Abstract
Objective
To compare the long-term outcome of combining hepatectomy with intraoperative ultrasound (IOUS)-guided open microwave ablation (MWA) versus hepatectomy alone in patients with colorectal cancer liver metastases (CRLM).
Method
A retrospective analysis of patients with CRLM who underwent hepatectomy alone (HT group; 380 patients) or hepatectomy combined with IOUS-guided open MWA (HT + MWA group; 57 patients) from April 2002 to September 2018 was conducted at our center. A propensity score-matched (PSM) analysis was used to reduce data bias between the two groups.
Results
The overall survival (OS) and disease-free survival (DFS) were not significantly different between the two groups after matching. Although intrahepatic recurrence was more frequent in the HT + MWA group in both the whole and matched cohort, the two groups exhibited similar rates of extrahepatic recurrence as well as concomitant intra- and extrahepatic recurrence. A higher number of CRLM (>3), larger maximum-size and absence of response to induction chemotherapy were independent risk factors for OS.
Conclusion
The oncological outcomes of hepatectomy combined with intraoperative open ablation was not significantly different to hepatectomy alone and should be considered as a safe and fair option for patients with difficultly resectable CRLM.
Introduction
Colorectal cancer is the third most common malignancy worldwide and the second most common cause of cancer-related deaths in developed countries [Citation1]. Liver metastases occur in approximately half of the patients [Citation2,Citation3]. According to the National Comprehensive Cancer Network clinical practice guidelines on colorectal cancer, hepatectomy is recommended for some patients with colorectal cancer liver metastases (CRLM) [Citation4]. Bellier et al. demonstrated that aggressive management of colon liver metastases, including repeated resections, may provide improved long-term outcome [Citation5]. However, only 10–15% of the patients with CRLM are eligible for hepatectomy as the majority are unresectable because of the difficult anatomical locations of the lesions, inadequate hepatic reserve, patient comorbidities or extrahepatic metastases [Citation6,Citation7]. Traditionally, these patients have been considered for only chemotherapy, thermal ablation or a combination of these treatments with hepatectomy [Citation2,Citation8,Citation9].
Thermal ablation techniques, which primarily include radiofrequency ablation (RFA) and microwave ablation (MWA) [Citation10] can be considered for unresectable lesions, deep-seated resectable lesions and some patients with poor health [Citation11]. Some studies have demonstrated the advantages of thermal ablation with respect to its parenchyma-sparing feature, leading to reduced treatment morbidity and allowing repeat ablation in cases of recurrent diseases [Citation12–15]. In a previous study, a 4-year overall survival (OS) of patients after HT and MWA was 70% and 41%, respectively [Citation7,Citation15]. Other reports have demonstrated a 3-year OS of 23% and 14% after HT and MWA, respectively (p = .83) [Citation15,Citation16]. Another study reported that a 1, 3 and 4-year disease-free survival (DFS) in the HT group was 55%, 42% and 35%, compared with 56%, 39% and 39% after HT and MWA, respectively (p = .86) [Citation15,Citation17]. Furthermore, Solbiati et al. demonstrated a 5-year OS of 47.8% in the ablation group [Citation18]. Moreover, previous analyses demonstrated that RFA can provide patients with DFS and OS rates that are comparable to or close to that of surgical candidates [Citation13,Citation15].
RFA has been considered to be a safe and effective method for patients with CRLM [Citation19]. However, several limitations to RFA, including increased impedance at 100 °C, limited and small effective heating zones, reduced carbonization and multiple antenna limitations, constrain its wide clinical application [Citation20,Citation21]. In addition, the minimum ablation margin of 10 mm surrounding the tumor is critical to achieve local tumor control as the margins represent one of the most relevant predictors for local tumor progression [Citation22–24]. Compared with RFA, the number of comparative studies of MWA is limited, especially with respect to open ablation [Citation13,Citation15]. Despite these limitations, recently developed thermal ablation techniques have several advantages including the ability to maintain higher, stable intra-tumoral temperatures and a larger ablation zone. In contrast, MWA includes a shorter ablation time and is more efficient at heating cystic tumors. MWA has the ability to deliver multiple ablations at the same time and has no obvious heat-sink effects [Citation21,Citation25].
