Abstract
Background
To investigate the safety, efficacy, and follow-up outcomes of microwave ablation (MWA) in patients with breast fibroadenoma.
Methods
An institutional review board-approved this study of patients treated with MWA for breast fibroadenoma from October 2017 to March 2019. Clinical features of patients and breast fibroadenoma were analyzed. At follow-up all patients received physical examination and ultrasound imaging.
Results
In total, 171 patients with 271 lesions were enrolled. The mean lesion diameter was 1.35 ± 0.47 cm. The results revealed differential lesion states, including stability, enlargement, reduction, and complete regression, at 1-6, 6-12, and >12 months of follow-up. The size was reduced in 22.14% (31/140), 26.36% (29/110), and 36.36% (16/44) of the lesions at 1-6, 6-12, and >12 months of follow-up, respectively. The proportion of lesions with complete regression was 24.29% (34/140) at 1-6 months, 45.45% (50/110) at 6-12 months, and 40.91% (18/44) at >12 months of follow up. There was no significant relationship between the curative effect and age, lesion location, and blood flow in patients with breast fibroadenoma after MWA (p > .05), but there was statistically significant relationship with lesion diameter (categorized as <1.5 cm and ≥1.5 cm) (p < .05).
Conclusions
The current evidence indicates that MWA is a safe and effective method for treating breast fibroadenoma. Nevertheless, further large-scale prospective trials and well-designed future studies are warranted to validate our findings.
Introduction
Breast fibroadenomas are commonly seen among women worldwide, with the incidence having increased over the past few decades [Citation1]. Breast cancer screening programs (i.e., physical examination, mammography, and ultrasound) and some improvements in diagnosishave increased the detection rate of breast fibroadenomas.
A previous study demonstrated that breast fibroadenoma arise due to proliferation of ductal or lobular tissue, and their treatment is controversial [Citation2]. Small asymptomatic breast lesions generally do not require treatment, and some surgeons tend to favor conservative management of breast fibroadenoma with regular follow-up, which should be accompanied by auxiliary examination (i.e., ultrasound and/or mammography and pathological assessment) every 3-6 monthsin order to dynamically monitor the status of breast lesions [Citation3,Citation4]. However, some patients with large-sized breast fibroadenomas suffering from pain or anxiety are not satisfied with the conservative management approach and seek for treatment [Citation5–7]. Thus, it is necessary to excise part of the breast fibroadenoma according to therapeutic options that depend on different presenting situations [Citation8].
Surgical resection is the most effective treatment option for patients suffering from breast lesions, although it is an invasive procedure. The patients much prefer abetter cosmetic outcome and fewer surgically-associated complications, which are commonly associated with minimally invasive procedures. Over the past few years, the introduction of the vacuum-assisted breast biopsy (VABB) system, which employs both ultrasound and stereotaxic guidance, has proven to be a valuable innovation in managing breast fibroadenoma, and the method is also considered minimally invasive, well-tolerated, and has a better cosmetic outcome than does traditional open surgery [Citation9–11]. Ultrasound-guided percutaneous microwave ablation (MWA) is a new minimally invasive treatment technology used in breast fibroadenoma, and has emerged as a hot topic of clinical exploration in recent years. MWA has already shown promising results in treating liver carcinomaand pulmonary tumors, and in the settings of adrenal and renal malignancies [Citation12–15]. Currently, in relation to benign lesions, MWA is considered a promising treatment option for patients with benign thyroid nodules given its advantage of being minimally invasive, scar-free, and relatively safe, and is associated with fewer post-operative complications [Citation16].
To the best of our knowledge, MWA, as a thermal ablation technique, is increasingly being accepted by surgeons and clearly needs to be evaluated. Thus, we conducted this retrospective study to analyze the characteristics of ultrasound-guided percutaneous MWA and to validate its clinical feasibility and efficacy prior to its broad clinical application.
