Abstract
Objective
To evaluate the feasibility and safety of using cone-beam CT (CBCT) to measure changes in parenchymal blood volume (PBV) of patients with hepatocellular carcinoma (HCC) after transcatheter arterial chemoembolization (TACE) and to guide microwave ablation (MWA) for residual tumors.
Methods
A retrospective study was performed on 42 patients with HCC who completed TACE and received CBCT-guided perfusion imaging. The residual active lesions after TACE were supplemented with MWA to complete the treatment process according to the residual PBV. The outcomes were analyzed, including PBV changes, interventional-related complications, local tumor progression (LTP) and overall survival (OS).
Results
Technical success was achieved in all lesions. Correlation analysis revealed that greater volume of residual PBV after MWA is negatively correlated with LTP. (p = .000); and the decrease of PBV was positively correlated with LTP (p = .000). All adverse events and complications were CTCAE Grade 1/2. After combination treatment, the 1-, 3-, and 5-year LTP-free survival were 97.6%, 69.0% and 15.1%, respectively, with a median LTP of 49.0 months (95% CI:43.129,54.871). Multivariate Cox regression revealed that the residual PBV > 13 ml/1000 was an independent factor predicting a shorter OS and LTP (Both p< .05). For LTP, multivariate Cox regression showed that a tumor in a single lesion were independently predicted to have a longer LTP in patients with HCC (p = .033).
Conclusion
CBCT is feasible and safe to use to measure changes in the PBV before and after TACE treatment, while it can also guide MWA for the treatment of residual tumors in one session
Introduction
The most recent iteration of the Barcelona Clinic Liver Cancer (BCLC) guidelines recommends surgical resection, ablation, or liver transplantation with a curative intent for early hepatocellular carcinoma (HCC), while transcatheter arterial chemoembolization (TACE) is recommended for intermediate stage and multifocal tumors [Citation1]. As a palliative treatment method, TACE can significantly improve the survival rate of patients with HCC by locally injecting a mixture of chemotherapeutic agents, blocking the blood supply of tumor arteries, and promoting the ischemia and necrosis of tumor tissue [Citation2–5]. However, TACE alone could be associated with low tumor necrosis and high recurrence rate [Citation6]. Therefore, TACE and radiofrequency ablation (RFA) or microwave ablation (MWA) have been considered effective palliative treatments for HCC[Citation7]. Of note, quantitative evaluation of the CT perfusion map is an important indicator of the response to TACE or ablation in patients with HCC [Citation8,Citation9]. Currently, researchers have revealed that perfusion imaging of HCC under cone-beam CT (CBCT) can analyze the hemodynamic changes of a tumor after TACE, which is of great clinical significance for monitoring the tumor response of the tumor after interventional treatment [Citation10]. Parenchymal blood volume (PBV) is a quantitative parameter to assess tumor perfusion. There is a strong correlation between PBV and CT perfusion in HCC (p < .001) [Citation11]. This research evaluates the feasibility of using CBCT to measure the PBV changes in patients with HCC after TACE and to guide MWA for residual tumors.
Materials and methods
The Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved the present study. The requirement for informed consent was waived because of the retrospective nature of the study.
Patients
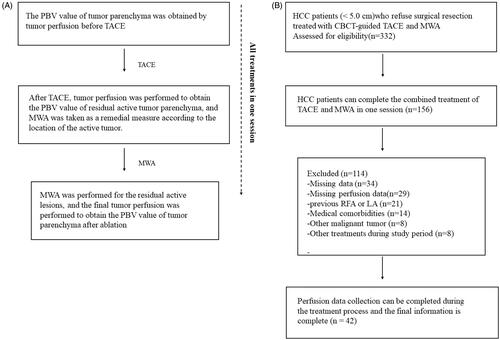
Between September 2014 and January 2016, A total of 332 patients with HCC underwent CBCT-guided TACE and MWA during the study period. A total of 156 patients were excluded from the study based on the exclusion criteria. Thus, 42 patients tumotwere ultimately included in the present study and received CBCT to measure changes in the PBV of HCC (tumor diameter <5 cm) before and after TACE and to simultaneously guide MWA for active residual tumor lesions (median age 52.3 ± 10.8 years; range 34-70 years). The inclusion and exclusion criteria are listed in ().