Compared with percutaneous ablation, open ablation is a more invasive approach which requires general anesthesia and a longer hospital stay [Citation13,Citation26]. However, there are benefits to open ablation including optimization of staging and better mobilization of the liver through detection of occult liver or peritoneal disease [Citation27]. In addition, the combination of ablation and hepatectomy may save patients from two-stage hepatectomy resulting in less morbidity [Citation27,Citation28]. Another potential benefit of adding MWA during hepatectomy is that it can retain uninvolved functional liver parenchyma. A previous study demonstrated that parenchyma-sparing hepatectomy improved survival in the event of liver recurrence, possibly because it increased the likelihood that the patient remained eligible for salvage hepatectomy [Citation14]. Therefore, for some patients and tumors, it is reasonable to perform open ablation and hepatectomy for the treatment of CRLM.
The aim of this study was to compare the long-term outcome of combining hepatectomy with intraoperative ultrasound (IOUS)-guided open MWA with hepatectomy alone in the patients with CRLM by using a propensity score-matched (PSM) analysis.
Patients and methods
Patients
Data for CRLM patients who underwent hepatectomy alone (HT group) or combined with IOUS-guided open MWA (HT + MWA group) from April 2002 to September 2018 at the Sun Yat-Sen University Cancer Centre were retrieved. The patients had been diagnosed with CRLM by biopsy before surgery. Inclusion criteria consisted of patients with colorectal liver metastases and primary tumor resected. Patients who underwent an R2 margin of resection or colorectal liver metastases were excluded (). Thoracoabdominal and pelvic imaging (ultrasound, computed tomography (CT) and/or magnetic resonance imaging (MRI)) was used to determine disease stage before hepatectomy. Using to the response evaluation criteria in solid tumors, the response to preoperative chemotherapy was classified as a partial response (PR), stable disease (SD) or progressive disease (PD) [Citation29]. Ethical approval was waived by the institutional research ethics board as this was a retrospective study.
Surgical strategy
The goal of hepatectomy was to remove all detectable lesions with a tumor-free margin. In the event tumor-free margins resulted from contact with any major vascular or biliary structures, resection was still considered as long as the tumors could be removed under the microscope [Citation30]. If a single hepatectomy could not be thoroughly complete treatment, even in combination with MWA, a two-stage hepatectomy was considered. For hepatectomy, MWA was used to treat tumors that could not be removed or were located deep in the remaining liver to preserve the parenchyma. Only in limited and treatable cases could extrahepatic disease be eliminated, by resection, ablation or radical radiotherapy [Citation13]. Open ablation and hepatectomy were performed simultaneously.
The interventional radiologists who performed the MWA procedures had more than 5 years of experience in interventional therapy. For the process of continuous electrophysiological monitoring using general anesthesia, MWA was performed under real-time ultrasound guidance.
Under the guidance of IOUS, a microwave system (FORSEA MTC-3C, Qinghai Microwave Electronic Institute, Nanjing, China) was used with a frequency of 2450 MHz and an output of 0–150 W. The nodule was inserted with an 18-cm 14 G cooled-shaft antenna, whereas the antenna tip was 5 mm beyond the deep margin of the nodule. For each application, according to the size and location of the nodules, 40–70 W of microwave energy for 5–20 min were administered. For tumors with a maximum diameter of ≤3.0 cm, a single antenna insertion was applied. For tumors >3.0 cm, multiple insertions were performed to ensure adequate ablative necrosis. After ablation, a hyperechoic area around the electrode tip covered an area larger than 10 mm around the lesions. At the end of the procedure, the needle track was ablated to prevent bleeding and tumor seeding (). In addition, ablation margins were measured on the PACS workstation using the first pre- and post-ablation contrast enhanced CT (CECT) scans and anatomic landmarks () [Citation22,Citation31]. The first post-MWA CECT was performed within 4–8 weeks. Distance from the tumor/ablation defect to the anatomic landmarks were chosen on the pre- and post-ablation CECT scans, respectively. The value subtracted from the distance between pre-ablation and post-ablation distance is the margin of ablation. The smallest value was considered the minimal margin. In this study, the ablation margins were more than 10 mm (A0).
