Abstract
Purpose
To evaluate the efficacy and safety of ultrasonography (US)-guided radiofrequency ablation (RFA) for treating T2N0M0 papillary thyroid cancer (PTC).
Methods
This retrospective study was approved by the ethics committee of Chinese PLA General Hospital (S2019-211-01). Twelve patients with T2N0M0 PTC (five men and seven women with a mean age of 41.0 ± 9.2 years (range, 21–61 years)), who were not eligible for or refused surgery, were included in our study. RFA was performed with the moving-shot technique, and the ablation area exceeded the tumor edge by at least 3 mm. US was performed before RFA, immediately, 1, 3 , 6 and 12 months after RFA, and every 6–12 months thereafter.
Results
All tumors were ablated as planned. The mean follow-up duration was 24.1 ± 6.9 months (range, 13–33 months). The tumor volume decreased significantly from 4.4 ± 2.8 ml to 0.3 ± 0.5 ml, and the volume reduction rate (VRR) was (93.7 ± 7.6)% at the final follow-up with two tumors (16.7%) disappearing. New or recurrent tumors were not found, and no local or distant metastasis were detected during follow-up. No life-threatening or delayed complications were observed.
Conclusion
RFA may be a potential alternative to surgery for the management of T2N0M0 PTC in select patients, especially for those who are ineligible for surgery.
Introduction
The incidence rates of thyroid cancer have increased globally over the last 50 years [Citation1]. Papillary thyroid cancer (PTC) is the most common, and least aggressive histologic type of thyroid cancer, accounting for most of the new cases [Citation2]. It has an excellent prognosis because of its indolent nature, and its mortality rate is low [Citation3].
Thyroid surgery is the first-line treatment of primary PTC recommended by several guidelines [Citation4–6]. However, surgery may lead to more harm than benefit due to surgery-related complications, such as permanent recurrent laryngeal nerve injury, inadvertent hypoparathyroidism, cosmetic problems and lifelong administration of levothyroxine [Citation7,Citation8], which have considerable effects on patients’ quality of life. Active surveillance was endorsed as an alternative to immediate surgery for select patients with very low-risk papillary thyroid microcarcinoma (PTMC) in 2015 by the American Thyroid Association (ATA) guidelines [Citation4]. Sakai et al. [Citation9] proposed active surveillance for the management of T1bN0M0 PTC in select patients. Although it is a safe alternative to immediate surgery, fewer patients would choose it due to various degrees of anxiety about the tumor-carrying state [Citation10]. In this scenario, minimally invasive image-guided thermal ablation may be a potentially attractive alternative. Besides, it might be a mean to compensate for overdiagnosis of small PTCs [Citation11].
With characteristics of minimal invasiveness, fast recovery and better appearance, image-guided thermal ablation, such as radiofrequency ablation (RFA), laser ablation (LA) and microwave ablation (MWA), has been used to treat benign thyroid nodules, low-risk papillary thyroid microcarcinomas and metastatic cervical lymph nodes in the past few years [Citation12–17]. RFA has been recommended for the treatment of benign thyroid nodules and recurrent thyroid cancers by several guidelines and consensus statements [Citation18–21]. Previous studies [Citation22,Citation23] reported that RFA for the treatment of T1N0M0 PTC achieved favorable results without major complications, and it was linked to a higher quality of life compared to surgery [Citation24]. However, little research has been performed on the validity of RFA for the management of T2N0M0 PTC (i.e., PTC >2 cm and ≤4 cm without clinical evidence of local region and distant metastases). Therefore, the purpose of this study was to investigate the efficacy and safety of ultrasonography (US)-guided RFA for the treatment of T2N0M0 PTC.
Materials and methods
Patients
This retrospective study was approved by the ethics committee of Chinese PLA General Hospital (S2019-211-01). A written informed consent was gained from each patient before RFA, which emphasized that surgery is the standard treatment for PTC, and that RFA cannot avoid the potential risks of recurrence, and eradicate occult local and distant metastasis.
Patients with T2N0M0 PTC treated with US-guided RFA from April 2014 to October 2018 were retrospectively reviewed. Follow-up ended in October 2020. The inclusion criteria were as follows: (1) a single nodule with the diameter >2 cm and ≤4 cm on US; (2) no clinical or radiological evidence of extrathyroidal extension, lymph node metastasis (LNM, cN0) or distant metastasis; (3) US-guided core needle biopsy (CNB) confirmed PTC; (4) no history of neck irradiation or surgery; and (5) more than 12 months of follow-up.