Table 1. Inclusion and exclusion criteria.
Procedure
TACE treatment
All TACE procedures were performed by two interventional radiologists (with more than 10 years of experience in interventional radiology) to ensure consistency (). Hepatic artery angiography was performed using a 5-Fr catheter to identify the tumor and feeder (s). Then, superselective catheterization of the feeding artery was performed using a 2.0 F microcatheter (Progreat, Terumo Corporation, Tokyo, Japan) followed by conventional TACE. The amount of pirarubicin (THP; 60-80 mg; Shenzhen Meirui Pharmaceutical Co., Ltd. China) and iodized oil (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) used was 10 to 20 mg (average 14.3 mg) and 4-6ml (average 5.1 ml), respectively. Finally, after the lipiodol was evenly deposited in the tumor, the tumor feeding artery was completely embolized with microspheres (100-300μm; Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) ().
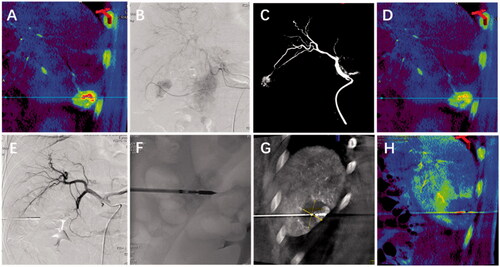
Figure 1. A patient with hepatocellular carcinoma (HCC) who refused surgical resection was treated with transcatheter arterial chemoembolization (TACE) followed by microwave ablation (MWA) under cone-beam CT(CBCT). Tumor perfusion images were obtained before TACE, after TACE and after MWA; (A) CBCT-guided tumor perfusion imaging showed that HCC with a diameter of 3.2 cm in the lower right corner measured an initial parenchymal blood volume of 102 ml/1000 ml. (B-C) Discover abnormal tumor clusters under the guidance of CBCT and complete tumor chemoembolization.(D) After TACE, the residual parenchymal blood volume was 43 ml/1000 ml. (E-G) Guide and plan residual tumor puncture paths and complete tumor ablation under CBCT navigation.(H) After MWA, PBV decreased to 2.4 ml/1000 ml, and there was no tumor blood vessel staining.
C-arm CBCT acquisition
A flat panel angiographic system (Artis zee BA Twin; Siemens AG, Germany) was used to acquire tumor PBV values three times; before TACE, after TACE, and after MWA. The PBV acquisition consisted of two consecutive spins both rotations are performed during one breath hold, including: initial rotation, first rotation without the addition of contrast agent (mask run), and injection of the contrast agent after completing the second rotation (fill run). Prior to the second rotation, a contrast agent was injected into the proper hepatic artery through a 2.0-F microcatheter using a power injector (Arterion®, Medrad, Inc.), and 18 ml of iodixanol (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) was injected at a rate of 2 ml/s.
MWA treatment
Ablations were conducted with the patient under local anesthesia in CBCT-guided (Syngo X-workplace with Syngo DynaCT; Siemens AG, Germany). The microwave antennas (ECO-100AI10, ECO Microwave System Co, Nanjing, China) were placed percutaneously under the CBCT reconstruction image. Specific power and time settings were determined by the physician operator based on the quality of the surrounding liver tissue, lesion depth and demarcation line length, but generally, tumors are ablated for 5–10 min at 65 W. Then, perfusion imaging was performed to determine the outcome of the treatment. Finally, the pre- and post-CT scans were overlayed to evaluate the ablation zone and immediate complications.