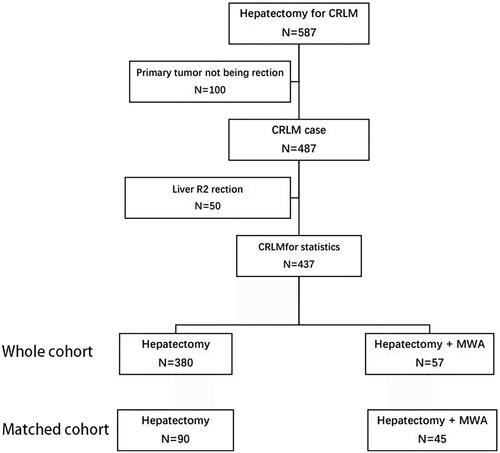
Figure 2. A 57-year-old male patient with CRLM. (A) CEMRI showed one lesion 14mm in diameter (thick arrow) with hyper-enhancement in the arterial phase. (B) Three months after ablation, the ablation area 43mm in diameter (thick arrow) showed that the ablated volume did not show contrast enhancement in the arterial phase.
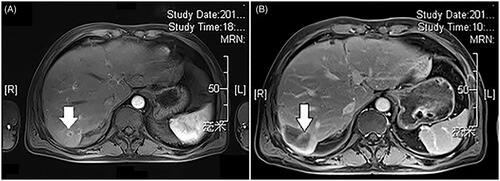
Figure 3. Example of microwave ablative margin measurement in a 63-years old man with a 1.6-cm colorectal liver metastasis in segment 6. (A) Axial portal venous phase computed tomography (CT) image shows the tumor before ablation; distances were measured from the edges of the tumor to the chosen landmarks. (B) Axial portal venous phase CT image obtained 7 weeks after ablation shows the ablation defect; the same distances were measured from the edge of the ablation defect to the same landmarks. The corresponding distances were subtracted, and the smallest value was chosen as the minimal margin—that is 22.1 mm–8.7 mm = 13.4 mm.
Follow-up
The classification of adverse events for the interventional radiology procedures, and post-treatment complications were recorded and stratified. The first follow-up was approximately one month after surgery to assess the effectiveness of the technique, and every 3 months thereafter until death or dropout. Patients would undergo CECT or contrast enhanced MRI (CEMRI) one month after hepatectomy to further assess the therapy efficiency of ablation. Absence of contrast enhancement was shown in the target tumor by IOUS imaging and post-ablation CT or MR imaging [Citation10]. At each follow-up, physical examination, serum carcinoembryonic antigen (CEA) measurement, and at least one imaging examination (abdominal CEMRI or CECT) were performed. Follow up with the same technique as before treatment. During the follow-up period, the size and number of recurrent tumors, recurrence pattern and follow-up treatments were recorded.
Statistical analysis
The distribution of continuous and categorical variables was compared using the Mann–Whitney U-test and χ2 test, respectively. OS curves and DFS curves were generated using the Kaplan–Meier method and compared with the log-rank test. The factors associated with intrahepatic recurrence and death were analyzed using the Cox regression model. The R statistical package (R software version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria) and SPSS statistical software package (version 25; IBM, Armonk, NY) were used to perform the statistical analyses. The R statistical package (R Foundation for Statistical Computing, Vienna, Austria) was used to match data, and the other statistical analyses were done with SPSS software (IBM, Armonk, NY). OS was calculated from the date of hepatectomy until death or the last follow-up. DFS was defined as the time between curative surgery (hepatectomy or resection of concomitant extrahepatic disease if present) and the first recurrence or death. p Values less than .05 were considered statistically significant.
Propensity score matching analysis
In order to reduce potential data bias, a PSM analysis was performed using the R statistical package [Citation32]. Logistic regression in a stepwise manner (six variables with p < .100) was used to identify the possible variables associated with treatment selection, including primary tumor location, primary tumor pathological type, tumor number at hepatectomy, preoperative chemotherapy, response to neoadjuvant chemotherapy and distribution of liver metastases. A one-to-two matching algorithm with an optimal caliper of 0.2 was used ().
Table 1. The characteristic of match cohort.
