Abstract
Objective
To investigate the long-term clinical outcomes of patients with adenomyosis treated by high-intensity focused ultrasound (HIFU).
Materials and methods
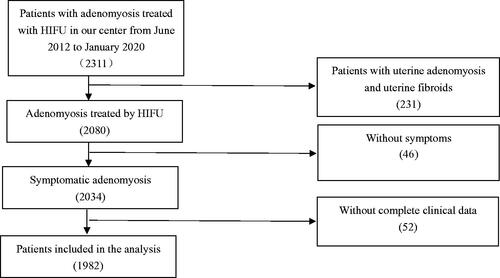
From June 2012 to January 2020, 2311 patients with adenomyosis were treated with HIFU at our center, 1982 patients who have complete clinical data were retrospectively reviewed. Among the patients who completed the follow-up, 485 were treated with HIFU alone, 289 were treated with HIFU followed by GnRH-a, 255 were treated with HIFU combined with Mirena and 594 were treated with HIFU combined with GnRH-a and Mirena. The dysmenorrhea severity pain score and average menorrhagia severity score before and at 3 months, 6 months, 1 year, 2 years, 3 years and 5 years after HIFU were compared. The adverse effects were recorded. In addition, the efficacy between patients treated with GnRH-a and/or Mirena were compared.
Results
After HIFU ablation, the dysmenorrhea severity pain score and the menorrhagia severity score were significantly decreased at each follow-up time point. However, it was observed that as the follow-up time increased, the effective rate of HIFU treatment in improving dysmenorrhea and menorrhagia decreased. The 6 months and 3 years follow-up results showed that the efficacy of HIFU combined with Mirena and HIFU combined with GnRH-a and Mirena were significantly higher than HIFU alone and HIFU combined with GnRH-a (p < 0.05). The major complications were rare.
Conclusion
HIFU is a safe and effective treatment for patients with adenomyosis. HIFU combined with Mirena or HIFU combined with GnRH-a and Mirena can significantly enhance the long-term treatment results.
Introduction
Adenomyosis is a common gynecological disease characterized by the presence of endometrial glands and stroma within the myometrium [Citation1]. Adenomyosis is more likely to affect women older than 30 years old, and it is reported that the mean frequency at hysterectomies is between 20% and 30% [Citation1–2]. Although women with adenomyosis may be asymptomatic, there are still approximately 70% of the patients suffering from the pelvic pain (including dysmenorrhea, dyspareunia and chronic pelvic pain), abnormal uterine bleeding (AUB) and impaired reproductive potential [Citation3–5]. In addition, due to its estrogen-dependent nature, treatment of adenomyosis, especially uterine sparing therapy, is often limited in effectiveness and has a high recurrence rate. In the past two decades, various uterine-sparing treatment modalities for adenomyosis have emerged, and among these methods, high-intensity focused ultrasound (HIFU) has become a promising option. Although many studies have shown that HIFU is safe and effective in the treatment of adenomyosis [Citation6–9], there is still a lack of studies with large sample sizes and long-term follow-up results. In this study, we aimed to retrospectively analyze the clinical and follow-up data of patients with symptomatic adenomyosis treated by HIFU in our center over the last 7 years to further investigate the long-term results of HIFU treatment for adenomyosis.
Materials and methods
The ethics committee of the Third Xiangya Hospital approved this study. All patients signed an informed consent form before HIFU treatment.
Patients
From June 2012 to January 2020, 2311 patients with adenomyosis were treated in the Third Xiangya Hospital, Central South University. Among them, 1982 patients with symptomatic adenomyosis who had complete clinical data were retrospectively reviewed in this study ().
The inclusion criteria were: (1) the diagnosis of adenomyosis was based on clinical evaluation, ultrasound and magnetic resonance imaging (MRI) (); (2) all patients had symptoms of dysmenorrhea and/or menorrhagia; (3) adenomyotic lesions were suitable for HIFU treatment based on ultrasound examination; (4) patients could communicate with the nurse or physician during the HIFU procedure;
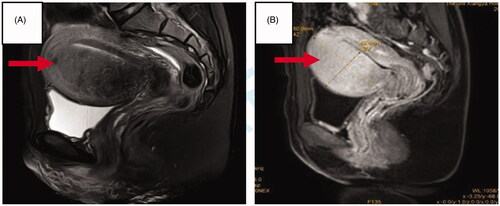
Figure 2. MR images obtained from a patient with adenomyosis. (A) T2WI obtained before HIFU showed the adenomyotic lesion located at anterior wall of the uterus (arrow); (B) Contrast-enhanced MRI showed perfusion of the adenomyotic lesion (arrow).
