Abstract
Purpose
To evaluate the safety and efficacy of a new internal cold circulation bipolar radiofrequency compared with Habib-4X bipolar radiofrequency device in the resection of liver tumors.
Methods
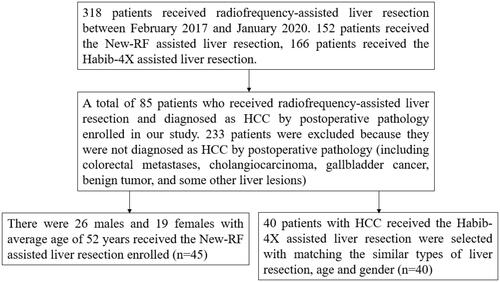
A total of 85 patients with hepatocellular carcinoma who received radiofrequency-assisted liver resection from February 2017 to January 2020 were retrospectively enrolled in our study, in which 45 patients received the new internal cold circulation bipolar radiofrequency (New-RF) and 40 patients received Habib-4X bipolar radiofrequency (Habib-4X). Primary outcome measures were the speed of liver transection, the width of coagulation tissue, hemorrhage volume, blood transfusion rate, and operation time.
Results
The baseline characteristics of patients in the New-RF and Habib-4X groups had no significant difference (p > 0.05). Compared to Habib-4X, the New-RF had a faster average speed of liver transection (4.81 ± 1.20 cm2/min vs 3.64 ± 1.08 cm2/min, p < 0.001), a narrower width of coagulation tissue (1.42 ± 0.23 cm2 vs 1.81 ± 0.20 cm2, p < 0.001), a less operation time (55.04 ± 16.12 min vs 64.02 ± 15.09 min, p = 0.010), a lower rate of needle path bleeding (13.3% vs 35.0%, p = 0.019), and a lower carbonization rate of electrode needle (22.2% vs 77.8%, p < 0.001). Hemorrhage during the transection (85.0 ml vs 105.0 ml, p = 0.438) and hemorrhage per square centimeter (3.28 ± 0.86 ml/cm2 vs 3.60 ± 1.12 ml/cm2, p = 0.141) in the New-RF group were smaller than those in Habib-4X group with no significant difference.
Conclusion
The new internal cold circulation bipolar radiofrequency was a safe and efficacious auxiliary device for liver resection with a faster speed of resection, lower carbonization rate of electrode needle, and more precise range of coagulation.
Introduction
Hepatectomy is the first-line therapy for primary and secondary liver tumors, especially for hepatocellular carcinoma (HCC), liver metastases of colorectal cancer, and hepatobiliary carcinoma [Citation1–3]. The application of advanced equipment can retain more liver tissue to decrease intraoperative hemorrhage and transfusion, reduce risks of liver failure and other complications, and provide the opportunity of re-excision after tumor recurrence [Citation4].
Recently, auxiliary liver resection devices such as Cavitron ultrasonic aspirators [Citation5], Tissuelink [Citation6], and BiClamp [Citation7] are developed to increase the safety of hepatectomy and reduce the hemorrhage. Besides, the advancement of vascular occlusion techniques and the application of low central venous pressure (CVP) anesthesia minimize the risk of hemorrhage during the operation [Citation8–10]. However, these interventions will reduce the blood perfusion of the liver, leading to regional ischemia. Previous studies revealed that ischemia and reperfusion were the main causes of liver injury or liver failure after surgery [Citation11,Citation12], and they could increase risks of early recurrence [Citation13]. Therefore, multiple techniques are developed to reduce intraoperative hemorrhage and transfusion, and avoid ischemia liver injury caused by blood reflux occlusion. For example, radiofrequency devices such as Habib-4X [Citation14], Tissuelink, and InLine [Citation15] can coagulate tissues before the resection to excise the liver tissue without bleeding. The radiofrequency energy released by electrodes is conducted to adjacent liver tissue, leading to the oscillation of intracellular ions and generation of heat. This mechanism can cause the coagulation and necrosis of the liver tissue and seal blood vessels and biliary ducts with a diameter of 3–7 mm through collagen fusion [Citation16,Citation17], thereby reducing the need of Pringle operation (extended intermittent clamp of the hepatic portal) and blood transfusion [Citation18,Citation19].