Patients and methods
Patients
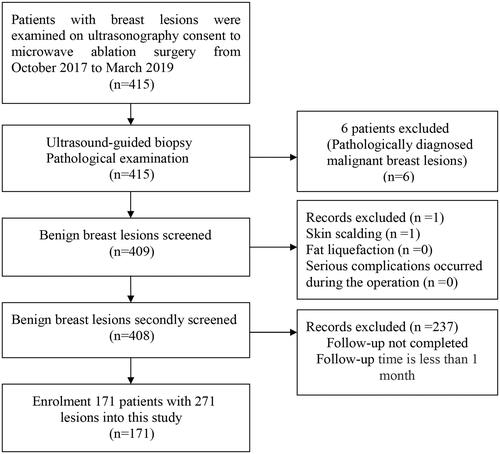
This study was approved by the Institutional Ethics Committees of The Third Hospital of Nanchang City (Nanchang, China). In the period from October 2017 to March 2019, a total of 171 patients were diagnosed with benign breast tumors, as determined by ultrasound-guided biopsy and histopathology examination. All patients signed an informed consent prior to participation in this study. The flow chart for managing benign breast lesions in this study is shown in .
Inclusion criteria included the following
(a) lesions pathologically diagnosed as fibroadenomas; (b) a single lesion of 30 mm or smaller; (c) breast lesions identified as having a score of 3 or less by the Breast Imaging Reporting and Data System (BI-RADS) category and proven to be benign by ultrasound (US)-guided core needle biopsy; (d) distance from the breast lesion to the skin and pectoralis major muscle exceeding 10 mm; (e) breast lesions positioned away from the nipple areola area; (f) follow-up time 1 month later.
Exclusion criteria included the following
(a) breast lesions larger than 30 mm in their greatest diameter; (b) BI-RADS score ≥4; (c) distance from the breast lesion to the skin and pectoralis major muscle ≤10 mm; (d) breast lesions positioned near the nipple areola area; (e) biopsy showing the presence of pathologically proven malignant tumors; (f) poor compliance for follow-up examinations anticipated; (g) evidence of coagulopathy, acute or active infectious disease, and serious chronic disease; and (h) ineligibility or refusal to undergo surgery for psychological or other reasons.
The procedure of MWA
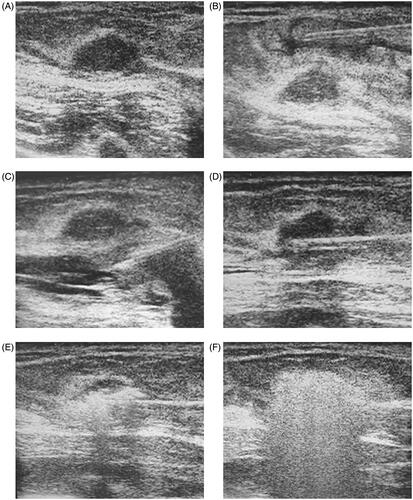
Prior to the procedure, all patients were subjected to routine blood tests to assess their coagulation status. Ultrasound imaging was applied by radiologists with more than 10 years of experience in breast tissue ultrasound to evaluate breast lesions before ultrasound-guided percutaneous MWA. Pre-operative biopsy and MWA were performed by doctors with more than 5 years of experience in breast surgery. The microwave unit (KY-2000, Kangyou Medical, Nanjing, China)can produce 100 Watts of power at 2450 MHz, and the MWA system used in this study was the same as that employed in previous studies [Citation17,Citation18]. The key procedure of MWA in our study is shown in . Patients were positioned supine or lateri-cumbent, which fully exposed the breast and permitted surgical disinfection. During the administration of a local anesthetic, we carefully administered a mixture of 2% lidocaine and 0.9% saline solution into the surrounding breast fibroadenoma to achieve a liquid isolated region to protect the skin, pectoralis, and areola from thermal injury. Under real-time ultrasound guidance, an MWA antenna was inserted into the breast fibroadenoma, and therapy was ceased until the hyperechoic area completely covered the entire breast fibroadenoma. On concluding surgery, an iced water bag was placed over the MWA area to avoid skin scalding.
