient developed a liver abscess and underwent percutaneous drainage and intravenous antibiotics. Severe hepatic bleeding occurred in one patient and hemostasis was successfully achieved after percutaneous ablation. One patient experienced acute pulmonary infection and recovered after antibiotic therapy. In the AA failure group, major complications occurred in one patient. The patient experienced colon perforation and recovered after emergency surgery. No AA or ablation related deaths occurred in either group.
Discussion
This large-scale study demonstrated that a history of hepatic resection, ablation, and upper abdominal surgery was the main cause of the unsuccessful introduction of AA. In addition, a larger volume of AA was needed when the tumor is located in the left lobe of the liver. Failure to separate hepatic tumor and perihepatic organs after the induction of AA are associated with poor local effectiveness.
It is generally believed that abdominal adhesions caused by previous abdominal surgery are one of the major limitations for introducing AA into the perihepatic space. In the present study, a previous history of hepatic resection, ablation and upper abdominal surgery was the sole risk factor associated with technical failure of AA formation. Laparotomy can cause destruction of the mesothelium, increased synthesis of fibrinolysis antagonists, hypoxia, and radical formation or bacterial infection, which may promote postoperative adhesion formation [Citation18,Citation19]. However, considering the pathogenesis of adhesions, which result from surgical trauma, they may be localized to the corresponding surgical field [Citation20]. Therefore, unlike the history of upper abdominal or hepatic surgery, a previous history of lower abdominal surgery was not related to technical failure of AA formation, which was confirmed by our multivariate analysis. Previous studies reported that peritoneal adhesions can be related to a history of TACE, which may cause unsuccessful induction of AA [Citation15,Citation21]. However, our study did not find a relationship between a previous history of TACE and the technical failure of AA formation. The reason might be that mild and local adhesions between the hepatic capsule and peritoneum caused by TACE may not be a substantial obstacle to filling the peritoneal free space with AA.
Most previous studies considered that the amount of AA introduced was not to exceed 1500 ml to avoid excessive fluid load. However, the median amount of liquid has not been clearly reported for tumors located in different hepatic locations. We demonstrated here that when the tumor was located in the left lobe of the liver, the AA success rate was lower and a larger amount of liquid was needed. The underlying reasons might be as follows. First, the posterior part of the perihepatic space is usually narrower than the anterior or lateral part, as the liver is attached to the bare area by triangular ligaments [Citation14,Citation22]. Massive amounts of AA around the bare area will be needed for tumors located on segment 4 of the liver in the hepatic dome. Second, for the tumor in the left external lobe of the liver (segments 2 and 3), it was difficult for the fluid to gathered in this perihepatic space, as the fluid can easily flow away into the hepatorenal space and perisplenic space. Thus, the amount of AA is usually variable depending on the location of the liver.
Complete ablation was achieved more frequently in the AA success group (98.7% 292/296) than in the AA failure group (92.4% 61/66), and the LTP rate was higher in the AA failure group (21.1%, 14/66) than in the AA success group (8.8%, 26/296). Multivariate analysis showed that AA failure was an independent risk factor associated with LTP (HR 0.38, 95% CI: 0.20–0.73). These results indicated that successfully introducing AA achieved a relatively optimistic clinical outcome. There might be two explanations for this benefit. First, AA improves ultrasonographic image clarity, allowing operators to perform accurate electrode placements to ensure complete ablation of larger tumors, which is crucial to delay tumor progression [Citation23]. Second, the insulating effect of the ascites allows for a more aggressive ablation procedure that may help achieve optimal ablation margins, whereas tumors in AA failure cases were treated with significantly lower power and less time due to operator caution prompted by the high-risk location [Citation23,Citation24]. Although we performed thermal ablation combined with ethanol injection for AA failure cases, it is still failed to obtain the same effect as AA success cases. Further studies are needed to improve the local efficacy for AA failure tumors, especially for MLC which is not proved to be efficient by using combined ablation.
Other factors associated with LTP were tumor size and tumor type. The current results showed that the frequency of LTP occurrence for ≤3.0 cm and 3.1–5.0 cm tumors was 8.5 and 30.2% (p < 0.001), respectively. Tumor size was an independent prognostic factor of LTP after thermal ablation, which may be related to larger tumors tending to have isolated septa and satellite nodules [Citation25]. Tumor type is another important factor influencing LTP. This finding was consistent with that noted in a previous report on LTP in different tumor types after thermal ablation [Citation5,Citation26]. Some of this discrepancy between HCC and MLC might be explained as follows: first, peri-metastasis and infiltration of cancer cells occurred more frequently in CRLM lesions than in HCC lesions [Citation27]. Second, HCC usually occurs in patients with hepatic fibrosis or cirrhosis. The fibrosis tissue around the tumor may behave like a thermal insulator, increasing the heat retention within the tumor and preventing heating outside the tumor, while for metastases without a thermal barrier, blood flow reduces the extent of the ablative lesion [Citation26,Citation28].