Weber et al. firstly applied a unipolar internal cold radiofrequency ablation electrode for the hepatectomy, reducing the intraoperative hemorrhage to 30 ± 10 ml [Citation20]. With the advancement of technology, the coagulation range of unipolar electrode radiofrequency increased, and multiple electrodes such as internal cold circulation electrode [Citation21,Citation22], bipolar electrode [Citation23], and cluster electrode [Citation15,Citation18] were developed to extend the coagulation range by regulating the deposition of radiofrequency energy in tissues. Although the cluster electrode could extend the coagulation range by overlapped effect, the increased risk of electrode-related puncture wounds cannot be ignored [Citation18]. Therefore, we designed a new internal cold circulation bipolar radiofrequency (New-RF) device by combining a bipolar radiofrequency electrode and an internal cold device, to minimize the damage of puncture wounds without affecting the efficiency of tissue coagulation. Previous studies suggested that an internal cold electrode had a high coagulation efficiency with a repeatable coagulation range [Citation16,Citation24–27]. Besides, the internal cold circulation electrode was demonstrated to have a higher coagulation efficiency compared to the cluster electrode both in theory and in vivo [Citation16,Citation28]. Cluster electrode (Habib-4X) has been safely and efficaciously used for decades with the advantage of reducing the need for Pringle operation and intraoperative hemorrhage [Citation14,Citation15,Citation18]. Although the unipolar internal cold circulation radiofrequency device is used in the assistance of hepatectomy [Citation29,Citation30], few research have explored the efficacy of the bipolar one in the open hepatectomy. This study was conducted to assess the efficacy of the New-RF compared to Habib-4X in the assistance of hepatectomy by analyzing the intraoperative hemorrhage, blood transfusion, and complications in the perioperative period.
Materials and methods
Enrollment of patients
A total of 85 patients with HCC who received radiofrequency-assisted liver resection from February 2017 to January 2020 were retrospectively enrolled in our study, in which 45 patients received the new internal cold circulation bipolar radiofrequency (New-RF) and 40 patients received Habib-4X bipolar radiofrequency (Habib-4X) (). Written informed consent was obtained from each patient and the study was approved by the Ethics Committee of The Affiliated Tumor Hospital of Zhengzhou University. All procedures were conducted in accordance with the Declaration of Helsinki. The inclusion criteria are as follows [Citation1]: patients diagnosed as primary liver cancer by two imaging examinations combined with the clinical conditions before surgery and diagnosed as HCC by postoperative pathology [Citation2]; operable liver tumor with sufficient liver tissue remaining [Citation3]; no extrahepatic metastasis [Citation4]; Child–Pugh classification A or B [Citation5]; ECOG (East Coast Oncology Group) Scale of Performance Status ≤2. The baseline characteristics of patients in the New-RF and Habib-4X groups had no significant difference (p > 0.05) ().
Table 1. Baseline characteristics of enrolled patients.
Ablation device
The ablation is conducted by an internal cold circulation radiofrequency ablation system (Taishang Lide LDRF-120S Mianyang Lide Electronics Co., Ltd. Mianyang, China). The system is composed of a radiofrequency generator with a maximum power of 200 W and a frequency of 400 kHz, a bipolar radiofrequency electrode, and a cold circulation pump. The length of the bipolar electrode is 10.0 cm, the distance between the needles is 1.2 cm, and the length of the tip of the needle (the exposed part for electric conduction) is 3.0 cm. The two-needle electrode contains dual channels that can pump cold saline through a cold circulation pump. The working temperature of the cold circulation electrode is maintained between 10–20 °C to dissipate the heat energy generated in the tip of the needle. The generator produces radiofrequency energy when the impedance ranges from 25 Ω to 30 Ω and the coagulation will continue until the impedance rises to 30 Ω. The used power is output at a constant power according to the recommendations of the equipment manufacturer, and the power is set according to the insertion depth of the tip of the electrode needle into the tissue. Generally, it is recommended to set the tissue depth of 1 cm to 40 W, 2 cm to 80 w and 3 cm to 120 W. The generator is programmed to be automatically stopped when the delivery energy is overloaded for the coagulation.
