Abstract
Objective
To evaluate the safety and efficacy of ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for desmoid tumors (DTs).
Method
A total of 111 patients with histologically proven DTs were included and treated by USgHIFU ablation. Adverse events were continuously evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 until 3 months after treatment. The incidence of non-perfused areas within the treated tumors, non-perfused volume rate (NPVR) and tumor volume reduction were evaluated using contrast-enhanced MRI before and one week and 3 months after the procedure.
Results
The enrolled patients (32 male, 79 female, mean age 29.5 ± 1.0 years) with 145 DTs (118 extra-abdominal, 16 abdominal wall, 11 intra-abdominal; median maximum diameter: 9.6 cm, range: 3–34.5 cm) underwent 188 sessions of HIFU ablation, and the mean number of ablations was 1.7 (range, 1–7) per patient. In majority of cases (143/145 cases, 98.6%), no serious adverse events were observed. There was no significant difference in the incidence of adverse events between patients who received a single treatment and those who received multiple treatments. Non-perfused area was observed within every treated tumor, and the median NPVR was 84.9% (range, 1.9–100%). The tumor volume reduction rate was 36.1 ± 4.2% at 3 months after treatment.
Conclusion
USgHIFU ablation, as a noninvasive and easily repeatable local treatment, is a promising treatment for DTs.
Introduction
Desmoid tumors (DTs), also known as aggressive fibromatosis (AF) and desmoid-type fibromatosis, are a rare intermediate soft tissue tumor with an incidence of 5.36 new diagnoses per 1 million individuals per year and are characterized by local invasion, non-metastasis, and a variable and often unpredictable clinical course [Citation1–3]. DTs are typically diagnosed in young adults with a peak age of about 30 years and a female predominance [Citation4]. They may develop at any anatomic site and are classified as extra-abdominal, abdominal wall and intra-abdominal types. Although the latter type is often related to familial adenomatous polyposis (FAP)-associated DTs (7.5–16%) with poor prognosis [Citation5,Citation6], most patients can achieve long-term survival [Citation7].
While surgical resection used to be the mainstay of treatment for DTs, its high recurrence rate, more aggressive recurrence and associated postoperative functional impairment became major concerns [Citation8]. Currently, it is no longer considered as a first-line treatment. Radiotherapy has been used in patients with unresectable lesions, incomplete resection or local recurrence. Shin et al. [Citation9] found that radiotherapy was able to delay tumor growth but did not influence the overall recurrence rate. Nevertheless, the complications (secondary sarcoma for young patients) induced by radiotherapy cannot be disregarded [Citation10,Citation11]. Recently, there has been a paradigm shift in the treatment of DTs toward more conservative management [Citation12,Citation13]. A joint global consensus guideline approach to the management of this disease has been undertaken, and active surveillance for 1–2 years is recommended as the first-line treatment for DTs [Citation14]. Depending on the locations, systemic treatments including nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy, cytotoxic chemotherapy and target therapy have moved increasingly into the focus of interest except for abdominal wall DTs with tumor progression. Nevertheless, systemic treatments with long treatment durations have resulted in significant side effects and modest overall response rates (6–40%) and thus have poor repeatability and insufficient evidence [Citation15–19]. Most DTs cannot be cured, and local recurrence and progression are almost inevitable. As a result, long-term survival accompanying DTs is the norm. At present, there is still a lack of effective treatment methods for DTs. Therefore, it is necessary to explore a safe, effective and repeatable therapy to control or delay tumor progression and reduce the impact of tumors on patients.
High-intensity focused ultrasound (HIFU) ablation is a noninvasive local treatment that has been successfully used for the treatment of various solid tumors, such as uterine fibroids, liver cancer, pancreatic cancer, bone tumors and soft tissue tumors [Citation20–25]. In the past decade, several small-sample studies have been reported, suggesting the safety and efficacy of HIFU ablation for the treatment of DTs [Citation26–32]. However, to our knowledge, there is no large-sample study to confirm the results and report the safety of multiple treatments. Thus, the aim of this study was to evaluate the safety and efficacy of HIFU ablation for the treatment of DTs.