AA is generally safe and well-tolerated, and no major AA related major complications occurred in our study. Theoretically, ascitic fluid has been thought to wash away thrombogenic substances, reduce the ‘tamponade’ effect from the opposing parietal peritoneum against the liver and eliminate thrombogenic substances at the puncture site [Citation12]; however, only one patient in AA success group developed procedure-associated hemoperitoneum, and no needle track implantation was observed during follow-up. In the literature, AA did not increase the rate of bleeding at the puncture site or needle track implantation metastasis [Citation29,Citation30]. Electrode tract thermocoagulation can induce a hemostatic effect, thereby accounting for the rarity of these types of complications. The major concern regarding the ablation of tumors at the liver margin is thermal damage to the adjacent organs. In the present study, no cases in the AA success group experienced major complications of diaphragmatic or gastrointestinal perforation. However, one case in the AA failure group experienced colon perforation, despite the use of an ethanol injection combined with thermal ablation. This result confirms that AA between the liver and surrounding organs can insulate thermal energy transmission and effectively protect surrounding organs from heat damage.
Despite these promising outcomes, there were several limitations in the present study. First, there were inherent limitations, as this was a retrospective single-center study. Because of our institution’s significant experience with thermal ablation for liver tumors, lower residual tumor and LTP rates were observed. Therefore, a prospective multicenter study is needed. Second, because the tumor pathologies were heterogeneous and comprised HCC as well as various types of MLC, this study exclusively focused on the local therapeutic effect. Other long-term outcomes such as overall survival or progression-free survival are needed to assess the treatment effect of AA assisted thermal ablation. Third, although the AA was created using a PTC needle, the method of creating AA is not standardized worldwide and can vary according to the preference of the operator at each institution.
In conclusion, our findings indicated that history of previous upper abdomen surgery and history of hepatic resection and ablation was the significant predictive factor for the technical failure of AA formation. The successful introduction of AA before thermal ablation can achieve better local tumor control efficacy.
Supplemental Material
Download PDF (19.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- Cirocchi R, Trastulli S, Boselli C, et al. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Db Syst Rev. 2012;6(6):D6317.
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
- Di Martino M, Rompianesi G, Mora-Guzmán I, et al. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur J Surg Oncol. 2020;46(5):772–781.
- Liu M, Huang G, Xu M, et al. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia. 2017;33(4):446–453.
- Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection-propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919.
- Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int. 2015;9(4):514–519.
- Yamakado K, Nakatsuka A, Akeboshi M, et al. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14(9):1183–1186.
- Simo KA, Sereika SE, Newton KN, et al. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104(7):822–829.
- Hirooka M, Kisaka Y, Uehara T, et al. Efficacy of laparoscopic radiofrequency ablation for hepatocellular carcinoma compared to percutaneous radiofrequency ablation with artificial ascites. Dig Endosc. 2009;21(2):82–86.
- Wang Y, Zhang L, Li Y, et al. Computed tomography-guided percutaneous microwave ablation with artificial ascites for problematic hepatocellular tumors. Int J Hyperthermia. 2020;37(1):256–262.
- Hsieh Y, Limquiaco JL, Lin C, et al. Radiofrequency ablation following artificial ascites and pleural effusion creation may improve outcomes for hepatocellular carcinoma in high-risk locations. Abdom Radiol. 2019;44(3):1141–1151.
- Zhang M, Liang P, Cheng ZG, et al. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia. 2014;30(2):134–141.
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95(8):996–1004.
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19(11):2630–2640.
- Rhim H, Lim HK, Kim YS, et al. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190(1):91–98.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–1705.
- DiZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7(6):547–555.
- Brüggmann D, Tchartchian G, Wallwiener M, et al. Intra-abdominal adhesions: definition, origin, significance in surgical practice, and treatment options. Dtsch Arztebl Int. 2010;107(44):769–775.
- Corona R, Verguts J, Schonman R, et al. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95(4):1224–1228.
- Seki S, Sakaguchi H, Hagihara A, et al. Transcatheter arterial chemoembolization for superficial hepatocellular carcinoma induces adhesion. Adv Med Sci. 2007;52:66–70.
- Iwai S, Sakaguchi H, Fujii H, et al. Benefits of artificially induced pleural effusion and/or ascites for percutaneous radiofrequency ablation of hepatocellular carcinoma located on the liver surface and in the hepatic dome. Hepatogastroenterology. 2012;59(114):546–550.
- Bhagavatula SK, Chick JFB, Chauhan NR, et al. Artificial ascites and pneumoperitoneum to facilitate thermal ablation of liver tumors: a pictorial essay. Abdom Radiol. 2017;42(2):620–630.
- Kitchin D, Lubner M, Ziemlewicz T, et al. Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia. 2014;30(5):299–305.
- Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95(9):1931–1937.
- Yu J, Liang P, Yu X, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25(4):1119–1126.
- Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249(1):364–374.
- Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008;15(10):2757–2764.
- Hakimé A, Tselikas L, Otmezguine Y, et al. Artificial ascites for pain relief during microwave ablation of subcapsular liver tumors. Cardiovasc Intervent Radiol. 2015;38(6):1557–1562.
- Kang TW, Lim HK, Lee MW, et al. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013;68(12):e641–e651.