PBV calculation
The Syngo workstation (Siemens Healthcare) in C-arm CBCT was used to complete the extraction of PBV data. In fact, PBV is an application that can determine the parenchymal blood volume when the steady-state contrast is enhanced. It mainly records the mask and filling volumes through a non-rigid motion correction algorithm and then subtracts them to obtain data. In the process, in order to calculate the arterial input function, the hepatic artery was segmented from the liver parenchyma, and then the arterial input function value was calculated from the automatic histogram analysis of the vessel tree. Finally, the arterial input function value was used as a scale factor to obtain the quantitative parenchymal blood volume map. The programme applies a smoothing filter to reduce pixel noise. In addition, the blood volume pixel value is presented to the reader as a parameterized color-coded image to calculate the parenchymal blood volume. In this study, the average value of residual PBV after MWA was obtained, and the survival analysis was completed according to different intervals (>mean VS ≤ mean).
Definitions
Local tumor progression describes the appearance of tumor foci at the edge of the ablation zone, after at least one contrast-enhanced follow-up study has documented adequate ablation and an absence of viable tissue in the target tumor and surrounding ablation margin by using imaging criteria. Technical success describes the tumors treated according to protocol. The ablation evaluation standards were the modified response evaluation criteria in solid tumors RECIST guideline (version 1.1). The intervention-related complications will be jointly evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Event (CTCAE Version 4.03) and Society of Interventional Radiology (SIR) classification system.
Follow up
Electronic medical records were reviewed for pre-and post-treatment laboratory values and treatment-related complications. Imaging follow-up was performed with contrast-enhanced MRI or CT at intervals of 1, 3, and 6 months with a 6-month interval follow-up thereafter. All post-procedure and follow-up images were reviewed for consensus between a senior radiology resident and a board-certified interventional radiology faculty member with 5 years of experience in oncologic imaging and interventions. If a recurrent active tumor was found during the period, the second TACE-MWA or MWA would be performed separately depending on the patient's condition.
Statistical analysis
Descriptive statistics were used to present patient characteristics and results. Continuous data are presented as total number, percentage, mean and standard deviation or median and range. Continuous variables were compared by independent t-test. Categorical data are reported as numbers with percentages, The local tumor progression (LTP) was estimated with Kaplan–Meier survival analysis using SPSS version 22.0 software (SPSS Inc., Chicago, IL). Use simple linear regression to analyze the relationship between residual parenchymal blood volume (PBV) and local tumor progression (LTP) after microwave ablation (MWA) within five years after treatment. Additionally, univariate and multivariate Cox’s proportional hazards regression analyses were used to predict prognostic factors of LTP. p < .05 was defined as statistically significant.
Result
Patient characteristics
The mean age of the patients was 52.3 ± 10.8 years (range, 34–70 years). Of the 42 patients, 19 (45.2%) patients were 60 years old or younger, 28 (66.7%) patients were male, and 29 (69.0%) patients also had hepatitis B (). In all, the number of HCC tumors with a diameter ≥3 cm but <5 cm was 36 (85.7%), and those with a diameter <3 cm numbered 6 (14.3%). In addition, 29 (69.0%) patients were considered to be Child-Pugh A, while 13 (31.0%) patients were considered to be Child-Pugh B. The mean energy and ablation duration per tumor were 53.7 ± 7.5 kJ and 8.5 ± 2.2 min, respectively. The median hospitalization after the intervention was one day (range: 1–13 days). Flow diagram shows exclusion criteria in ().
Table 2. Patient characteristics.
PBV values before and after treatment
The success rate of TACE with sequential MWA for all patients under CBCT conditions is 100%. We evaluated 65 lesions in 42 patients. PBV measurement results of local tumors before TACE demonstrated a mean value of 115.1 ml/1000 ml ± 41.1; after TACE, the PBV immediately dropped to 50.8 ml/1000 ml ± 18.4. With regard to the blood perfusion of the residual tumor, the PBV was reduced to 13.8 ml/1000 ml ± 10.3 using sequential MWA. The decrease in PBV before and after the combination therapy was statistically significant (p < .001) (). With regard to the relationship between residual PBV after combination therapy LTP within five years after treatment, linear regression analysis revealed that more residual PBV after combination therapy was negatively correlated with LTP. (r = −0.622, p = .000) (). In addition, correlation analysis showed that the decrease in PBV before and after combination therapy was positively correlated with LTP (r = 0.393, p = .000) ().