Figure 2. A 28-year-old woman had a fibroadenoma with a right breast of 1.3 × 1.0 × 1.0 cm3 in size (A). The key procedure of the MW treatment is shown here (A–F). The spacer fluid (a mixture of 2% lidocaine and 0.9% saline solution) was injected into the surrounding fibroadenoma to achieve a liquid isolating region to protect the skin (B), pectoralis and areola from thermal injury (C); the MW antenna is inserted into the center of fibroadenoma for ablation (D); extensive air bubble formation is observed on ultrasound during the ablation, and shows the typical hyperechoic region surrounding the antenna (E, F).
Follow-up
Clinical follow-up consisted of questionnaires, which were mainly in the form of telephone calls, through which it was assessed whether there were postoperative complications and these calls also included reminders for regular follow-ups, and ultrasonography. We categorized the cases into three groups based on key follow-up periods that included the MWA period and the periods of 1-6, 6-12, and >12 months, in order to observe changes in ultra-sonographic imaging. After the MWA, physical examination and conventional ultrasound imaging was performed to evaluate the efficacy of the procedure. Ultrasound examinations assisted in classification of blood flow patterns and determination of the lesion size. Blood flow classification was assessed by Color Doppler Flow Imaging. The patients were also asked about the presence of pain and nipple discharge at each follow-up. Post-intervention complications, including pricking, skin scalding, local erythema and swelling, and fat liquefaction, were also evaluated and recorded for each lesion.
Data and statistical analysis
Median, mean, range, and standard deviation were analyzed for continuous variables (ablation time and ablation power). Age, lesion location, maximum lesion diameter, and blood flow were compared among groups by Student’s t-test, and the significance was set at 0.05. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
From October 2017 to March 2019, a total of 171 patients (age range, 21–63 years; mean age, 37.3 years) with 271 lesions were enrolled in this study (see flow chart, ). The baseline characteristics are summarized in . Hundred and three patients had one lesion, 47 patients had two lesions, and 21 patients had more than two lesions. The mean lesion diameter was 1.35 ± 0.47 cm, with 81 (29.9%), 166 (61.2%), and 24 (8.9%) patients having lesions with diameter <1.0 cm, ≥1.0 and <2.0 cm, and 2.0-3.0 cm, respectively. Blood flow was classified as ‘no flow’ in 184 (67.9%) lesions, minimal in 72 (26.6%) lesions, moderate in 8 (2.9%) lesions, and marked in 7 (2.6%) lesions.
Table 1. Clinical data basic characteristics.
Therapeutic response and clinical outcomes
The median duration of MWA for all lesions was 240 s, ranging from 30 to 900 s. The median power was 30 W and ranged from 25 to 40 W. During ablation, 25 patients suffered from slight to moderate pain and three experienced severe pain. MWA-related complications were also recorded during the follow-up period and included skin scalding (n = 1), fat liquefaction (n = 0) and serious complications that occurred during MWA (n = 0) (). There was no significant association of the efficacy of MWA with age, lesion location, and blood flow patterns in patients with breast fibroadenomas (p > .05; ), but there was a statistically significant association with the diameter of lesions (categorized as <1.5 cm and ≥1.5 cm) (p < .05; ). depicts three cases of patients with different responses to treatment. Ultrasound before the MWA showed three lesions with clear boundary (). At 8, 9, and 11 months after the MWA, ultrasound showed enlargement of one lesion (), reduction of the other (), and complete regression of the third lesion ().
Figure 3. Depicts three cases of patients with different responses to treatment (see figure A, B and C). Ultrasound before MWA showed three lesions with clear boundary (A.a, B.a and C.a). 8 months, 9 months and 11 monthsafter the MWA, showed the results of enlargement (A.b), reduction (B.b) and complete regression (C.b) on ultrasound, respectively.