Pre-ablation evaluation
All patients underwent US and contrast-enhanced ultrasonography (CEUS) before RFA using the Siemens Acouson Sequoia 512 (Siemens, Mountain View, CA) scanner with a 7.0 MHz linear array transducer, the Siemens S2000 (Siemens, Mountain View, CA) scanner with a 8.0 MHz linear array transducer, or the Mindray Resona 7 (Mindray, Shenzhen, China) scanner with a 5.6–10 MHz linear array transducer. The size, volume, location and US features of each nodule were recorded. Nodule volume (ml) was calculated using the ellipsoid volume formula: length × width × depth × 0.524, with measurements expressed in cm. US features, including composition, echogenicity, shape, margin and echogenic foci, were evaluated and recorded according to the American College of Radiology Thyroid Imaging, Reporting, and Data System [Citation25].
CEUS was used to evaluate the tumor’s blood supply. SonoVue (Bracco International, Milan, Italy) suspension was bolus injected at a dose of 2.4 ml, immediately flushing with 5 ml of normal saline. At the initiation of the injection of SonoVue, timer was turned on, and the video clip was digitally recorded for 3 min to further analyze the CEUS enhancement pattern. It was divided into three patterns, that is, low, equal and high enhancement, by comparing the echo intensity within the tumor with that in the surrounding thyroid parenchyma at peak enhancement [Citation26].
In addition, complete blood count, coagulation test, thyroid function, calcitonin and serum thyroglobulin were also performed, and all of these were within normal limits. All patients were forbidden to take antiplatelet or anticoagulant medications for at least 1 week prior to RFA. All patients were diagnosed as PTC via US-guided CNB before RFA.
US-guided RFA procedure
One US physician (Y.K.L., more than 20 years of experience in thyroid US and interventional US) performed all RFA procedures in an outpatient US interventional room. A bipolar radiofrequency generator (CelonLabPOWER; Olympus Surgical Technologies Europe, Hamburg, Germany) and an 18-gauge straight-type electrode with a 1.5 cm active tip (CelonProSurge micro 100-T15; Olympus Surgical Technologies Europe, Hamburg, Germany) were used.
Patients were placed in the supine position with the neck extended during the RFA procedure, and a multiparametric monitor was connected to patients in order to monitor their breath rate, blood pressure, heart rate and PO2. After skin sterilization, 1% lidocaine was administrated at the subcutaneous puncture site and the thyroid anterior capsule for local anesthesia. A mixture of 1% lidocaine and normal saline was continuously injected using another 23 gauge needle to form at least 1 cm distance between thyroid nodule and critical structures (i.e., the trachea, esophagus, recurrent laryngeal nerve and blood vessels) to prevent thermal injury [Citation22]. After insertion of the electrode into the deepest and most remote part of thyroid nodule under US guidance, ablation was commenced at 3 W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the output power was increased, up to 9 W. The trans-isthmus approach and moving-shot technique were used during the procedure. The ablated zone exceeded the margin of the thyroid nodule at least 3 mm to prevent marginal recurrence [Citation24]. The procedure was not terminated until a transient hyperechoic zone covered the whole tumor, and the needle track was coagulated to prevent tumor cell seeding when withdrawing the electrode. At the end of each procedure, CEUS was performed to evaluate the ablation zone; if residual enhancement was present, additional ablation was performed during the same treatment session. After the procedure, patients were observed for 1–2 h with compression of the neck for 20–30 min for any discomfort or complications before discharge.
Post-ablation follow-up
US was performed 1, 3, 6 and 12 months after RFA, and every 6–12 months thereafter. The size, volume and vascularity of the ablation zones as well as new tumors in the thyroid were carefully evaluated. The volume reduction rate (VRR) was calculated as follows: VRR=([initial volume – final volume] × 100)/initial volume. If there was new suspicious tumor in thyroid, US-guided CNB was performed to exclude PTC. Cervical lymph nodes were carefully evaluated. In case that there were any suspicious characteristics (i.e., microcalcifications, cystic aspects, peripheral vascularity, hyperechogenicity and/or round shape) [Citation27] in cervical lymph nodes, US-guided CNB was performed to determine whether the nodes were metastatic or reactive. If there were suspicious symptoms of distant metastasis, computed tomography (CT), positron emission tomography or bone scan was performed.