Habib-4X bipolar radiofrequency ablation system (Generator 1500X, RITA Medical Systems, Inc. CA, USA) is a handheld bipolar radiofrequency device designed for hepatectomy, which can release radiofrequency energy with a maximum power of 150 W and a frequency of 500 kHz by two pairs of needles (2 × 2 arrangement like a rectangle). The system can monitor the output power, tissue impedance, temperature, and duration time of the radiofrequency generator. Besides, the radiofrequency generator can be operated in manual or automatic modes. The Habib-4X can control the output power in the impedance feedback mode, in which the coagulation will continue until the impedance rises to 35 Ω [Citation31].
Operation process
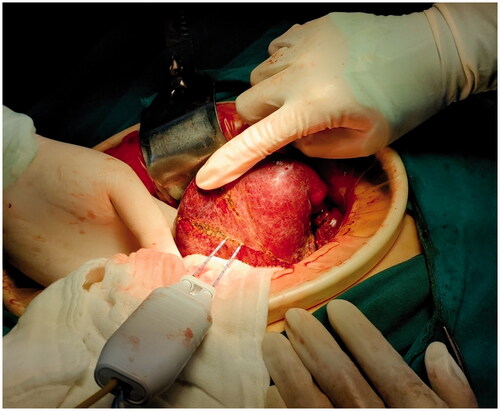
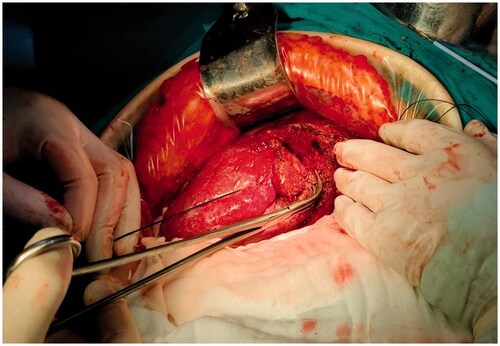
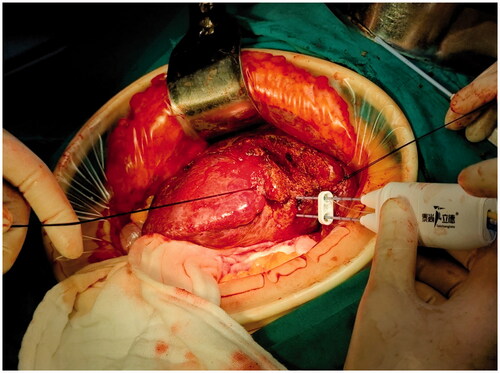
The patient received laparotomy under general anesthesia. The liver was dissociated based on the location and range of the lesion. The relationship between the lesion and important anatomical structures such as blood vessels and biliary ducts was revealed by the ultrasound examination. Blood vessels to be preserved and the liver to be resected were marked under ultrasound guidance. The electrode was inserted along the marked line to coagulate the liver tissue, forming a ‘no blood circulation’ tissue plane (). Then, we used vascular forceps or tissue scissors to excise the liver along the ‘no blood circulation’ tissue plane, close to and parallel to the outer edge of the liver tissue that needed to be preserved, remaining an ablation edge of about 0.8 cm (). Blood vessels and biliary ducts with relatively large diameters were identified and ligated. Little hemorrhage was detected during the excision. A limited number of sutures or clips were required to control the blood flow of the trunk or the branches of the portal vein. Although there was no need to clamp the influx and efflux channels, we still preset a blocking band at the hepatic portal as a backup. The process obeyed the sequence of ‘coagulation to excision’, which allowed us to change the direction during the liver excision according to the position, size, and shape of the tumor (). Besides, sufficient liver tissue would be preserved during the liver resection in this way.