Results
A total of 587 patients with CRLM who underwent hepatectomy were identified. One hundred and fifty were excluded because of primary tumor not being resection (100) or liver R2 resection (50). Finally, MWA + HT and HT were included in 57 and 380 cases, respectively. The characteristics of the CRLM patients are shown in . Patients in the HT + MWA group were characterized by a larger tumor burden before PSM, including bilobar metastases (p < .001), multiple hepatic metastases (>3, p < .001) and more neoadjuvant chemotherapy compared with the HT group (82.5% vs. 45.8%, p < .001). In addition, three characteristics including pathological type of primary tumor (p = .013), response to neoadjuvant chemotherapy (p = .033) and number of neoadjuvant chemotherapy cycles (p < .001), were statistically significant. In addition, PSM analysis was performed to reduce potential data bias as baseline variables were different, whereupon 45 patients were allocated to the MWA + HT group and 90 to the HT group ().
Table 2. The characteristic of whole cohort.
Major complications (Clavien–Dindo classification III or above)
Among the entire cohort, 10 patients in the HT + MWA group and 29 patients in the HT group experienced major complications (Clavien–Dindo classification III or above) (17.50% vs. 7.60%, p = .014, ). No significant difference in major postoperative complications between the two groups in the matching cohort was observed (seven patients in the HT + MWA group (15.60%) vs. 10 patients in the HT group (11.10%), p = .463, ). All patients with complications received pertinent and appropriate therapy and achieved the discharge criteria.
Table 3. The complications of the whole cohort.
Table 4. The complications of the match cohort.
Survival and tumor recurrence
The median follow-up time for the HT + MWA group was 36.0 months and 30.0 months for the HT group. Before matching, OS () and DFS () in the HT + MWA group were poor compared with the HT group (all p < .001). For the entire population, the 1- and 3-year OS rates for the HT + MWA group were 88.9% and 54.7%, respectively, whereas in the HT group, they were 94.6% and 77.5%. The 1- and 3-year DSF rates for the HT + MWA group were 45.1% and 14.9%, respectively, whereas in the HT group, they were 67.20% and 46.0%.
Figure 4. Overall survival in patients who underwent hepatectomy alone and following microwave ablation (MWA) in combination with hepatectomy: A whole cohort and B match cohort (p<.001 and p=.180, respectively) (log rank test).
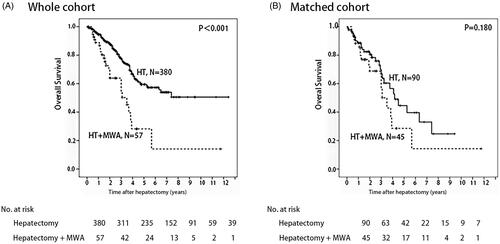
Figure 5. Disease-free survival in patients who underwent hepatectomy alone and following microwave ablation (MWA) in combination with hepatectomy: A whole cohort and B match cohort (p<.001 and p=.082, respectively) (log rank test).
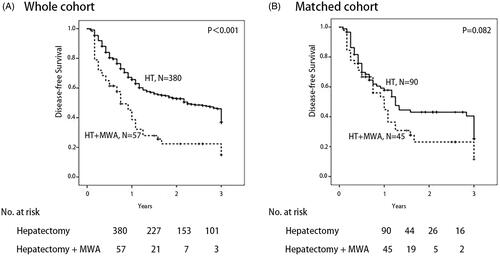
In the matched cohort, no significant difference in OS () or DFS () between the two groups was observed (p = .180 and p = .082, respectively). For the entire population, the 1- and 3-year OS rates for the HT + MWA group were 88.2% and 56.3%, respectively, whereas in the HT group, they were 88.6% and 66.9%. The 1- and 3-year DSF rates of the HT + MWA group were 53.2% and 11.5%, respectively, whereas they were 57.8% and 40.5% in the HT group.
In the entire cohort, 27 of 57 patients (47.4%) in the HT + MWA group and 97 of 380 patients (25.5%) in the HT group experienced intrahepatic recurrence (p = .001). Extrahepatic recurrence was observed in seven patients (12.3%) from the HT + MWA group and 39 patients (10.3%, p = .644) from the HT group. Concomitant intra- and extrahepatic recurrence occurred in four patients (7.0%) and 31 patients (8.2%) (p = .767) in the HT + MWA and HT groups, respectively.