Data analysis
Technical feasibility, technical success, technique efficacy and rate of complications at follow-up [Citation28,Citation29] were analyzed.
Technical feasibility was defined as the ability to target the nodule and perform the ablation as planned. Technical success was defined as complete absence of enhancement in the tumor on CEUS immediately after RFA. Technique efficacy was evaluated by local tumor progression, which is defined as (1) new or persistent PTC; (2) LNM; or (3) enlargement of the ablation zone [Citation30]. Technical safety was evaluated by the rate of complications, which were reported according to the reporting standards of the Society of Interventional Radiology [Citation29].
Results
From April 2014 to October 2018, a total of 12 patients with T2N0M0 PTC treated with US-guided RFA were included in this study (). The demographic and clinical characteristics in 12 patients are listed in . There were five men and seven women with a mean age of 41.0 ± 9.2 years (range, 21–61 years). Among them, eight patients refused surgery because of cosmetic reason and concern of surgery-related complications, while four patients were ineligible for surgery owing to a high risk associated with general anesthesia as follows: renal insufficiency (n = 1), poor pulmonary function (n = 1) and hypertension (n = 2).
Figure 1. Flowchart summarizing the patient inclusion process. PTC: papillary thyroid cancer; PTMC: papillary thyroid microcarcinoma; RFA: radiofrequency ablation.
Table 1. Demographic and clinical characteristics of T2N0M0 papillary thyroid carcinoma.
All patients underwent one session of RFA, and the RFA treatment parameters are summarized in . All tumors were correctly targeted, and RFA was performed as planned. On CEUS immediately after RFA, all ablation zones showed non-enhancement, which encompassed the target tumors (i.e., the technical success rate was 100%).
Table 2. Radiofrequency ablation treatment parameters.
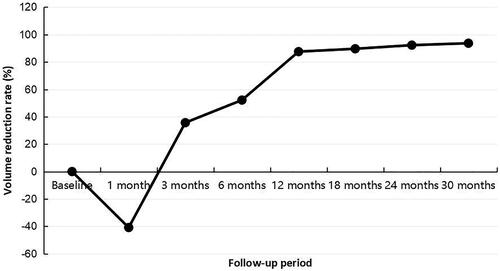
The mean follow-up duration was 24.1 ± 6.9 months (range, 13–33 months). The index tumor volume decreased gradually from 4.4 ± 2.8 ml (range, 1.0–11.4 ml) before RFA to 0.3 ± 0.5 ml (range, 0.0–1.0 ml) at the final follow-up (, ). The VRR at 1-month visit was (–40.8 ± 202.5)%. After that, it changed to positive, and increased gradually (, ). At last follow-up, the VRR was (93.7 ± 7.6)%.
Figure 2. Sonograms of a 36-year-old male with T2 papillary thyroid carcinoma. (A) A hypoechoic nodule with an initial volume of 11.39 ml was detected in the right thyroid lobe (white arrow). (B) The nodule was covered by a hyperechoic area (white arrow) during the radiofrequency ablation (RFA) procedure, and hydrodissection (arrow head) was used to protect the vital organs from thermal injury. (C) Immediately after RFA, the ablated zone showed non-enhancement on contrast-enhanced ultrasonography. (D–G) The volume of the ablated zone was 4.91 ml, 3.76 ml, 2.40 ml and 1.81 ml, respectively, at 1, 3, 6 and 27 months after RFA.
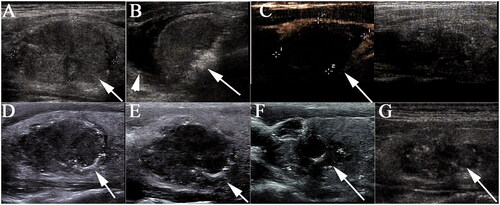
Table 3. Changes in mean volume at each follow-up point.
During follow-up, two ablation zones disappeared (). US-guided CNB was performed 6 months after RFA in seven of these patients, and no viable neoplastic cells were found in the center and edge of the ablated zone as well as the surrounding thyroid parenchyma. At the end of follow-up, no local tumor progression or distant metastasis was detected.