Figure 3. (A) Relationship between residual parenchymal blood volume (PBV) after microwave ablation (MWA) and local tumor progression (LTP) within five years after treatment. Linear regression analysis revealed that the residual PBV after MWA was negatively correlated with time to LTP (r = −0.622, p = .000). In detail, the smaller the residual PBV value, the later the local tumor progression; (B) The relationship between the percentage decrease in PBV (Ratio of PBV after MWA to PBV before TACE) and LTP. Linear regression analysis showed that the decrease of PBV was positively correlated with time to LTP (r = 0.393, p = .000). In brief, the smaller the decrease of PBV after treatment, the earlier the local tumor progression.
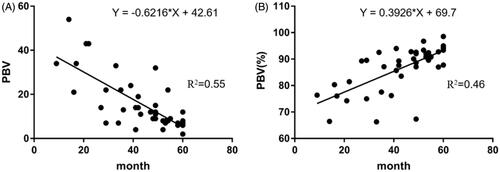
Table 3. Changes in PBV values before and after procedure.
Adverse events and complications
Postoperative pain, fever and liver dysfunction were the most common adverse events after treatment, with 26 (62.0%), 18 (42.9%) and 24 (57.1%) patients, respectively. According to the CTCAE classification, the number of people with fever Grades 1 and 2 was 11 (26.1%) and 7 (16.7%), respectively. The proportion of patients who needed or did not require opioid oral analgesics to treat pain was 5 (12.0%) and 21 (50.0%), respectively. Meanwhile, the proportions of total bilirubin elevation, hypoalbuminemia and liver abscess were 18 (42.9%), 3 (7.1%) and 9 (21.4%), respectively. In addition, one patient (2.4%) had asymptomatic intrahepatic bile duct dilatation, and two patients (4.8%) had asymptomatic perihepatic effusion ().
Table 4. Adverse events and complications.
Ltp
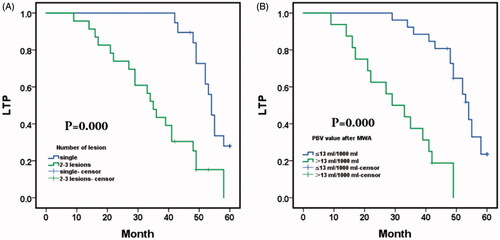
After the combination procedure, the 1-, 3-, and 5-year LTP-free survival were 97.6%, 69.0% and 15.1%, respectively, with a median LTP of 49.0 months (95% CI: 43.129,54.871) (). Kaplan-Meier LTP between single tumor and multiple tumors (2-3 lesions) after TACE with sequential MWA treatment revealed a median LTP of 54.0 months (95% CI: 51.945, 56.055) in procedures with a single lesion versus 35.0 months (95% CI: 30.305, 39.695) with 2-3 lesions (p = .000, log-rank test). The 1-, 3-, and 5-year LTP-free survival in patients with a single lesion were 100.0%, 100.0% and 28.0%, respectively; and the 1-, 3- and 5-year LTP-free survival with 2-3 lesions were 95.7%, 43.5% and 0.00%, respectively (). Comparison of LTP in residual PBV after MWA between PBV ≤13 ml/1000 ml and the PBV > 13 ml/1000 ml. Median LTP was 54.0 months (95% CI: 51.074, 56.926) in the residual PBV ≤13 ml/1000 ml versus 29.0 months (95% CI: 17.240, 40.760) in PBV > 13 ml/1000 ml (p = .000, log-rank test). The 1-, 3-, and 5-year LTP-free survival with residual PBV ≤13 ml/1000 ml were 100.0%, 88.5% and 23.5%, respectively, and the 1-, 3- and 5-year LTP-free survival with residual PBV > 13 ml/1000 ml were 93.8%, 37.5% and 0.00%, respectively ().