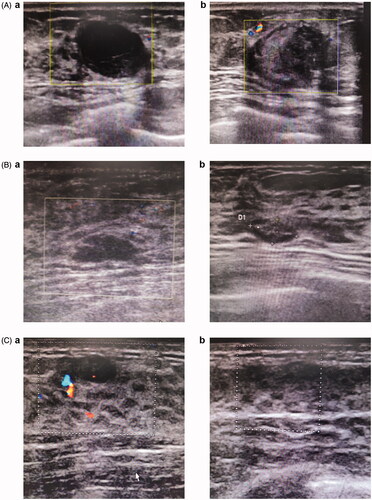
Table 2. Therapeutic response and clinical outcomes.
Table 3. Relevant factors of curative effect (include reduction and complete regression) in patients with breast fibroadenoma after microwave ablation.
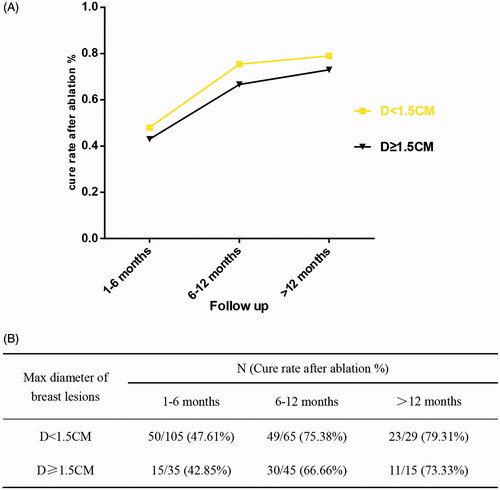
shows the percentages of the following four states of the lesions the post-MWA state at 1-6 months, 6-12 months, and >12 months: stability, enlargement, reduction, and complete regression. After ablation, the percentages of lesions with enlarged diameters decreased with the follow-up time as follows: 61 lesions (43.57%, 61/140), 27 lesions (24.55%, 27/110), and 7 lesions (15.91%, 7/44) at 1-6 months, 6-12 months, and >12 months, respectively. The lesions had a stable status for 14 lesions (10.00%, 14/140) at 1-6 months, 4 lesions (3.64%, 4/110) at 6-12 months, and 3 lesions (6.82%, 3/44) at >12 months of the MWA follow-up. After ablation, the percentages of lesions with reduced diameters and complete regression clearly increased with the follow-up time. Specifically, the diameter was reduced in 22.14% (31/140) of the lesions at 1-6 months, 26.36% (29/110) at 6-12 months, and 36.36 (16/44) at >12 months of follow-up. Moreover, 34 lesions (24.29%, 34/140) had complete regression at 1-6 months, 50 (45.45%, 50/110) had complete regression at 6-12 months, and 18 lesions (40.91%, 18/44) had complete regression at >12 months of follow-up. shows the correlation between changes in the cure rate and the diameter of lesions (categorized as <1.5 cm and ≥1.5 cm) at 1-6 months, 6-12 months, and >12 months post-ablation. The ablation rate increased with the extension of follow-up time in both lesions with a diameter <1.5 cm and those with a diameter ≥1.5 cm ().
Figure 4. The graph reveals different states of lesions at 1–6 months, 6–12 months and more than 12-months follow-up visit after MW ablation, included stability, enlargement, reduction and complete regression (A). The state of reduction and complete regression ration of the lesions in the 6-12 months and more than 12 months follow-up increased than that in the 1–6 months follow-up (B).
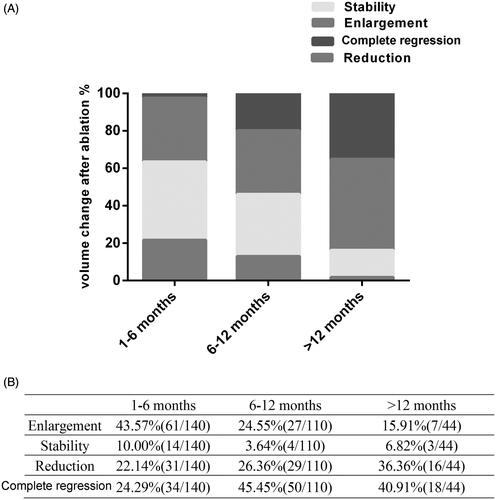
Figure 5. Graph shows correlation between the changes of the cure rate and two groups of diameter of lesions (diameter <1.5 cm, diameter ≥ 1.5 cm) 1–6 months post-ablation, 6–12 months post-ablation and more than 12-months post-ablation (A). Both groups with a diameter of < 1.5 cm and groups with a diameter of ≥1.5 cm showed an increasing ablation rate with the extension of follow-up time (B).