For the matched cohort, 20 of 45 patients (44.4%) in the HT + MWA group and 23 of 90 patients (25.6%) in the HT group experienced intrahepatic recurrence (p = .026). Extrahepatic recurrence was observed in five patients (11.1%) in the HT + MWA group and seven patients (7.8%) (p = .521) in the HT group. Concomitant intra- and extrahepatic recurrence occurred in three HT + MWA group patients (6.7%) and nine HT group patients (10.0%, p = .521) ().
Table 5. Pattern of recurrence after hepatectomy.
The prognostic factors of survival and tumor recurrence
Cox regression analyses revealed that the number of hepatic metastases (HR = 1.596 [95% CI: 1.020–2.498]), maximal size of metastases (HR = 1.631 [95% CI: 1.153–2.307]) and response to neoadjuvant chemotherapy (HR = 1.143 [95% CI: 1.019–1.283]) were independent risk factors for OS (p < .001). The maximal size of metastases (HR = 1.089 [95% CI: 1.025–1.157]) (p = .006) and neoadjuvant chemotherapy (HR = 1.899 [95% CI: 1.447–2.491]) (p < .001) were independent risk factors for DFS (log-rank test).
Discussion
In this retrospective study, the short- and long-term outcomes of the HT + MWA group were similar to that of the HT group in the PSM analysis. The OS and DFS rates of the HT + MWA group were not different compared with those of the HT group after PSM. In addition, the complications were not significantly different between the two groups in the matched cohort. Although patients from the HT + MWA group were more prone to intrahepatic recurrence, for both the whole and matched cohort, the two groups exhibited similar rates of extrahepatic recurrence as well as both intrahepatic and extrahepatic recurrence.
Previous studies have shown that a tumor size less than 3 cm [Citation23,Citation26] and ablation margins greater than 5 mm [Citation22–24,Citation31,Citation33–35] yield the best outcomes. Kurilova et al. demonstrated the minimal margin of 6–10 mm, especially in patients who have received HAI, can offered 76% of local tumor control and 4% of biliary complications [Citation36]. In the present study, although the margin of ablation was larger than 10 mm, may increase the risk of biliary complications in certain populations [Citation36]. There were more patients with >3 cm tumor size in the HT + MWA group compared with the HT group (42.1% vs. 34.5%). These will affect the survival of patients. The COLLISION trial is an ongoing phase III randomized trial that compares thermal ablation and hepatectomy in patients with small CRLM (<3 cm), and it may provide more evidence regarding this dilemma [Citation3].
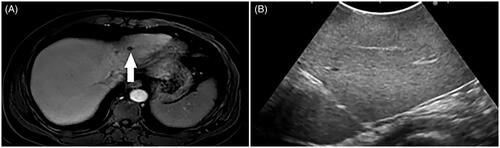
In this study, the higher intrahepatic recurrence rate observed in the HT + MWA group may be due to ‘disappearing lesions’. ‘Disappearing lesions’ represent viable tumor cells that are too small for visualization with radiologic methods, may be due to the following reasons. First, a phase II trial demonstrated that aggressive local treatment such as RFA + oxaliplatin can prolong OS in patients with unresectable colorectal liver metastases [Citation37]. However, most of the HT + MWA group patients received preoperative chemotherapy, which may have caused the liver metastases to shrink or disappear, resulting in a reduction in sensitivity to IOUS. Previous reports have shown that after effective preoperative chemotherapy, most patients present with viable tumors at the site of the ‘disappearing lesions’ in a series of images [Citation38–40] (). Multiple courses of preoperative chemotherapy, such as FOLFIRI which causes hepatic sinusoidal obstruction syndrome or FOLFOX, which causes steatohepatitis, can produce changes similar to that of early liver cirrhosis. This may affect the IOUS judgment of tumors. Similarly, the biliary tract injury caused by chemotherapy, leads to changes in chronic cholangitis, which may affect the valuation of tumors by IOUS. This could have affected the IOUS observations of liver metastases, resulting in an ultrasound showing that the tumor was not as clear as before chemotherapy (), and may have led to a failure in achieving a better ablation site during tumor puncture ablation. In addition, the proportion of metastases that were reported as a complete pathologic response (CPR) ranged from 18.22% [Citation41] to 73% [Citation42]. Thus, several sites with ‘disappearing lesions’ may still exhibit tumor activity. Limitations in the ability to locate or visualize these ‘disappearing lesions’ with IOUS may have prevented these lesions, from being treated, and their presence may have led to the observed tumor recurrence. Second, hepatic thermal ablation promotes colon cancer metastasis at the site of injury. One study has shown that the zone of subacute injury and inflammatory response caused by ablation can increase local metastasis, so the method of ablation energy transfer promotes local metastasis [Citation43].