Method
Study population
This study was approved by the institutional ethics committee. Written informed consent was obtained from all patients or their parents/guardians.
The inclusion criteria for this study were as follows: (1) patients with a histologically confirmed diagnosis of DT by thick needle puncture or surgical resection; (2) patients with symptomatic DTs, or patients with asymptomatic DTs with progression after active surveillance or conservative therapy, or patients with recurrent DTs after surgical resection and strong treatment requirements; (3) patients with tumors that could be visualized on ultrasound; and (4) patients with a tumor depth (the distance from the deep surface of a tumor to the surface of the skin) greater than 1.5 cm.
The exclusion criteria were as follows: (1) local skin in the acoustic pathway had become hard and fibrous due to previous radiotherapy; (2) the presence of extensive scars (>1.5 cm in width, >1.0 cm in height from skin) with obvious acoustic attenuation in the acoustic pathway; (3) ruptured skin and soft tissues in the tumor region, unhealed defects and local infection; (4) DTs in which major nerves were completely encased, and nerve functions needed to be preserved; (5) patients who were unable to maintain the treatment position for 1 h; (6) patients with diseases of vital organs; (7) patients allergic to imaging contrast agents; (8) patients with contraindications for sedation and analgesia; and (9) pregnant and lactating patients.
The elimination criteria were as follows: (1) the treatment and follow-up were not completed as planned and (2) patients received other physical therapy within 3 months after HIFU ablation.
HIFU ablation procedure
The ultrasound-guided HIFU ablation procedure was performed with the Model-JC Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) [Citation33]. Strict bowel preparations were performed for the patients with abdominal wall and intra-abdominal DTs the day before treatment, and skin preparations were performed for all of the patients [Citation28].
The procedure was performed under intravenous conscious sedation and analgesia using midazolam and fentanyl for the majority of patients [Citation33], whereas general anesthesia was used for a few patients who were too young to cooperate or whose tumors were far from important nerves. The patients were placed in a proper position according to the tumor location and the favorable acoustic pathway. The target tumor was divided into sections with an interlayer spacing of 5 mm using real-time ultrasound. A power output of 200 W was used initially, each exposure lasted 1–2 s to ablate one target spot. The therapeutic energy could be adjusted according to the patient’s response and the grayscale-changes in the target area on ultrasound. If the patient complained intolerable pain during the treatment, the power was reduced or the cooling time was increased; if there were not visible grayscale-changes on ultrasound, the power was increased at an increment of 20 W and the sonication time was increased until the treated spot became hyperechoic on ultrasound or the sonication time of the target spot was 50 s. After ablation of the spot, a nearby spot was treated. The treatment began from the maximum plane of the tumor, from the deep to surface region and was performed slice by slice. The focus was at least 1.0 cm away from the margin of the tumor and at least 2.0 cm away from the neurovascular bundle to prevent injuring the surrounding normal tissue. When the hyperechoic area covered the entire tumor on real-time ultrasound or the target tumor was treated completely according to the treatment plan, the ablation effect was evaluated with contrast-enhanced ultrasound, and non-perfused area within the tumor was regarded as a successful ablation [Citation34]. During the procedure, vital signs were continually monitored, and skin thermal damage of the acoustic pathway was closely observed on real-time ultrasonographic imaging to adjust the cooling time in time to avoid skin burn. Multiple treatments were adopted for single tumors with a diameter of ≥10 cm or multiple tumors, with an interval of 1–3 months. All procedures were performed by the same experienced doctor.
Safety evaluation
Adverse events were continuously monitored until 3 months after treatment, and patients with adverse events were followed up until it was determined that the adverse events had resolved or stabilized. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [Citation35]. The skin in the acoustic pathway and tumor-adjacent nerve functions (the sensory and motor functions) were examined by physical examinations and recorded immediately after the treatment, and then the symptoms and signs (such as fever, local pain, skin and soft tissue conditions in the treatment region, and limb sensorimotor function) of the patients were monitored for 1 or 2 days in the ward. After discharge, each patient was followed up by telephone interviews and outpatient services.