Figure 4. Kaplan–Meier local tumor progression (LTP) and overall survival (OS) with hepatocellular carcinoma (HCC); (A) Mean LTP was 49.0 months (95% CI: 43.129,54.871); and the 1-, 3-, and 5-year LTP-free survival with hepatic malignant tumors were 97.6%, 69.0% and 15.1%, respectively; (B) Mean OS was 53.140 months (95% CI: 49.639,56.588); and the 1-, 3-, and 5-year OS with hepatic malignant tumors were 97.6%, 88.1% and 62.9%, respectively.
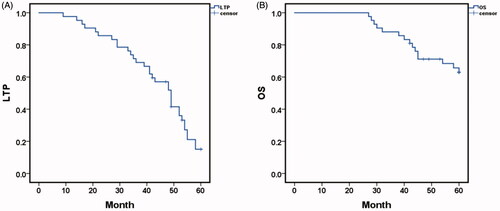
Figure 5. (A) Comparison of local tumor progression (LTP) between single tumor and multiple tumors(2–3lesions) after TACE sequential MWA treatment. Median LTP was 54.0 months (95% CI: 51.945, 56.055) in procedures with single lesion versus 35.0 months (95% CI: 30.305, 39.695) with 2–3 lesions (p=.000, log-rank test). The 1-, 3-, and 5-year LTP-free survival in patients with a single lesion were 100.0%, 100.0% and 28.0%, respectively; and the 1-, 3- and 5-year LTP-free survival with 2-3 lesions were 95.7%, 43.5% and 0.00%, respectively; B. Comparison of LTP in parenchymal blood volume (PBV) after MWA between PBV ≤13 ml/1000 ml and the PBV > 13 ml/1000 ml. Median LTP was 54.0 months (95% CI: 51.074, 56.926) with residual PBV ≤13 ml/1000 ml versus 29.0 months (95% CI: 17.240, 40.760) with PBV > 13 ml/1000 ml (p=.000, log-rank test). The 1-, 3-, and 5-year LTP-free survival with residual PBV ≤13 ml/1000 ml were 100.0%, 88.5% and 23.5%, respectively, and the 1-, 3- and 5-year LTP-free survival with residual PBV > 13 ml/1000 ml were 93.8%, 37.5% and 0.00%, respectively.
Os
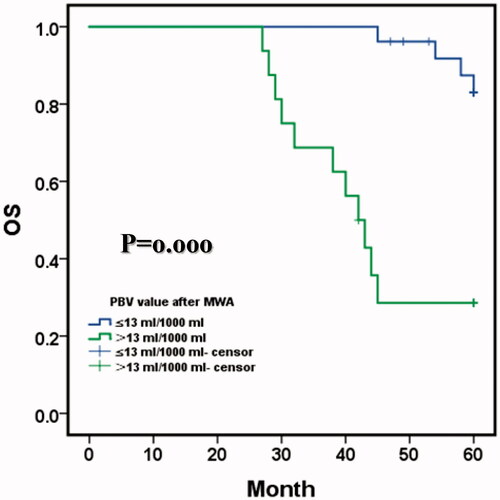
After the combination procedure, the mean OS was 53.140 months (95% CI: 49.639,56.588); and the 1-, 3-, and 5-year OS with hepatic malignant tumors were 97.6%, 88.1% and 62.9%, respectively (). Comparison of OS in PBV after MWA between PBV ≤13 ml/1000 ml and the PBV > 13 ml/1000 ml. Mean OS was 59.0 months (95% CI: 57.686, 60.460) with residual PBV ≤13 ml/1000 ml versus 43.196 months (95% CI: 37.195, 49.198) with PBV > 13 ml/1000 ml (p = .000, log-rank test). The 1-, 3-, and 5-year OS with residual PBV ≤13 ml/1000 ml were 96.2%, 96.2% and 83.0%, respectively, and the 1-, 3- and 5-year OS residual PBV > 13 ml/1000 ml were 93.8%, 68.8% and 28.6%, respectively ().