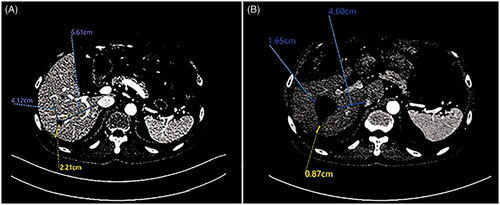
Figure 6. A 64-year-old male patient with CRLM. (A) CEMRI showed colorectal metastases in the liver (thick arrow). (B) after induction chemotherapy, CEMRI demonstrating no lesion at the site of metastases on the prior exam (thick arrow).
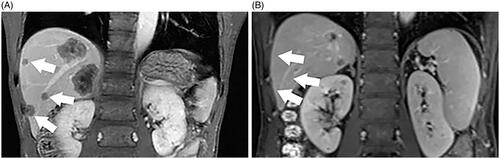
Figure 7. A 65-year-old male patient with CRLM. (A) MRI showed colorectal metastases in the liver at pre-surgical staging (thick arrow). (B) After preoperative chemotherapy, the tumor was not as clear as before chemotherapy during IOUS guidance.
Owen et al. demonstrated that gadoxetic acid-enhanced MRI was the most sensitive preoperative imaging modality for patients with CRLM [Citation38]. Improved detection of disappearing lesions occurred in patients that received gadoxetic acid-enhanced MRI during the preoperative chemotherapy or HT period. Arita et al. observed disappearing lesions with a sensitivity of 99%, a positive-predictive value of 98% and an accuracy of 97% by using contrast-enhanced intraoperative ultrasonography (CE-IOUS) [Citation44]. Therefore, the combination of preoperative gadoxetic acid-enhanced MRI and CE-IOUS may improve the CPR of disappearing lesions.
Previous studies of CRLM comparing hepatectomy with thermal ablation have experienced serious selection bias because of the absence of a randomization process and the retrospective collection of data. Other studies have shown that confounding factors between the ablation group and the hepatectomy group, such as the number or size of CRLM, RAS status, ki-67, clinical risk score (CRS), CEA and extrahepatic diseases, can affect the results [Citation16,Citation34,Citation37,Citation45–55]. In addition, the origin of rectal cancer has an impact on the survival and prognosis of liver metastases [Citation50,Citation52,Citation56]. These confounding factors resulted in data bias between the two groups. The patients who underwent MWA had a greater tumor burden, including bilobar metastases, multiple hepatic metastases (>3) and preoperative chemotherapy, compared with the patients who underwent hepatectomy alone. To overcome this issue, a PSM analysis was used. After PSM analysis, the data bias between the two groups was reduced.
There were a relatively small, number of patients in the HT + MWA group and the random selection conditions were not as rigorous compared with the present study. Despite the PSM analysis, there was still data bias. To further reduce data bias, increasing the number of patients and conducting randomized trials for the HT + MWA group will be necessary.
Conclusion
The oncological outcomes of hepatectomy combined with intraoperative open ablation was not significantly different to hepatectomy alone and should be considered as a safe and fair option for patients with difficultly resectable CRLM.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22.
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94(7):982–999.
- Puijk RS, Ruarus AH, Vroomen L, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) – a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821.
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369.
- Bellier J, De Wolf J, Hebbar M, et al. Repeated resections of hepatic and pulmonary metastases from colorectal cancer provide long-term survival. World J Surg. 2018;42(4):1171–1179.
- Feliberti EC, Wagman LD. Radiofrequency ablation of liver metastases from colorectal carcinoma. Cancer Control. 2006;13(1):48–51.
- Engstrand J, Nilsson H, Jansson A, et al. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases – a safety and feasibility study of a new concept. Eur J Surg Oncol. 2014;40(11):1488–1493.
- Pan Z, Peng J, Lin J, et al. Is there a survival benefit from adjuvant chemotherapy for patients with liver oligometastases from colorectal cancer after curative resection? Cancer Commun (Lond). 2018;38(1):29.