Efficacy evaluation
The primary outcome measures included the incidence of non-perfused areas within the treated tumor, non-perfused volume rate (NPVR) and tumor volume reduction at 3 months after HIFU ablation. Follow-up was carried out up to 3 months after the last treatment. Contrast-enhanced magnetic resonance imaging (MRI) was performed before and at 1 week and 3 months after HIFU ablation. All images were read, and all measurements were performed by the same experienced radiologist.
A new non-perfused area within the treated tumor was judged as effective ablation. The incidence of non-perfused areas within the treated tumors was defined as the number of tumors effectively ablated divided by the number of tumors treated, indicating the probability of technical success. Three-D Imaging Software v 1.0 (Chongqing Weihai Software Development Co., Ltd., Chongqing, China) was used to measure the tumor volume and non-perfused volume (NPV). NPVR was defined as follows: (1) for a single treatment, NPV divided by tumor volume; (2) for multiple treatments, NPV after the last treatment divided by tumor volume at the last treatment. Tumor volume reduction rate at 3 months after HIFU ablation was calculated as follows: tumor volume before treatment minus that three months after treatment, and then divided by that before treatment.
Statistical analysis
All data were analyzed using SPSS 26.0 (IBM, Armonk, NY). Normally distributed continuous variables are presented as the mean ± standard deviation. Non-normally distributed continuous variables are presented as the median and extreme values. Categorical variables are presented as counts and percentages. The differences in adverse events were evaluated with the chi-square test or paired chi-square test. p< .05 was considered statistically significant.
Results
Patients and tumors
A total of 111 patients (male to female ratio 1:2.5) with 145 DTs were enrolled from the First Affiliated Hospital of Chongqing Medical University and Chongqing Haifu Hospital from March 2013 to March 2020. A total of 76.6% (85/111) of patients had undergone surgical resection. The mean number of surgical resections was 1.7 ± 0.1 (range, 1–7). After surgical resection, the mean time to recurrence was 13.3 ± 1.4 months, and the median time to recurrence was 10 months (range, 2–72). The median maximum diameter of tumors was 9.6 cm (range, 3.0–34.5), and the median tumor volume was 131 cm3 (range, 5.4–3997.3). The characteristics of the patients and tumors are listed in .
Table 1. The characteristics of the patients and tumors.
HIFU ablation results
A single session of HIFU treatment was planned for 64 patients, and multiple sessions of HIFU treatment were planned for 47 patients with large tumors and/or multiple tumors. Of the 111 patients included, 109 patients successfully completed 186 sessions of HIFU ablation, and the mean number of treatments was 1.7 ± 0.1 (range, 1–7) per patient. The patient who had four DTs (the size of the largest one: 34.5 cm) in left lower limbs underwent seven sessions of HIFU ablation, and the largest one was treated for four sessions. The median hospitalization time was three days (range, 1–15). The non-perfused area was observed in every treated lesion, shown on the post-HIFU contrast-enhanced MRI (), and the median NPVR was 84.9% (range, 1.9–100%). The treatment parameters and HIFU ablation results are listed in . Two patients did not complete HIFU treatment as planned. One of the two patients, whose DT located in left hip, complained pain in the innervated areas during the treatment of all planned areas; another one with intra-abdominal DT frequently complained skin burning and pain, and there was obvious ultrasound attenuation after several minutes of sonication. In order to avoid nerve injury and skin burns, HIFU ablation was discontinued.
Figure 1. Contrast-enhanced MRI of three types of desmoid tumors (DTs). Before HIFU ablation, the areas of significant enhancement (white arrow) were shown in extra-abdominal DT (A), abdominal wall DT (B) and intra-abdominal DT (C). One week after HIFU ablation, non-perfused areas (red arrow) were observed within the extra-abdominal DT (A1), abdominal wall DT (B1) and intra-abdominal DT (C1), and the areas of peripheral enhancement representing residual tumors (blue arrow) were observed in the extra-abdominal DT (A1) and intra-abdominal DT (C1).
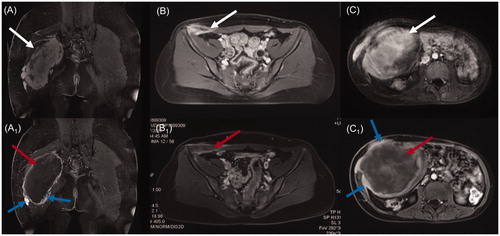
Table 2. The treatment parameters and HIFU ablation results.