Outcome measures
Patients received examinations such as liver function, kidney function, and blood routine before surgery and 1, 3 and 7 days after surgery. Each patient would be followed up for at least 3 months. The safety of the New-RF device was evaluated based on the incidence of complications and mortality after surgery; its feasibility was assessed by the speed of liver transection per square centimeter (cm2/min) and hemorrhage per square centimeter (ml/cm2). Resection time was defined as the time from the beginning to the end of the transection. The hemorrhage was evaluated by the weight of the suction fluid, blood-soaked cotton pads, and gauze pieces subtracting the weight of lavage fluid, dry blood-soaked cotton pads, and gauze pieces (1 g ≈ 1 ml). A piece of sterile paper was stuck on the surface of the liver as a mark of the resection area. After surgery, the contour of the sterile paper was duplicated to a piece of dry white paper. Then, an identical white paper was used to calculate the surface area of the liver section (cm2) [Citation7]. The resection speed was defined as the resection area divided by resection time (cm2/min). The width of coagulation tissue was measured directly using a sterilized steel ruler in centimeters. Needle path bleeding was defined as continuous bleeding in the needle path when the electrode needle was inserted into and pulled out from the liver tissue. The carbonization of the needle tip was defined as the adhesion of burnt tissue in the tip of the electrode needle. The biliary fistula was defined as the bilirubin concentration in the effluent after surgery at least 3 times the serum bilirubin concentration, or bile accumulation or biliary peritonitis that required surgical intervention [Citation32]. Indications for blood transfusion were intraoperative hemorrhage >1500 ml or hemoglobin <70 g/l. Surgery-related death was termed as death within 30 days after hepatectomy. Primary outcome measures were the speed of liver transection, the width of coagulation tissue, hemorrhage volume, blood transfusion rate, and operation time. Secondary outcome measures were total hospitalization time, 30-day mortality rates, and complications such as biliary fistula, abdominal abscess, pleural effusion, and liver failure after surgery.
Statistical analysis
All statistical analyses were conducted using SPSS 25.0. Quantitative data obeying Gaussian distribution were represented by the mean ± standard deviation; the comparison of differences was performed by student’s t-test. Quantitative data disobeying Gaussian distribution were represented by the median with range in the parentheses; the comparison of differences was performed by Mann–Whitney U test. Qualitative data were represented as rates (%) or proportion; Pearson Chi-square test, Pearson continuous correction Chi-square test, or Fisher exact probability test was used to compare the differences. Two-sided p < 0.05 was considered statistically significant.
Results
Intraoperative data of enrolled patients
In the New-RF group, 33 (77.3%) patients received minor liver resection (<3 liver segments) whereas 12 (26.7%) patients received major liver resection (≥3 liver segments); in the Habib-4X group, 33 (77.3%) patients received minor liver resection whereas 12 (26.7%) patients received major liver resection (). No significant difference was detected between the two groups (p = 0.861). Compared to Habib-4X, the New-RF had a faster average speed of liver transection (4.81 ± 1.20 cm2/min vs 3.64 ± 1.08 cm2/min, p < 0.001), a narrower width of coagulation tissue (1.42 ± 0.23 cm2/min vs 1.81 ± 0.20 cm2/min, p < 0.001), a less operation time (55.04 ± 16.12 min vs 64.2 ± 15.09 min, p = 0.010), a lower rate of needle path bleeding (13.3% vs 35.0%, p = 0.019), and a lower carbonization rate of electrode needle (22.2% vs 77.8%, p < 0.001). However, no significant difference was detected in the total hemorrhage, hemorrhage during the transection, hemorrhage per square centimeter, total operation time, device usage time, Pringle operation rate, and blood transfusion rate between the two groups. As a result, most patients in the two groups had no necessary to receive blood transfusion, whereas very few patients received the transfusion of no more than six units of red blood cells.