Discussion
Conventional surgical treatments of breast fibroadenoma are associated with greater tissue trauma, which could result in scar formation and eventually undesirable cosmetic outcomes. Over the past few decades, in order to obtain improved clinical decisions regarding therapy and cosmetic outcomes, researchers have focused on research and development of minimally invasive approaches in treating breast fibroadenoma.
As such a minimally invasive approach, ultrasound-guided MWA is currently being used in clinical practice and has emerged as an attractive treatment option for breast fibroadenoma. Some studies have shown the efficacy and safety of ultrasound-guided MWA in treating breast fibroadenoma and the advantages of this minimally invasive approach, including better cosmetic outcomes, decreased post-operative pain, early recovery, and enhanced quality of life [Citation1,Citation17–22].
Previous studies have reported that MWA is a feasible and efficacious treatment strategy for many solid tumors, including tumors of the liver, kidney, adrenal glands, pulmonary system, as well as locally recurrent colorectal cancer, thyroid microcarcinoma, and pancreatic head cancer [Citation12–15,Citation23–25]. MWA is also an effective treatment option for patients with benign lesions including benign thyroid nodules, uterine leiomyoma; and benign focal liver lesions [Citation16,Citation26,Citation27]. Particularly the mammary gland is a superficial organ that is very suitable for ablative therapy. This is partly because it is covered only by skin, has no complex anatomic structures, and has less-involved vascular structures as compared with the thyroid, lung, kidney, and liver. The proposed principle of MWA is that it uses electromagnetic waves and generates high temperatures. This results in a series of biochemical changes that induce protein denaturation and coagulation and in cellular metabolic deficits that eventually provoke the destruction of the extracellular matrix and cellular necrosis when treating breast fibroadenoma [Citation18,Citation28–30]. Peek et al. reported on normal breast tissue with lower-water-content such as fat and glandular tissues, and showed that during MWA, the procedure induced mainly heat effects and damage to the high-water-content of tumor cells; however, the lower-water-content of tissues remained unharmed [Citation31]. Therefore, MWA therapy usually has a low rate of complications, as shown in previous studies [Citation19–21]. In order to improve the efficiency of ablation of breast fibroadenoma while trying to minimize complications, the surgeon should master skills such as pull-back and hydro-dissection techniques.
Clearly, surgical skills affect the outcome of ablation, previous studies have described the pull-back technique, which is frequently implemented during thyroid ablation[Citation32,Citation33]. Yu et al. and Zhou et al. adopted the hydro-dissection technique in the MWA procedure of breast fibroadenoma; owing to the administration of 2% lidocaine or saline injected into the subcutaneous and retro-mammary spaces, they reported no patients displayed serious complications, such as hemorrhage, serious pain or fat necrosis, skin burns and chest wall injuries, observations that were consistent with those in our study [Citation17,Citation30].