Figure 6. Comparison of OS in parenchymal blood volume (PBV) after MWA between PBV ≤13 ml/1000 ml and the PBV > 13 ml/1000 ml. Mean OS was 59.0 months (95% CI: 57.686, 60.460) with residual PBV ≤13 ml/1000 ml versus 43.196 months (95% CI: 37.195, 49.198) with PBV > 13 ml/1000 ml (p=.000, log-rank test). The 1-, 3-, and 5-year OS with residual PBV ≤13 ml/1000 ml were 96.2%, 96.2% and 83.0%, respectively, and the 1-, 3- and 5-year OS with residual PBV > 13 ml/1000 ml were 93.8%, 68.8% and 28.6%, respectively.
Factors affecting LTP and OS
Univariate Cox proportional hazards regression indicated that the residual PBV ≤ 13 ml/1000 ml and a single tumor in the liver were associated with a longer OS and LTP (All p < .05). Furthermore, multivariate Cox regression revealed that the residual PBV > 13 ml/1000 was an independent factor predicting a shorter OS and LTP (p = .030 and p = .000, respectively). For LTP, multivariate Cox regression showed that a tumor in a single lesion were independently predicted to have a longer LTP in patients with HCC (p = .033) ().
Table 5. Factors affecting LTP and OS.
Discussion
TACE used alone always leads to a low necrosis rate but a high intrahepatic recurrence rate of HCC[Citation12–14]. At present, TACE combined with ablation has become a favorable option, which controls tumor lesions significantly and improve patient survival[Citation15–17]. However, how to quickly evaluate the effect after TACE and provide individualized therapies according to the treatment information of different patients has always been a problem that puzzles clinicians. Investigators have studied the effectiveness of computed tomography perfusion imaging (CTPI) for the response of HCC to TACE therapy, suggesting that perfusion imaging can accurately measure tumor blood perfusion and thus would be used to evaluate the response after TACE [Citation18–20]. Moreover, the complete disappearance of tumor enhancement on final dual-phase (CBCT) is linked to the success of TACE [Citation21]. Thomas et al. found a significant correlation between initial blood volume and changes in blood volume between the initial and last PBV measurements (rho = 0.61; p < .001). Subgroup analysis further revealed that tumors with large differences before and after PBV had a better response to TACE [Citation22]. Olivier et al. analyzed the results of chemoembolization in patients with liver metastases from colorectal cancer and found that higher PBV values before chemoembolization correlated with greater tumor shrinkage, but only if the PBV decreases by more than 70% after treatment [Citation10]. To increase the effect of treatment and patient survival time, this research evaluate the feasibility of using CBCT to measure PBV changes in patients with HCC after TACE and to guide MWA for residual tumors.
Previous studies have suggested that the initial complete response represents an independent predictive factor for both recurrence-free and disease-specific survival in patients undergoing ablation for HCC [Citation23]. Of note, the accurate placement of the ablation probes in the tumor targets is a crucial factor affecting ablation for HCC [Citation24,Citation25]. Recently, sequential combination therapy has been frequently adopted, and ablation is usually performed 2-4 weeks following TACE under the guidance of CT [Citation26]. Nevertheless, CBCT using the Artis zee DSA system (SIMENS, Germany) can obtain synchronized acquisition with a flat-panel detector during C-arm rotation, and TACE and MWA can be performed sequentially on the same working bed. Following TACE, CBCT can be simultaneously used to scan the tumor and reconstruct 3 D images to plan the puncture site and the route [Citation27]. Wang et al. employed TACE combined with simultaneous CBCT -guided RFA to treat large HCCs with a technical success rate of 100% [Citation28]. In the study of Yuan et al, under the guidance of CBCT, the ablation needles accurately punctured the target lesions in forty-six patients and achieved a favorable tumor response [Citation29]. Therefore, CBCT can be used to guide accurate puncture with the aid of the DSA system, which not only achieves the simultaneous combination of TACE and MWA but also maximizes their synergistic effect, thereby improving the therapeutic efficacy.