The exclusion criteria were: (1) adenomyotic lesion could not be visualized or could not be reached by the focused ultrasound; (2) patients during pregnancy, lactation or menstruation; (3) patients with acute pelvic inflammatory disease; (4) patients with suspected or confirmed uterine malignancy; (5) patients with uterine fibroids demanding treatment.
Preparation before HIFU ablation
Before HIFU ablation, all patients received specific bowel preparation for 3 days, including ingesting semi-liquid for 2 days, followed by liquid food the day before HIFU treatment, and a mandatory enema the morning of HIFU treatment. Skin preparation included shaving, degreasing and degassing the skin of the anterior abdominal wall from the umbilicus to the upper margin of the pubic symphysis. In order to optimize the acoustic pathway, a urinary catheter was inserted to control the size of the bladder through infusion of warm saline during the treatment. If necessary, suction curettage was performed under hysteroscopic guidance to rule out endometrial disease.
HIFU ablation
The procedure of HIFU treatment was performed under sedation and analgesia (Fentanyl at 0.8–1μg/kg administered at 30–40 min intervals; midazolam hydrochloride, at 0.02–0.03 mg/kg, administered at 30–40min intervals) using JC-200 focused ultrasound tumor therapeutic system (Chongqing Haifu Tech Co., Ltd., Chongqing, China). A transducer measuring 20 cm in diameter, with a focal length of 12 cm, was used to produce therapeutic ultrasound energy. An ultrasound imaging probe (My-Lab70, Esaote, Italy), situated in the center of the transducer, was used to monitor the response of the adenomyotic lesion to HIFU during the procedure. Every patient was carefully positioned prone on the HIFU treatment table with the anterior abdominal wall in contact with degassed water. The sagittal view of the ultrasound scanning mode was selected, and the treatment plan was made by dividing the lesion into different slices with a thickness of 5 mm each. The ablation procedure began from the innermost slice, and the focal point at least 1.5 cm away from the endometrium and 1 cm from the margin of the lesion. The site and intensity of the treatment were adjusted according to the patient’s reactions and the grayscale changes. Contrast-enhanced ultrasound was performed to evaluate the ablation volume and non-perfused volume (NPV) ratio of adenomyotic lesions (). The ablation volume was calculated according to the ellipsoid volume formula, V = (A × B × C)/6, A, B and C are the long diameter, wide diameter and thickness diameter of the lesion respectively. NPV ratio (%) = NPV/Adenomyotic lesion ×100%. After HIFU ablation, cold saline was used to wash the bladder, and NSAIDs and antibiotics were prescribed as needed. All patients were required to stay in the hospital for observation of the side effects until 24 h after treatment. The hospital stay should be extended if necessary.
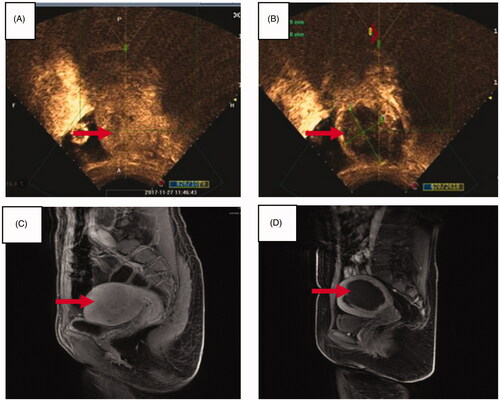
Figure 3. Pre- and post-HIFU contrast-enhanced ultrasound images and MR images obtained from a patient with adenomyosis. (A) Pre-HIFU contrast-enhanced ultrasound image showed perfusion of adenomyotic lesion (arrow); (B) post-HIFU contrast-enhanced ultrasound image showed non-perfused area (arrow); (C) pre-HIFU contrast-enhanced MRI showed perfusion of the adenomotic lesion (arrow); (D) post-HIFU MR image showed the non -perfused area (arrow).