Adverse events
One hundred and eleven patients underwent 188 sessions of HIFU ablation. The total incidence of adverse events was 30.9% (58/188), of which CTCAE grade 1–2 adverse events accounted for 95.3% and CTCAE grade 3 adverse events accounted for 4.7%. No CTCAE grade 4–5 adverse events occurred. The types and incidences of adverse events are listed in . There was no significant difference in the incidence of adverse events (37.93%, 31.3% and 27.3%, respectively) in patients with extra-abdominal, abdominal wall and intra-abdominal DTs (p= .846). There was no significant difference in the incidence of adverse events between patients who received a single session of HIFU treatment and those who received multiple sessions of HIFU treatment (p= .561) (). There was no significant difference in the incidence of adverse events (38.3% vs. 38.3%) between the first ablation treatment and the subsequent ablation treatments in the 47 patients who received multiple sessions of HIFU treatment (p= 1.000). There was no significant difference in the incidence of adverse events (38.9% vs. 25%) between the first ablation treatment and the subsequent ablation treatments in the 36 tumors that were treated by multiple sessions of HIFU treatments (p= .383).
Table 3. The types and incidences of adverse events in 188 sessions of HIFU ablation.
Table 4. Comparison of the incidences of adverse events between patients with a single session of HIFU ablation and those with multiple sessions of HIFU ablation.
The treatment was interrupted due to adverse events in three patients. One of these patients developed hypothermia (minimum body temperature of 33.7 °C), the patient’s body temperature quickly returned to normal, and the treatment was completed as planned after keeping the patient warm. The other two patients had elevated blood pressure and continued to complete the treatment as planned after nitroglycerin administration. After HIFU ablation, the skin overlying the treated region became slightly swollen in all patients. Seven patients experienced 1st or 2nd degree skin burns without special treatments except for keeping the areas dry, which recovered within 2 weeks. Twenty patients complained of mild pain in the treated region requiring no analgesics, and six patients complained of moderate pain requiring tramadol administration, which subsided after 24 h. Three patients experienced mild pain in the innervation region requiring no intervention due to local tumor edema and inflammation stimulating adjacent nerves after HIFU ablation, and the symptoms disappeared within seven days. One patient suffered from brachial plexus injury after HIFU ablation. However, the function of the right upper limb was not fully recovered after 11 months of follow-up. Two patients with DTs adjacent to the bladder experienced gross hematuria, which disappeared after 12 h by bladder irrigation with cold normal saline and hemostatic drug administration. One patient had intratumoral hemorrhage with low-grade fever (maximum 37.9 °C) four days after treatment, which improved within 1 week after local puncture, hemostatic drug administration and preventive anti-infective treatment. The bone adjacent to the tumor of 18 patients showed banded hyperintensity on the post-HIFU ablation contrast-enhanced MRI (). Two patients with superficial DTs (with distances from the superficial surface of the tumor to the surface of the skin of 0.1 cm and 0.3 cm, separately) had local secondary infections of tumors 20 days after treatment, one of whom improved within 2 weeks after antibiotic therapy. However, another patient with a foot DT had to undergo amputation due to uncontrolled infection.
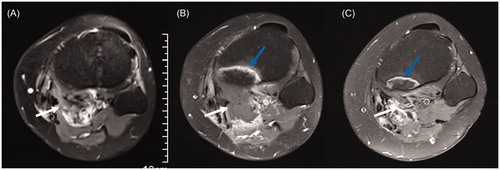
Figure 2. Contrast-enhanced axial MRI obtained from a 26-year-old woman with recurrent DT in the popliteal fossa before and after HIFU ablation. (A) Before HIFU ablation, the DT showed significant enhancement (white arrow), and the tibia showed no abnormal signal. (B) Three months after HIFU ablation, the DT without enhancement shrank (white arrow), and the tibia adjacent to the DT showed banded hyperintensity (blue arrow). (C) Fifteen months after HIFU ablation, the DT disappeared (white arrow), and the area of hyperintensity in the tibia (blue arrow) shrank.