Table 2. Intraoperative data of enrolled patients.
Lab examinations of patients after surgery
The total bilirubin (TBIL), prothrombin time (PT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) of patients in the two groups were elevated one day after surgery and returned to normal three to seven days after surgery. However, the TBIL, PT, ALT, and AST of patients in the two groups at 1, 3 and 7 days after surgery had no significant difference (p > 0.05) ().
Table 3. Lab examinations of patients after surgery.
Complications of patients after surgery
The total incidence of complications of patients in the New-RF group was lower than those in the Habib-4X group but with no significant difference (p = 0.256) (). Moreover, intensive care unit (ICU) time (0 vs 0.5 days, p = 0.604), total hospitalization time (14.61 ± 2.52 vs 15.40 ± 2.84 days, p = 0.178), and 30-day mortality rates (0.0% vs 2.5%, p = 0.953) of patients in the two groups had no significant difference. Notably, one patient in the Habib-4X group was transferred to ICU due to liver failure with a continuously deteriorated condition on the 5th day after surgery. On the 7th day after surgery, he died of multiple organ dysfunction syndromes (MODS); therefore, the mortality in the perioperative period of Habib-4X was 2.5% (1/40). However, no patient died in the New-RF group within 30 days after surgery.
Table 4. Complications of patients after surgery.
Discussion
Surgical resection of liver tumors is required to minimize intraoperative hemorrhage and prevent liver dysfunction after surgery. Although non-anatomical liver resection can preserve more liver tissue, it also leads to more hemorrhage compared to anatomical liver resection [Citation33]. It is hard for patients with liver cirrhosis to choose anatomical or non-anatomical liver resection since these patients need more liver tissue to be preserved. However, the radiofrequency can assist the liver resection to decrease intraoperative hemorrhage and blood transfusion rates while preserving more liver tissue as much as possible, thereby reducing the risks of liver failure after surgery and accelerating the recovery of the organ [Citation34]. In the meantime, the reduction of hemorrhage and blood transfusion was also helpful for patients receiving preoperative chemotherapy [Citation35]. A previous study suggested that complications such as ascites, transient liver failure, and transient renal failure are more common in patients receiving blood transfusions [Citation36]. Therefore, minimized hemorrhage and blood transfusion are the primary objective of hepatobiliary surgery.
Initially, the development of radiofrequency device was to treat unresectable liver lesions by local thermal ablation, but the application of radiofrequency energy gradually extended to conventional liver resection [Citation20], The invention of the Habib-4X bipolar radiofrequency device, by Professor Habib, for the assistance of liver resection has further promoted the application of radiofrequency technology in liver resection [Citation14]: firstly, the Habib-4X can coagulate the liver tissue and occlude the blood vessels, reducing intraoperative hemorrhage and the number of ligation and hemostasis processes, leading to a shortened operation time; secondly, there is no need to completely dissociate the liver and occlude blood vessels using Habib-4X, so that it is possible to alternatively perform the hepatectomy; thirdly, the bipolar electrode of Habib-4X can avoid the occurrence of complications during electrical conduction [Citation19,Citation31,Citation37]. However, we found that the range of coagulated tissue was wide and carbonized tissues would be attached on the tip of the electrode needle, which damages normal liver tissues and cause the needle path bleeding, leading to the decreased efficiency of liver resection.