Adjuvant therapy after HIFU
In order to consolidate the efficacy of HIFU in the treatment of adenomyosis, some patients received adjuvant therapy after HIFU, which included:
Adjuvant treatment using GnRH-a: for the patients whose uterus were enlarged (greater than 10 weeks of gestation) or the length of the uterine cavity was more than 9cm, GnRH-a was recommended after HIFU ablation. The first injection of GnRH-a was given on the first day of the first menstruation following HIFU treatment, with an interval of 28 days. Based on the size of the uterus, 3–6 courses of injection of GnRH-a were administered.
Adjuvant treatment using Mirena: If the patient had no fertility requirement, Mirena was inserted 1-3 months after HIFU.
Adjuvant treatment using GnRH-a + Mirena: For those patients with uterus greater than 10 weeks of gestation, GnRH-a was administered before the insertion of Mirena to reduce the uterine volume.
Follow-up
After HIFU ablation, the patients were required to follow up at 1 month, 3 months, 6 months and then every 12 months to assess for side effects and improvement of the symptoms.
The visual analog scale (VAS) score was used to evaluate the degree of the dysmenorrhea [Citation10]. The left end of the VAS was scored as 0 to represent ‘no pain’, while the right end was scored as 10, representing the ‘most severe pain imagined’. If the VAS score decreased by more than 20% after HIFU treatment, then the treatment was defined as effective for dysmenorrhea.
The menorrhagia was categorized by a 5-point categorical scale [Citation11]. In this categorical scale, 1 indicates not affected; 2 indicates a little increased; 3 indicates somewhat increased; 4 indicates greatly increased; 5 indicates very greatly increased. Because menorrhagia in the range of 1–2 often has no effect on patients, we defined the menorrhagia severity score decrease from 3–5 to lower than 2 as effective.
If patients became pregnancy, developed menopause or carried out other treatments except GnRH-a and/or Mirena for adenomyosis, the subsequent follow-up results were not included in the statistical analysis.
Statistical analysis
SAS 9.4 was used for statistical analysis. Data were presented as mean ± standard deviation or frequency (proportion). The score of dysmenorrhea and menorrhagia before and after HIFU ablation was compared by Wilcoxon signed rank test. A Chi-square test was employed to compare the efficacy between different treatment groups, and Bonferroni method was used to adjust p values. A significant difference was defined as a two-sided p value <0.05.
Results
Baseline characteristics
The baseline characteristics of the 1982 patients enrolled in this study were summarized in . The mean age was 40.6 ± 5.5 years old. The mean body mass index (BMI) was 22.6 ± 3.0 kg/m2. The average size of the uterus before HIFU ablation was 220.1 ± 118.4 cm3. The average size of the adenomyotic lesions before HIFU ablation was 72.7 ± 66.1 cm3. 36.3% of the lesions were classified as focal adenomyosis and 63.7% were classified as diffuse adenomyosis ().
Table 1. Baseline characteristics of patients.
Peri-HIFU evaluation
All the patients completed HIFU ablation for adenomyotic lesions in one session. However, 12 patients received a second session of HIFU treatment because of recurrence of symptoms. The averaged sonication power used was 387.4 ± 27.0 W and the averaged sonication energy used was 199,139.5 ± 171,825.2 J. The mean treatment time was 63.0±30.6 min, and the mean sonication time was 492.6 ± 333.9s. During the HIFU ablation procedure, massive gray scale change was observed in 66.1% of the adenomyotic lesions, and uniform gray scale change was observed in 33.9% of the lesions. Contrast-enhanced ultrasound showed NPV in all of the lesions with an average NPV ratio of 64.5 ± 20.4% ().
Table 2. HIFU treatment parameters.
Follow-up results
In this study, the last follow-up data were collected at the end of August 2020. Among the 1982 patients, all of them should have completed 3-month and 6-month follow-ups, 1761 patients should have completed the 1-year follow-up, 1357 patients should have completed the 2-year follow-up, 993 patients should have completed the 3-year follow-up and 429 patients should have completed the 5-year follow-up. But in fact, 1623 patients completed their 3-month follow-up, 1622 patients completed their 6-month follow-up, 1416 patients completed their 1-year follow-up, 992 patients completed their 2-year follow-up, 597 patients completed their 3-year follow-up, and 179 patients completed their 5-year follow-up, with 359, 360, 345, 365, 396, and 250 patients were lost to 3 months, 6 months, 1 year, 2 years, 3 years, and 5 years follow-up, respectively. Fifty cases reported pregnancy, 103 cases reported menopause, and 156 cases had re-intervention during the follow-up period ().