Discussion
DTs are characterized by non-metastasis, local invasion and easy recurrence after surgery. The chance of local radical therapy is very low. Therefore, most patients have long-term survival accompanying these tumors, and local recurrence or tumor progression is almost inevitable. It is important to find a local treatment that can be repeatable to control local recurrence or tumor progression throughout the patient’s lifetime. HIFU ablation is a noninvasive thermal ablation technique that uses nonionizing radiation ultrasound as the physical therapy factor. It is also a conformal thermal ablation technique [Citation21]. Therefore, it not only has the potential of being a repeatable treatment but also has the potential to safely ablate more tumor tissue. Although a few studies have reported the safety and efficacy of HIFU ablation in the treatment of DTs, showing the prospect of HIFU ablation, the sample size was too small, and the convincing evidence was far from sufficient. In addition, DTs can occur in any part of the body, including the extra-abdominal, abdominal wall and intra-abdominal types. The safety and efficacy of HIFU ablation for different types of DTs also need to be explored. To the best of our knowledge, this is the largest reported series of HIFU ablation for DTs with the most comprehensive tumor sites. Our findings demonstrate that HIFU ablation is safe and effective in the treatment of DTs.
When developing a repeatable treatment method, safety should be the primary concern. Previous studies have suggested that the adverse events of HIFU ablation are mild [Citation26, Citation28, Citation30, Citation31], but there is no study reporting the impacts of a single session of HIFU ablation and multiple sessions of HIFU ablation on adverse events. In this study, we found that the incidence of adverse events was 30.9%, of which 95.3% were CTCAE grade 1 and 2 adverse events, and there were no serious CTCAE grade 4 and 5 adverse events. Although the sample size was significantly expanded, in majority of cases (143/145 cases, 98.6%), no serious adverse events were observed. This result was similar to those of previous studies [Citation26–32], still mainly reporting minor adverse events, suggesting that HIFU ablation in the treatment of DTs is safe. We further analyzed and found, for the first time, no difference in the incidence of adverse events between a single session of HIFU ablation and multiple sessions of HIFU ablation. The results preliminarily suggested that HIFU ablation is safe to repeat for multiple treatments of DTs. A further study with a larger sample size is needed.
Although HIFU ablation is a noninvasive treatment, the skin barrier function is reduced due to local edema after treatment, and the risk of secondary infection may occur in some tumors that are very close to the skin. The complication has not been reported in previous studies, but two patients experienced local secondary infection in our study. Therefore, postoperative local care should be provided to patients with superficial DTs (distance from the superficial surface of the tumor to the surface of the skin of less than 0.4 cm), and therapy techniques need to be improved. In addition, an abnormal signal of bone adjacent to the tumor was found on the post-HIFU ablation MRI, which was similar to the abnormal signal change of the adjacent sacrum in some patients with uterine fibroids after HIFU ablation [Citation36]. However, these patients had no pain or fractures, and the abnormal signal region gradually shrank in the follow-up. In previous study, one case with signal change of bone was reported, which may be related to the fact that the tumor is close to the bone and to the absorption of ultrasound energy on the surface of the bone when the acoustic pathway goes through the bone during treatment [Citation31]. Therefore, the long-term influence of abnormal bone response on patients remains to be further investigated. Two patients with neck DTs experienced elevated blood pressure during the treatment. Apart from the overstress reason, the ultrasound beam stimulating the cervical sympathetic nerve might account for this result. Therefore, it is advisable to place the sympathetic nerve outside of the acoustic pathway during treatment. One patient experienced hemorrhage in the tumor region after treatment, which may be related to the abnormal blood vessels of the DT or the use of ultrasound contrast agent.