Our study revealed that the New-RF had a lower carbonization rate of electrode needle (22.2% vs 77.8%, p < 0.001) and a faster average speed of liver transection (4.81 ± 1.20 cm2/min vs 3.64 ± 1.08 cm2/min, p < 0.001) compared to the Habib-4X device. One reason was that the internal cold circulation in the New-RF device reduced the heat concentrated at the tip of the electrode needle, which could decrease the carbonization of tissue. The carbonized tissue could act as an insulator to increase the impedance and reduce the energy deposition at the tip of the electrode needle [Citation38]. Therefore, the internal cold radiofrequency device empowered the sufficient diffusion of the radiofrequency energy by reducing the carbonization rates. In contrast, although the Habib-4X had an extra electrode needle, it would contact with more liver tissues, which increased the tissue carbonization and dispersed the energy deposition at the tip of the electrode needle. Another reason was that during the application of Habib-4X, sometimes we had to suspend the operation and wipe the carbonized tissue. When the electrode was taken out of the liver, the electrode needle was likely to stick to the tissue and cause the hemorrhage [Citation19]. These would bring extra operations and we might need to reinsert the electrode to stop bleeding, which reduced the efficiency of liver transection and affected the clarity of surgical field. Besides, our study found that the needle path bleeding rate of the Habib-4X was significantly higher than that of the New-RF device (35.0% vs 13.3%, p = 0.019), which suggested that the New-RF could significantly reduce the needle path bleeding rate. Since the continuous needle path bleeding could blur the boundary between the cross-sectional liver tissue and the critical ducts and the vision of surgical field, the application of the New-RF could facilitate the delicate operation and endow the operators with confidence. Moreover, the width of coagulation tissue of the New-RF was significantly narrower than that of Habib-4X (1.42 ± 0.23 cm2 vs 1.81 ± 0.20 cm2, p < 0.001), which suggested that the isolation band for coagulation tissue of the New-RF was narrower. This could improve the safety of operation, meet the requirement of precise liver resection, and reduce risks of damaging important anatomical structures such as blood vessels, inferior vena cava, and gallbladder, etc. Additionally, hemorrhage during the transection (85.0 ml vs 105.0 ml, p = 0.438), hemorrhage per square centimeter (3.28 ± 0.86 ml/cm2 vs 3.60 ± 1.12 ml/cm2, p = 0.141) and transfusion rates (11.1% vs 12.5%, p = 1.000) exhibited no significant difference in the two groups, which indicated that although the width of coagulation tissue of the New-RF was lower than that of Habib-4X, the application of the New-RF could block the ducts with high efficiency. In fact, the significance of radiofrequency-assisted device for liver transection was to induce a coagulation plane with no blood circulation and facilitate the isolation of the liver tissue. Therefore, there was no need to make a wide coagulation plane.
A previous study revealed that the remaining coagulated necrotic tissue after surgery increased the risk of abdominal infection [Citation31]. In our study, the incidence of abdominal infection of the New-RF was lower than that of Habib-4X (2.2% vs 7.5%, p = 0.526). Although there was no significant difference, the incidence of abdominal infection was lower than a previous study [Citation37]. Therefore, further studies are required to investigate the correlation between residual coagulated necrotic tissues and the risk of abdominal infection. Besides, the incidence of biliary fistula in the two groups was relatively low (2.2% vs 5.0%, p = 0.917), which suggested that both devices had potent abilities in tissue coagulation and pipe sealing. Moreover, the incidence of complications, ICU time, total hospitalization time, and 30-day mortality rates in the two groups were relatively low with no significant difference. Therefore, the safety of the New-RF was consistent with Habib-4X.
There are several limitations in our study. The effects of the New-RF on tumor recurrence and metastasis and long-term survival rate of patients require further investigation. Besides, the sample size of our study is limited. The large-scale and multicenter studies are needed to determine the efficacy of the New-RF device.
To sum up, we developed a new internal cold circulation bipolar radiofrequency device, of which the safety and efficacy were demonstrated in our study. This would provide a potential therapeutic approach with high efficiency in the assistance of liver resection.