Table 3. Summary of patients’ follow-up details.
Symptom improvement
Among the patients enrolled in this study, 1484 patients complained of dysmenorrhea before HIFU treatment. As shown in , the average dysmenorrhea severity pain scores in every follow-up time point after HIFU ablation were significantly decreased than that before HIFU ablation (p < 0.001). According to the criteria for effectiveness of dysmenorrhea, the effective rates of HIFU ablation for dysmenorrhea at 3 months, 6 months, 1 year, 2 years, 3 years, and 5 years were 89.0%, 82.7%, 74.8%, 74.2%, 74.2%, and 65.8%, respectively ().
Table 4. Efficacy of HIFU ablation for dysmenorrhea in patients with adenomyosis.
Among the enrolled patients in this study, 793 patients complained of menorrhagia with a menorrhagia severity score equal to or greater than 3. As shown in , the average menorrhagia severity scores in every follow-up time point after HIFU ablation were significantly decreased than that before HIFU ablation (p < 0.001). According to the criteria for effectiveness, the effective rates at 3 months, 6 months, 1 year, 2 years, 3 years, and 5 years after HIFU were 85.4%, 78.5%, 72.7%, 73.9%, 72.4%, and 68.4%, respectively ().
Table 5. Efficacy of HIFU ablation for menorrhagia in patients with adenomyosis.
Evaluation of adjuvant therapies after HIFU
Based on the different adjuvant therapies, the patients were divided into 4 groups: HIFU alone (H), HIFU combined with Marina (H + M), HIFU combined with GnRH-a (H + G), and HIFU combined with GnRH-a and Mirena (H + G+M). Among the patients who completed the follow-up, 485 were treated with HIFU alone, 289 were treated with HIFU followed by GnRH-a, 255 were treated with HIFU combined with Mirena, and 594 were treated with HIFU combined with GnRH-a and Mirena.
As shown in , the 6-month effective rates for dysmenorrhea in H + G, H + M, H + G+M and H group were 70.9%, 91.6%, 95.6%, and 69.0%, respectively. The 3-year effective rates for dysmenorrhea in H + G, H + M, H + G+M and H group were 51.3%, 88.6%, 90.6%, and 56.3%, respectively. Compared with H and H + G groups, the effective rates of H + M and H + G+M groups were significantly higher (p < 0.05) ().
Table 6. Comparison of the effective rates among patients treated with different adjuvant therapies.
And the 6-month effective rates for menorrhagia after HIFU in H + G, H + M, H + G+M and H group were 59.6%, 96.1%, 94.2%, and 53.5%, respectively. The 3-year effective rates for menorrhagia after HIFU in H + G, H + M, H + G+M and H group were 36.0%, 88.6%, 87.5%, and 48.5%, respectively. Compared with H and H + G groups, the effective rates in H + M and H + G+M groups were significantly higher (p < 0.05) ().
Adverse effects
HIFU treatment was performed under conscious sedation with a Ramsay Score around 4 points, and the patients tolerated HIFU treatment well. The most common intraprocedural side effects included pain in the treatment area, transient leg pain, inguinal pain, sacrococcygeal pain, with an incidence of 88.3% (1750/1982). A burning sensation of the skin in the lower abdomen occurred in 469 patients, but no skin burns occurred. In addition, three patients reported small amounts of vaginal bleeding and four cases of drastic changes in blood pressure were observed. Based on the Society of Interventional Radiology (SIR) classification system, all of these adverse effects were classified as minor complications (class A) and resolved without any treatment ().
Table 7. Adverse effects of HIFU.
Three months after HIFU treatment, we observed these adverse effects: 521 patients complained of mild lower abdominal pain, 190 patients had mild sacrococcygeal pain, 17 had low-grade fever, 48 cases reported abnormal vaginal discharge and 9 cases had lower limb numbness or pain. We encountered 1 case of lower limb movement disorder (class C), 1 case of urinary retention (class C), 2 cases of vaginal bleeding caused by endometrial injury (class D) and 1 case of bowel perforation (class D). The major complications (class C above) accounted for 0.63% of all the adverse effects after HIFU ablation ().