In our current study, we assessed the differential states of lesions at 1-6, 6-12, and >12 months of follow-up after MWA, which included assessments of lesion stability, enlargement, reduction, and complete regression. Our study revealed that a higher proportion of lesions showed reduced size or complete regressionin the follow-up periods of 6-12 and >12 months than of 1-6 months, indicating that the follow-up time represents a key factor critically affecting lesion absorption. Treatment efficiency was also encouraging, since lesions completely regressed in 50/110 (45.45%) patients and reduced in size in 29/110 (26.36%) patients during the 6-12-month follow-up period. In their study, Yu et al. demonstrated an 80% mean volume reduction ratio for lesions ≤2 cm and a 50% ratio for lesions >2 cm at the 12-month follow-up period [Citation17], an observation that was consistent with our current research work. After ablation, the percentages of lesions with reduced diameters and complete regression clearly increased with the follow-up time. In this study, we demonstrated a correlation between changes in the cure rate and the diameter of lesions (categorized as <1.5 cm and ≥1.5 cm) at 1-6 months, 6-12 months, and >12 months post-ablation. The ablation rate increased with the extension of follow-up time for both lesions with a diameter <1.5 cm and ≥1.5 cm. Interestingly, Zhou et al., [Citation18] suggested that 93.0% of lesions were found to have complete regression at 18 months follow-up, and that the results of lesion absorption were significantly higher than found in other studies; probably because of the high proportion of histological types of adenosis (220/397, 55.4%). An interesting finding of this current study, was that at 1-6 months after MWA, ultrasound images showed that the largest diameter of ablative lesions were exceeded in 61 of 140 (43.57%) lesions as compared to that found prior to ablation. Zhang et al., also showed that the largest lesion diameters were increased one hour after MWA, and had gradually decreased, and even completely regressed by 12 months thereafter, with a success rate of 82.93% for MWA-mediated treatment of breast fibroadenoma [Citation20]. Therefore, the elimination of breast fibroadenoma after MWA has emerged as a key focus area of clinical concern, which has prevented the technology from being widely used. During the process of MWA, the conditions of high temperature cause the periphery of breast fibroadenoma tissues to undergo a series of biochemical changes, including pro-inflammatory reactions and cellular edema, which increase the size of the breast fibroadenoma and provoke the appearance of a hypoechoic halo (edema-zone) on ultrasound [Citation20]. In our study, we also demonstrated that there was no significant association of the efficacy of MWA with age, lesion location, and blood flow patterns in patients with breast fibroadenomas, but a significant association with the diameter of lesions (categorized as <1.5 cm and ≥1.5 cm).
There are some limitations to our study. First, at the period of >12 months after MWA, only 10 of the 44 lesions were maintained at a stable state (7 showing enlargement and 3 showing stability), and only 18 of 44 lesions (40.91%) completely regressed. In the future, we propose that factors related to the elimination of benign breast lesions should be explored for patient inclusion. Second, our study had a relatively short follow up and small sample size. Hence, studies with longer follow-up periods and a larger sample size should be considered to ensure and confirm our results. Third, the largest diameter of the lesions included was 30 mm; thus, the feasibility and efficacy of ultrasound-guided MWA should be further investigated for larger lesions (i.e., with the largest diameter >30 mm).
Conclusions
Our study reports promising clinical outcomes for MWA under ultrasound guidance, which represents a feasible and efficacious treatment strategy for patients with breast fibroadenoma. We propose that lesion size, lesion location, and surgical skills are factors that might influence the elimination of breast fibroadenoma after MWA treatment. Consequently, screening of patients and a greater practical experience with this technique are required before this approach is considered a widely acceptable clinical procedure. Nonetheless, further large-scale prospective trials and well-designed studies are warranted to validate its clinical feasibility and efficacy in the near future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg. 2014;3(2):142–148.
- Ackerman LV, Mucciardi AN, Gose EE, et al. Classification of benign and malignant breast tumors on the basis of 36 radiographic properties. Cancer. 1973;31(2):342–352.
- Carty NJ, Carter C, Rubin C, et al. Management of fibroadenoma of the breast. Ann R Coll Surg Engl. 1995;77(2):127–130.
- Dixon JM. Conservative management of fibroadenoma of the breast. Br J Surg. 1996;83(12):1798–1799.
- Alle KM, Moss J, Venegas RJ, et al. Conservative management of fibroadenoma of the breast. Br J Surg. 1996;83(7):992–993.