Despite strict criteria for inclusion and exclusion, two patients who were fully compliant with the criteria did not complete HIFU treatment as planned, mainly because of the risk of nerve injury and skin burn during treatment. DTs are not malignant, and treatment should not bring patients more serious risks than the disease itself [Citation37]. Therefore, it is necessary to improve the level of pre-HIFU ablation screening to exclude tumors that are not suitable for HIFU ablation, such as DTs with nerves wrapped in them or severe scars with significant ultrasound attenuation in the acoustic pathway. The location of nerves and severe scars are unavoidable problems in the HIFU ablation procedure. Although the inclusion and exclusion criteria were as detailed as possible, problems could still be encountered. Although some nerves can be displayed on ultrasound and/or MRI in some cases, not all important nerves can be displayed, especially in patients with abnormal anatomy after surgery, where the location of the nerve is difficult to determine. Therefore, finding a way to determine the location of the nerve is needed to more accurately formulate indications. Although the morphology of the scar can be quantified, it still cannot reflect the scar's ability to absorb ultrasound energy. Finding a physical measurement method that objectively reflects the ultrasound absorption ability of scars is needed to optimize the indications.
The effectiveness of HIFU ablation in the treatment of DTs can be evaluated by the incidence of non-perfused areas within the treated tumors and NPVR. The former indicates the probability of technical success, and the latter represents the range of ablation, implying the control of the tumor. In this study, the DTs are characterized by complex sites, diverse shapes, different sizes (3–34.5 cm), diverse neighboring relationships and mostly recurrent DTs after surgery (118/145) as common clinical cases. Although these features pose challenges for obtaining safe and effective HIFU ablation results, non-perfused area was observed within every treated tumor, suggesting that almost all tumors that meet the inclusion criteria can be ablated. The NPVR was 84.9%, which is similar to that in previous reports [Citation26, Citation28]. The tumor volume decreased by 36.1 ± 4.2% at 3 months after treatment, suggesting that DTs can shrink quickly after HIFU ablation.
In this study, most of DTs in the abdominal wall (10/16) and some extra-abdominal DTs far away from important nerves (26/118) could be completely ablated, suggesting that there may be a chance of ‘local radical treatment’. The median NPVRs of extra-abdominal and abdominal wall DTs are similar to the results reported in previous studies [Citation26, Citation27], confirming the effectiveness of the HIFU ablation of extra-abdominal and abdominal wall DTs. However, the NPVR of intra-abdominal DTs was significantly lower than that of the other two types. This result is also significantly lower than that of the two studies by Zhao et al. [Citation28] and Shi et al. [Citation29], which may be related to the sizes of the DTs and the influence of the intestinal tract on the acoustic pathway. Further studies with a larger sample size are needed.
This study confirmed the effectiveness of HIFU ablation in the treatment of DTs and the safety of multiple treatments. However, the symptomatic improvement, the long-term control of HIFU ablation for DTs, and the timing and methods of re-intervention need further study. The safety comparison with traditional treatments such as surgery and chemotherapy also needs further study. In addition, although the overall sample size was large, the sample sizes of the abdominal wall and intra-abdominal types were still relatively small, which cannot fully reflect the response of these two types to HIFU ablation. Therefore, further comparative studies would enlarge the sample size to assess the difference of different sites of DTs.
Conclusion
The biological characteristics of DTs result in long-term survival accompanying these tumors, and the purpose of treatment is to control tumor growth. HIFU ablation has the advantages of noninvasiveness, fewer complications, fast recovery and repeatable treatment, making it a promising treatment method for DTs.
Disclosure statement
Wen-Zhi Chen and Lian Zhang are senior consultants to Chongqing Haifu Medical Technology Co., Ltd. The other authors have no conflict of interest to declare.
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. editors. WHO classification of tumours of soft tissue and bone. Pathology and genetics of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
- van Broekhoven DL, Grunhagen DJ, den Bakker MA, et al. Time trends in the incidence and treatment of extra-abdominal and abdominal aggressive fibromatosis: a population-based study. Ann Surg Oncol. 2015;22(9):2817–2823.
- Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399–2408.
- Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682–693.
- Clark SK, Neale KF, Landgrebe JC, et al. Desmoid tumours complicating familial adenomatous polyposis. Br J Surg. 1999;86(9):1185–1189.
- Sinha A, Tekkis PP, Gibbons DC, et al. Risk factors predicting desmoid occurrence in patients with familial adenomatous polyposis: a meta-analysis. Colorectal Dis. 2011;13(11):1222–1229.