Acknowledgments
The authors would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240(4):698–708.
- Honda G, Ome Y, Yoshida N, et al. How to dissect the liver parenchyma: excavation with CUSA. J Hepatobiliary Pancreat Sci. 2020;27(11):907–912.
- Di Carlo I, Barbagallo F, Toro A, et al. Hepatic resections using a water-cooled, high-density, monopolar device: a new technology for safer surgery. J Gastrointest Surg. 2004;8(5):596–600.
- Zhao YJ, Zhou DC, Liu FB, et al. BiClamp(R) vessel-sealing device for open hepatic resection of malignant and benign liver tumours: a single-institution experience. BMC Cancer. 2017;17(1):554.
- Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226(6):704–711.
- Fu SY, Lau WY, Li GG, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201(1):62–69.
- Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187(6):620–625.
- Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59(5):1094–1106.
- Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev. 2014;2014:906965.
- Liu S, Li X, Li H, et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol. 2016;114(1):112–118.
- Pai M, Jiao LR, Khorsandi S, et al. Liver resection with bipolar radiofrequency device: Habib 4X. HPB. 2008;10(4):256–260.
- Daylami R, Kargozaran H, Khatri VP. Liver resection using bipolar InLine multichannel radiofrequency device: impact on intra- and peri-operative outcomes. Eur J Surg Oncol. 2012;38(6):531–536.
- de Baere T, Denys A, Wood BJ, et al. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001;176(1):187–192.
- Ihnat P, Ihnat Rudinska L, Zonca P. Radiofrequency energy in surgery: state of the art. Surg Today. 2014;44(6):985–991.
- Pai M, Kyriakides C, Mikhail S, et al. Radiofrequency-assisted hepatic resection. Ann Surg Oncol. 2011;18(12):3391.
- Reccia I, Kumar J, Kusano T, et al. Radiofrequency-assisted liver resection: technique and results. Surg Oncol. 2018;27(3):415–420.
- Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236(5):560–563.
- Cedeno DL, Vallejo A, Kelley CA, et al. Comparisons of lesion volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician. 2017;20(6):E915–E22.
- Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636–644.
- McGahan JP, Gu WZ, Brock JM, et al. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996;3(5):418–422.
- Pereira PL, Trubenbach J, Schenk M, et al. Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology. 2004;232(2):482–490.
- Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209(2):371–379.
- Burdio F, Guemes A, Burdio JM, et al. Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology. 2003;229(2):447–456.
- Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3(7):556–563.
- Quaranta V, Manenti G, Bolacchi F, et al. FEM analysis of RF breast ablation: multiprobe versus cool-tip electrode. Anticancer Res. 2007;27(2):775–784.
- Quesada R, Poves I, Berjano E, et al. Impact of monopolar radiofrequency coagulation on intraoperative blood loss during liver resection: a prospective randomised controlled trial. Int J Hyperthermia. 2017;33(2):135–141.
- Navarra G, Lorenzini C, Curro G, et al. Early results after radiofrequency-assisted liver resection. Tumori. 2004;90(1):32–35.
- Zacharoulis D, Tzovaras G, Rountas C, et al. Modified radiofrequency-assisted liver resection: a new device. J Surg Oncol. 2007;96(3):254–257.
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680–688.
- DeMatteo RP, Palese C, Jarnagin WR, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4(2):178–184.
- Milicevic M, Bulajic P, Zuvela M, et al. A radiofrequency-assisted minimal blood loss liver parenchyma dissection technique. Dig Surg. 2007;24(4):306–313.
- Pulitano C, Aldrighetti L, Arru M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;244(5):833–835.
- Ercolani G, Ravaioli M, Grazi GL, et al. Use of vascular clamping in hepatic surgery: lessons learned from 1260 liver resections. Arch Surg. 2008;143(4):380–387.
- Pai M, Frampton AE, Mikhail S, et al. Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol. 2012;38(3):274–280.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–331.