Accurate lesion visualization after microwave ablation (MWA) remains a challenge. Computed tomography perfusion (CTP) has been proposed to improve visualization [Citation30]. The changes in perfusion immediately after the combination of TACE and MWA procedures, were found and captured by the blood volume (BV) of CBCT perfusion. It shows the potential of using quantitative values of BV to evaluate the treatment response during the procedure and guide further decisions for other palliative strategies to improve the prognosis. In this retrospective study, we evaluated 65 lesions in 42 patients. PBV measurement results in local tumors before TACE demonstrated a mean value of 115.1 ml/1000 ml ± 41.1; after TACE, the PBV immediately dropped to 50.8 ml/1000 ml ± 18.4. With regard to the residual tumor, the PBV was reduced to 13.8 ml/1000 ml ± 10.3 using sequential MWA. The decrease in PBV before and after the combination therapy was statistically significant (p < .001). Correlation analysis revealed that more residual PBV after combination therapy was negatively correlated with LTP (r = −0.622, p = .000). Additionally, the decrease in PBV before and after combination therapy was positively correlated with progression free survival (r = 0.393, p = .000). Compared with the last PBV value, the patients in this study had an average decline of more than 70%, and obtained a favorable response in tumor control.
Of note, there are several limitations to our research. First, the study was retrospective and the study population was small which may lead to biased results. Second, there are pitfalls in liver perfusion imaging with CBCT, and the density of iodinated oil may cause artifacts in perfusion maps and measurements. Moreover, breath-holding and immobility is clearly a significant factor for CBCT perfusion. As this is a retrospective analysis, patients unable to cooperate were probably not included. This means that the technical success of the method is biased. Additionally, the researchers selected only well-delineated tumors as target lesions, while those surrounded by streak artifacts from catheters or located in the truncated segment of the liver were not considered. However, prudent precautions have been taken to reduce the impact on the results.
In short, CBCT-based liver perfusion maps could provide reliable images to assess the technical success after HCC treatment by qualitative visual assessment and quantitative perfusion values, and a lower PBV value seems to predict better LTP. More specifically, tumor residual PBV or the rate of PBV decrease could be an effective indicator for predicting TACE or ablation therapy. Furthermore, HCC perfusion imaging guided by CBCT could analyze the hemodynamic changes in tumors before and after TACE and/or MWA treatment, which has important clinical significance for monitoring tumor response after interventional therapy. In addition, with the help of the DSA system (Syngo workstation), CBCT could be used to guide accurate puncture, which helps with decision-making in interventional procedures and improves the safety of treatment by reducing the risks of treatment.
Author contributions
ZNL and DCJ were responsible for writing the manuscript. YHL, JFL,GYS and JL participated in recruitment of the patients and collection of data. WGZ,ZQL and XLZ conducted statistical analyses. XWH and ZNL designed and supervised the study.
Acknowledgements
We would like to thank the patients and all employees of the First Affiliated Hospital of the Zhengzhou University for interventional radiology. Thanks to Ms. Chen Xinyue from CT collaboration NE Asia, Siemens Healthcare, Beijing, china, she contributed strong technique support as well as language polish to this article.
Disclosure statement
All authors declare that they have no conflict of interest.
Data availability statement
The clinical data were obtained from the interventional department of the First Affiliated Hospital of Zhengzhou University. The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171.
- Takayasu K, Arii S, Ikai I, Liver Cancer Study Group of Japan, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469.
- Song DS, Nam SW, Bae SH, et al. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. WJG. 2015;21(8):2395–2404.
- Llovet JM, Real MI, Montana X, Barcelona Liver Cancer Group, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739.
- Li W, Ni CF. Current status of the combination therapy of transarterial chemoembolization and local ablation for hepatocellular carcinoma. Abdom Radiol (NY). 2019;44(6):2268–2275.