Discussion
Uterine-sparing treatment of adenomyosis has been an intense challenge. Surgical removal of adenomyosis often results in large trauma and high incidence of recurrence due to ill-defined margins of adenomyotic lesions. Therefore, gynecologists have been seeking a new therapeutic technique with minimal trauma in the treatment of adenomyosis. As a noninvasive therapeutic technique, HIFU has been widely used in the treatment of adenomyosis in recent years. The therapeutic mechanism of HIFU treatment is to focus the ultrasound beams generated by a transducer outside the body on the targeted lesion in the body. The mechanical effect of ultrasound is converted into thermal and cavitation effects, so that the temperature of the tumor tissue at the focal point rises to above 60 °C instantly and coagulative necrosis occurs without damaging the surrounding structures [Citation12]. The goal of HIFU treatment in the management of adenomyosis is to selectively ablate the adenomyotic lesion, thus to reduce the size of the uterus and to achieve symptom relief.
Dysmenorrhea and menorrhagia are often the primary causes of treatment for adenomyosis [Citation1]. Several clinical studies have indicated that HIFU is an effective treatment in improving the symptoms of dysmenorrhea and menorrhagia of patients with adenomyosis. Zhang et al. retrospectively reviewed 202 patients with adenomyosis who underwent ultrasound-guided HIFU, and the results showed that the dysmenorrhea severity pain score and the menorrhagia severity score of patients with adenomyosis decreased significantly after HIFU (p < 0.001) [Citation13]. Recently, a systematic review and a meta-analysis also showed a significant reduction in dysmenorrhea 3 months (SMD = 1.83) and 12 months (SMD = 2.37) after HIFU ablation, and a significant improvement in quality of life at 6 months (SMD = 3.0) and 12 months (SMD = 2.75) [Citation8]. This phenomenon may be caused by inducing coagulation necrosis of adenomyotic lesions and reducing the volume of the uterus. In addition, HIFU may also decrease the local inflammatory factors and the expressions of VEGF [Citation14–Citation16] in adenomyotic lesions, which are considered the causes of dysmenorrhea and menorrhagia in adenomyosis [Citation17–19]. In this study, among the 1623 patients who completed the 3-month follow-up, 1484 patients had dysmenorrhea, and 793 patients complained of menorrhagia with a menorrhagia severity score equal to or greater than three points. Our results showed that the dysmenorrhea pain score and the menorrhagia severity score significantly decreased after HIFU. In addition, HIFU has a relatively high short-term and long-term efficacy for dysmenorrhea and menorrhagia. The effective rate for these two symptoms was over 85% in the 3-month and 65% in the 5 years follow-up after treatment. These findings demonstrated that HIFU is effective in relieving dysmenorrhea and menorrhagia.
However, in this study, we noted that HIFU treatment had a relatively poor long-term efficacy in treating adenomyosis. The results showed that with the extension of follow-up time, the effective rate of HIFU treatment in relieving symptoms of dysmenorrhea and menorrhagia gradually decreased. So, in clinical practice in order to maintain the efficacy of HIFU treatment and reduce the recurrence rate, HIFU combined with GnRH-a and/or Mirena was adopted. In this study, we compared the effective rates in dealing with dysmenorrhea and menorrhagia in patients using different post-HIFU adjuvant therapies. The results showed that the H + M and H + M+G group had higher effective rates than the other groups. These indicated that Mirena as a post-HIFU adjuvant therapy can significantly improve the treatment results of HIFU for adenomyosis. The role of GnRH-a was limited, as it seemed to only improve the short-term efficacy and did not improve the long-term treatment results. Therefore, for patients with adenomyosis without fertility requirements, HIFU treatment should be combined with Mirena for as long as possible. If the uterus is too big to insert the Mirena directly, GnRH-a can be used before Mirena insertion. For patients who want to conceive, GnRH-a is recommended after HIFU treatment. However, patients should be informed of the poor long-term efficacy combined with GnRH-a alone and should be guided to get pregnant as soon as possible after treatment.