- Koskenvuo L, Ristimäki A, Lepistö A. Comparison of sporadic and FAP-associated desmoid-type fibromatoses. J Surg Oncol. 2017;116(6):716–721.
- Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347–353.
- Shin SH, Ko KR, Cho SK, et al. Surgical outcome of desmoid tumors: adjuvant radiotherapy delayed the recurrence, but did not affect long-term outcomes. J Surg Oncol. 2013;108(1):28–33.
- Bishop AJ, Zarzour MA, Ratan R, et al. Long-term outcomes for patients with desmoid fibromatosis treated with radiation therapy: a 10-year update and re-evaluation of the role of radiation therapy for younger patients. Int J Radiat Oncol Biol Phys. 2019;103(5):1167–1174.
- Guadagnolo BA, Zagars GK, Ballo MT. Long-term outcomes for desmoid tumors treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):441–447.
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563.
- Park JS, Nakache YP, Katz J, et al. Conservative management of desmoid tumors is safe and effective. J Surg Res. 2016;205(1):115–120.
- Desmoid TW. G. The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96–107.
- Azzarelli MDA, Gronchi MDA, Bertulli MDR, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92(5):1259–1264.
- Li S, Fan Z, Fang Z, et al. Efficacy of vinorelbine combined with low-dose methotrexate for treatment of inoperable desmoid tumor and prognostic factor analysis. Chin J Cancer Res. 2017;29(5):455–462.
- Shimizu K, Hamada S, Sakai T, et al. Efficacy of low-dose chemotherapy with methotrexate and vinblastine for patients with extra-abdominal desmoid-type fibromatosis: a systematic review. Jpn J Clin Oncol. 2020;50(4):419–424.
- Chugh R, Wathen JK, Patel SR, et al. Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res. 2010;16(19):4884–4891.
- Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379(25):2417–2428.
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253(5):981–987.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol. 2004;30(2):245–260.
- Chen W, Zhu H, Zhang L, et al. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology. 2010;255(3):967–978.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;27(11):1422–1428.
- Vidal-Jove J, Perich E, Del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: the Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem. 2015;27:703–706.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–W252.
- Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia. 2011;27(7):648–653.
- Ghanouni P, Dobrotwir A, Bazzocchi A, et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. Eur Radiol. 2017;27(2):732–740.
- Zhao WP, Han ZY, Zhang J, et al. Early experience: high-intensity focused ultrasound treatment for intra-abdominal aggressive fibromatosis of failure in surgery. Br J Radiol. 2016;89(1062):20151026.
- Shi Y, Huang Y, Zhou M, et al. High-intensity focused ultrasound treatment for intra-abdominal desmoid tumors: a report of four cases. J Med Ultrason (2001). 2016;43(2):279–284.
- Najafi A, Fuchs B, Binkert CA. Mid-term results of MR-guided high-intensity focused ultrasound treatment for relapsing superficial desmoids. Int J Hyperthermia. 2019;36(1):538–542.
- Griffin MO, Kulkarni NM, OʼConnor SD, et al. Magnetic resonance-guided focused ultrasound: a brief review with emphasis on the treatment of extra-abdominal desmoid tumors. Ultrasound Q. 2019;35(4):346–354.
- Avedian RS, Bitton R, Gold G, et al. Is MR-guided high-intensity focused ultrasound a feasible treatment modality for desmoid tumors? Clin Orthop Relat Res. 2016;474(3):697–704.
- Peng S, Zhang L, Hu L, et al. Factors influencing the dosimetry for high-intensity focused ultrasound ablation of uterine fibroids: a retrospective study. Medicine (Baltimore). 2015;94(13):e650.
- Peng S, Hu L, Chen W, et al. Intraprocedure contrast enhanced ultrasound: the value in assessing the effect of ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids. Ultrasonics. 2015;58:123–128.
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute; 2017 [cited 2017 Nov 27]. Available from: https://www.meddra.org/
- Cun JP, Fan HJ, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2019;36(1):21–28.
- Gronchi A, Jones RL. Treatment of desmoid tumors in 2019. JAMA Oncol. 2019;5(4):567–568.