- Biederman DM, Titano JJ, Bishay VL, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology. 2017;283(3):895–905.
- Ippolito D, Fior D, Bonaffini PA, et al. Quantitative evaluation of CT-perfusion map as indicator of tumor response to transarterial chemoembolization and radiofrequency ablation in HCC patients. Eur J Radiol. 2014;83(9):1665–1671.
- Pereira PL, Kruger K, Hohenstein E, et al. Intraprocedural 3D perfusion measurement during chemoembolisation with doxorubicin-eluting beads in liver metastases of malignant melanoma. Eur Radiol. 2018;28(4):1456–1464.
- Pellerin O, Pereira H, Moussa N, et al. Can cone-beam CT tumor blood volume predicts the response to chemoembolization of colorectal liver metastases? Results of an observational study. Eur Radiol. 2019;29(9):5022–5031.
- Kim KA, Choi SY, Kim MU, et al. The efficacy of cone-beam CT-based liver perfusion mapping to predict initial response of hepatocellular carcinoma to transarterial chemoembolization. J Vasc Interv Radiol. 2019;30(3):358–369.
- Luo Y, Jiang Y. Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer. J Coll Physicians Surg Pak. 2019;29(5):414–417.
- Zhu D, Yuan D, Wang Z, et al. Efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with radiofrequency ablation versus DEB-TACE alone in Chinese hepatocellular carcinoma patients. Medicine (Baltimore). 2019;98(26):e15682.
- Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. GUT. 2020;69(8):1492–1501.
- Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol. 2019;44(6):2283–2292.
- Wang Y, Ma L, Yuan Z, et al. Percutaneous thermal ablation combined with TACE versus TACE monotherapy in the treatment for liver cancer with hepatic vein tumor thrombus: a retrospective study. Plos One. 2018;13(7):e201525.
- Shimose S, Tanaka M, Iwamoto H, et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 2019;49(8):919–928.
- Chen G, Ma DQ, He W, et al. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14(37):5738–5743.
- Shaw CM, Eisenbrey JR, Lyshchik A, et al. Contrast-enhanced ultrasound evaluation of residual blood flow to hepatocellular carcinoma after treatment with transarterial chemoembolization using drug-eluting beads: a prospective study. J Ultrasound Med. 2015;34(5):859–867.
- Reiner CS, Gordic S, Puippe G, et al. Histogram analysis of CT perfusion of hepatocellular carcinoma for predicting response to transarterial radioembolization: value of tumor heterogeneity assessment. Cardiovasc Intervent Radiol. 2016;39(3):400–408.
- Loffroy R, Lin M, Yenokyan G, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266(2):636–648.
- Vogl TJ, Schaefer P, Lehnert T, et al. Intraprocedural blood volume measurement using C-arm CT as a predictor for treatment response of malignant liver tumours undergoing repetitive transarterial chemoembolization (TACE). Eur Radiol. 2016;26(3):755–763.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328.
- Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US Multicenter HCC Transplant Consortium. Ann Surg. 2017;266(3):525–535.
- Li Z, Jiao D, Han X, et al. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided microwave ablation in the treatment of small hepatocellular carcinoma. Cancer Imaging. 2020;20(1):13.
- Liu Z, Gao F, Yang G, et al. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumor Biol. 2014;35(8):7407–7413.
- Kato K, Abe H, Ika M, et al. C-arm cone beam computed tomography guidance for radiofrequency ablation in hepatocellular carcinoma. Oncology. 2017;92(3):142–152.
- Wang ZJ, Wang MQ, Duan F, et al. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. WJG. 2013;19(26):4192–4199.
- Yuan H, Liu F, Li X, et al. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided radiofrequency ablation in the treatment of solitary large hepatocellular carcinoma. Radiol Med. 2019;124(1):1–7.
- Bressem KK, Vahldiek JL, Erxleben C, et al. Comparison of different 4D CT-Perfusion algorithms to visualize lesions after microwave ablation in an in vivo porcine model. Int J Hyperthermia. 2019;36(1):1098–1107.