Safety is always a main concern. In this study, we found the main adverse effects during and after the HIFU treatment were classified as SIR class A/B. Major complications were rare, including 1 case of lower limb movement disorder (class C), 1 case of urinary retention, 2 cases of vaginal bleeding caused by endometrial injury (class D) and 1 case of bowel perforation (class D). The lower limb movement disorder may be explained by sciatic nerve injury; fortunately, she recovered in 6 months after taking neurotrophic drugs, non-steroidal anti-inflammatory drugs, and physical treatment. Urinary retention occurred in this patient because the bladder was excessively filed with normal saline during the procedure of HIFU treatment. The patient recovered after having an indwelling catheter for three days. Vaginal bleeding was caused by endometrial injury during HIFU treatment; avoiding excessive ablation is the key to minimizing this complication. So the focus should be kept at 1.5 cm away from the endometrium during treatment. These two patients underwent hysteroscopy to clear the necrotic tissue and the bleeding subsided. Bowel perforation is a rare complication that can occur after HIFU treatment with an incidence of 0.02%, and often has to be treated by surgery [Citation20]. In this study, the bowel perforation occurred 4 days after HIFU and the patient underwent a surgery. We carefully reviewed this case and found an adhesion of the bowel and the uterus; the lesion was over treated.
This study is limited because it is a retrospective study with many patients lost during long-term follow-up, and this may cause bias in the results. Another limitation of this study is that we only analyzed the symptom improvement. We did not get to compare the volume changes of the uterus and the lesions after HIFU treatment because many patients lived in different cities far away from the hospital and chose to visit their local hospital to check the ultrasound; it is difficult to obtain imaging results by telephone follow-up. Also, the patients treated with different adjuvant therapies were non-randomized when enrolled; these results may also be biased. Therefore, in the future, a randomized study is needed to validate these findings.
Based on the results of this study, we concluded that HIFU is a safe and effective treatment in improving the symptoms of dysmenorrhea and menorrhagia of patients with adenomyosis. HIFU combined with Mirena or Mirena + GnRH-a can significantly enhance the long-term treatment results. Future randomized studies with large number of subjects are needed to investigate the efficacy of HIFU treatment combined with adjuvant therapies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185.
- Vercellini P, Parazzini F, Oldani S, et al. Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod. 1995;10(5):1160–1162.
- Peric H, Fraser IS. The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):547–555.
- Gordts S, Grimbizis G, Campo R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril. 2018;109(3):380–388.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26(3):392–411.
- Jeng CJ, Ou KY, Long CY, et al. 500 cases of high-intensity focused ultrasound (hifu) ablated uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol. 2020;59(6):865–871.
- Haiyan S, Lin W, Shuhua H, et al. High-intensity focused ultrasound (HIFU) combined with gonadotropin-releasing hormone analogs (GnRHa) and levonorgestrel-releasing intrauterine system (LNG-IUS) for adenomyosis: a case series with long-term follow up. Int J Hyperthermia. 2019;36(1):1179–1185.
- Marques ALS, Andres MP, Kho RM, et al. Is high-intensity focused ultrasound effective for the treatment of adenomyosis? A systematic review and meta-analysis. J Minim Invasive Gynecol. 2020;27(2):332–343.
- Huang X, Yu D, Zou M, et al. The effect of exercise on high-intensity focused ultrasound treatment efficacy in uterine fibroids and adenomyosis: a retrospective study. BJOG. 2017;124(3):46–52.
- Karcioglu O, Topacoglu H, Dikme O, et al. A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 2018;36(4):707–714.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11(3–4):149–154.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124(3):207–211.
- Dababou S, Marrocchio C, Scipione R, et al. High-intensity focused ultrasound for pain management in patients with cancer. Radiographics. 2018;38(2):603–623.
- Yang X, Bai J, Yu T, et al. Effects of high intensity focused ultrasound on vascular endothelial growth factor in melanoma bearing mice. Technol Cancer Res Treat. 2004;3(5):499–503.
- Wang RS, Liu LX, Gu YH, et al. The effect of endostatin and gemcitabine combined with HIFU on the animal xenograft model of human pancreatic cancer. Biomed Pharmacother. 2010;64(5):309–312.
- Mao X, Wang Y, Carter AV, et al. The retardation of myometrial infiltration, reduction of uterine contractility, and alleviation of generalized hyperalgesia in mice with induced adenomyosis by levo-tetrahydropalmatine (l-THP) and andrographolide. Reprod Sci. 2011;18(10):1025–1037.
- Carrarelli P, Yen CF, Funghi L, et al. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci. 2017;24(3):369–375.
- Oh SJ, Shin JH, Kim TH, et al. β-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol. 2013;231(2):210–222.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714.