- Jang JY, Kim SM, Kim JH, et al. Clinical significance of interval changes in breast lesions initially categorized as probably benign on breast ultrasound. Medicine (Baltimore). 2017;96(12):e6415
- Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149(3):569–575.
- Orr B, Kelley JL, 3rd. Benign breast diseases: evaluation and management. Clin Obstet Gynecol. 2016;59(4):710–726.
- Jiang Y, Lan H, Ye Q, et al. Mammotome® biopsy system for the resection of breast lesions: Clinical experience in two high-volume teaching hospitals . Exp Ther Med. 2013;6(3):759–764.
- Pan S, Liu W, Jin K, et al. Ultrasound-guided vacuum-assisted breast biopsy using Mammotome biopsy system for detection of breast cancer: results from two high volume hospitals. Int J Clin Exp Med. 2014;7(1):239–246.
- Liberman L, Gougoutas CA, Zakowski MF, et al. Calcifications highly suggestive of malignancy: comparison of breast biopsy methods. AJR Am J Roentgenol. 2001;177(1):165–172.
- Li X, Fan WJ, Zhang L, et al. CT-guided percutaneous microwave ablation of liver metastases from nasopharyngeal carcinoma. J Vasc Interv Radiol. 2013;24(5):680–684.
- Liang P, Wang Y, Zhang D, et al. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol. 2008;180(3):844–848; discussion 848.
- Wang Y, Liang P, Yu X, et al. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25(6):455–461.
- Belfiore G, Ronza F, Belfiore MP, et al. Patients' survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82(1):177–181.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Yu J, Chen BH, Zhang J, et al. Ultrasound guided percutaneous microwave ablation of benign breast lesions. Oncotarget. 2017;8(45):79376–79386.
- Zhang W, Jin ZQ, Baikpour M, et al. Clinical application of ultrasound-guided percutaneous microwave ablation for benign breast lesions: a prospective study. BMC Cancer . 2019;19(1):345
- Zhou W, Wang R, Liu X, et al. Ultrasound-guided microwave ablation: a promising tool in management of benign breast tumours. Int J Hyperthermia. 2017;33(3):263–270.
- Zhang W, Li JM, He W, et al. Ultrasound-guided percutaneous microwave ablation for benign breast lesions: evaluated by contrast-enhanced ultrasound combined with magnetic resonance imaging. J Thorac Dis. 2017;9(11):4767–4773.
- Xu J, Wu H, Han Z, et al. Microwave ablation of benign breast tumors: a prospective study with minimum 12 months follow-up. Int J Hyperthermia. 2018;35(1):253–261.
- Li C, Ge H, Liang M, et al. Technical analysis of US imaging for precise microwave ablation for benign breast tumours. Int J Hyperthermia. 2018;34(8):1179–1185.
- Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24(10):1513–1520.
- Li W, Ye X, Yang X, et al. Microwave ablation as palliative treatment of locally recurrent colorectal cancer. Indian J Cancer. 2015;52(6):e61-63.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30(2):150–157.
- Goldberg J, McCrosson S, Kaulback KR. Delayed leiomyoma degeneration after microwave endometrial ablation. Obstet Gynecol. 2005;106(5 Pt 2):1176–1178.
- Cheng Z, Liang P, Yu X, et al. Percutaneous microwave ablation for benign focal liver lesions: Initial clinical results. Oncol Lett. 2017;13(1):429–434.
- Todorova VK, Klimberg VS, Hennings L, et al. Immunomodulatory effects of radiofrequency ablation in a breast cancer model. Immunol Invest. 2010;39(1):74–92.
- Widenmeyer M, Shebzukhov Y, Haen SP, et al. Analysis of tumor antigen-specific T cells and antibodies in cancer patients treated with radiofrequency ablation. Int J Cancer. 2011;128(11):2653–2662.
- Zhou W, Jiang Y, Chen L, et al. Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol. 2014;83(10):1771–1777.
- Peek MCL, Douek M. Ablative techniques for the treatment of benign and malignant breast tumours. J Ther Ultrasound. 2017;5:18
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.