- Joo JH, Park J-h, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99(4):876–883.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION Trial Group. Cancers (Basel). 2020;12(7):1779.
- Hof J, Wertenbroek MWJLAE, Peeters PMJG, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103(8):1055–1062.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an International Panel of Ablation Experts, the Interventional Oncology Sans Frontières Meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Mise Y, Aloia TA, Brudvik KW, et al. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263(1):146–152.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89(2):276–284.
- Tanaka K, Shimada H, Nagano Y, et al. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139(2):263–273.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Wang Y, Zheng J, Chen H, et al. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int J Hyperthermia. 2018;34(6):853–862.
- Song P, Sheng L, Sun Y, et al. The clinical utility and outcomes of microwave ablation for colorectal cancer liver metastases. Oncotarget. 2017;8(31):51792–51799.
- Smolock AR, Lubner MG, Ziemlewicz TJ, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol. 2015;204(1):197–203.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.e1.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes – a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Wu W, Xue Y, Wang D, et al. A simulator for percutaneous hepatic microwave thermal ablation under ultrasound guidance. Int J Hyperthermia. 2014;30(7):429–437.
- Tinguely P, Dal G, Bottai M, et al. Microwave ablation versus resection for colorectal cancer liver metastases – a propensity score analysis from a population-based nationwide registry. Eur J Surg Oncol. 2020;46(3):476–485.
- Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9(1):49–58.
- Philips P, Groeschl RT, Hanna EM, et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg. 2016;103(8):1048–1054.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216.
- de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248(4):626–637.
- Wang X, Sofocleous C, Erinjeri J, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104(5):570–579.
- Cornelis FH, Petre EN, Vakiani E, et al. Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med. 2018;59(9):1360–1365.
- Odisio B, Yamashita S, Huang S, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29(5):2698–2705.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2020:S1533–0028(20)30134–1. DOI:https://doi.org/10.1016/j.clcc.2020.09.005. [Online ahead of print]. PMID: 33246789.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
- Owen JW, Fowler KJ, Doyle MB, et al. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB (Oxford). 2016;18(3):296–303.
- Auer RC, White RR, Kemeny NE, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer. 2010;116(6):1502–1509.
- van Vledder MG, de Jong MC, Pawlik TM, et al. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg. 2010;14(11):1691–1700.
- Tanaka K, Takakura H, Takeda K, et al. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250(6):935–942.
- Elias D, Youssef O, Sideris L, et al. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol. 2004;86(1):4–9.
- Jones EL, Halpern AL, Carmichael H, et al. Hepatic ablation promotes colon cancer metastases in an immunocompetent murine model. Ann Surg. 2019;270(4):675–680.
- Arita J, Ono Y, Takahashi M, et al. Routine preoperative liver-specific magnetic resonance imaging does not exclude the necessity of contrast-enhanced intraoperative ultrasound in hepatic resection for colorectal liver metastasis. Ann Surg. 2015;262(6):1086–1091.
- Pak LM, Gagniere J, Allen PJ, et al. Utility of image guidance in the localization of disappearing colorectal liver metastases. J Gastrointest Surg. 2019;23(4):760–767.
- Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81(1):25–34.
- Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26(10):1635–1641.
- Peng S, Huang P, Yu H, et al. Prognostic value of carcinoembryonic antigen level in patients with colorectal cancer liver metastasis treated with percutaneous microwave ablation under ultrasound guidance. Medicine (Baltimore). 2018;97(10):e0044.
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–2734.
- Yamashita S, Odisio BC, Huang SY, et al. Embryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastases. Eur J Surg Oncol. 2017;43(6):1040–1049.
- Shady W, Petre E, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8(39):66117–66127.
- Marques MC, Ribeiro HSC, Costa WL, et al. Is primary sidedness a prognostic factor in patients with resected colon cancer liver metastases (CLM)? J Surg Oncol. 2018;117(5):858–863.
- Creasy JM, Sadot E, Koerkamp BG, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163(6):1238–1244.
- Sofocleous C, Garg S, Petrovic L, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–4269.
- Sofocleous C, Petre E, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761.
- Zhou F, Yu X, Liang P, et al. Does primary tumor location impact the prognosis of colorectal liver metastases patients after microwave ablation? Lessons from 10 years' experience. Oncotarget. 2017;8(59):100791–100800.