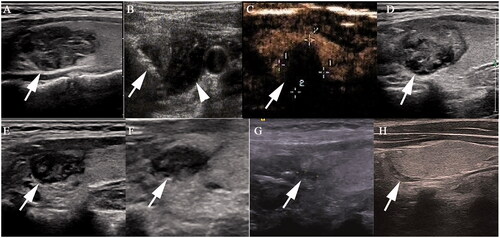
Figure 4. Radiofrequency ablation (RFA) treatment and follow-up of a 29-year old woman with T2 papillary thyroid carcinoma. (A) A heterogeneously hypoechoic nodule with an irregular margin, size 2.4 × 1.3 × 1.4 cm, was detected in the upper part of left thyroid lobe (white arrow). (B) The nodule was covered by a hyperechoic area (white arrow) on ultrasonography during the RFA procedure, and hydrodissection (arrow head) was used to protect the vital organs from thermal injury. (C) Contrast-enhanced ultrasonography immediately after RFA showed non-enhancement in the ablation zone. (D–G) The ablation area decreased gradually to 2.0 × 1.1 × 1.3 cm, 1.3 × 0.7 × 0.8 cm, 0.9 × 0.6 × 0.8 cm and 0.5 × 0.2 × 0.4 cm 1, 3, 6 and 12 months, respectively, after ablation. (H) Ultrasonography 18 months after ablation showed a needle track.
All patients tolerated the RFA procedure well. Some patients experienced slight burning sensation, mild pain or both, but no one asked to stop the procedure. No major complications were observed. There were no cases of local infection, skin burning or hematoma.
Discussion
This study was the first case series to describe the treatment of T2N0M0 PTC with US-guided RFA. It indicated that RFA may be a potential management for controlling T2N0M0 PTC. Tumor size reduced significantly with a VRR of 93.7% at 30-month follow-up after RFA, and a disappearance rate of 16.7%. All patients with T2N0M0 PTC tolerated the RFA procedure without major complications, and no cases of local tumor progression and distant metastasis were observed.
The initial surgical procedure recommended by ATA guidelines in 2009 was a near-total or total thyroidectomy for patients with PTC >1 cm [Citation31]. The overall survival was not different in a comparison of total thyroidectomy with lobectomy [Citation32]. Therefore, ATA guidelines revised in 2015 recommended thyroid lobectomy as an acceptable option for T2 PTC without extrathyroidal extension and LNM [Citation4]. The extent of surgery for PTC has a tendency to decrease.
In recent years, US-guided thermal ablation has been introduced to treat low risk PTC [Citation17,Citation22,Citation23], and obtained excellent results. A large cohort study with a mean follow-up of 42.15 months [Citation14] has reported the efficacy of RFA in the treatment of PTMC with a mean VRR of 98.8%, and tumor disappearance rate of 88.41%. In addition, a multicenter study [Citation23] on thermal ablation for the treatment of T1bN0M0 PTC showed a VRR of 99% at 48-month visit, and complete disappearance rate of 61.6% during a mean follow-up of 24.9 months. In the present study, the mean VRR at last follow-up (93.7%) was similar to those previous studies, but the disappearance rate (16.7%) was much lower. The disappearance rate correlated with the larger initial tumor size, and comparatively shorter follow-up period.
Thermal ablation is safe for the management of low risk PTC. Tong et al. reported that the pooled incidence of complications after MWA and RFA of PTMC was 6.0% and 1.7%, respectively [Citation33]. The incidence rates of overall and major complications after RFA of benign thyroid nodules and recurrent thyroid cancers were 3.5% and 1.6%, respectively [Citation34]. In our study, no major or life-threatening complications were encountered. The lack of major complications and local tumor progression in our study was likely because of the application of hydrodissection to protect the vital organs, and the extensive ablation to prevent recurrence. On the other hand, experience of US physician in thermal ablation is also important to reduce complications and tumor recurrence.
Recurrences after thermal ablation for PTC were low. A recent meta-analysis demonstrated that the pooled proportion of recurrence after thermal ablation was 0.4% [Citation35]. Compared with MWA and LA, RFA had a lower pooled proportion of recurrence (0.01%) [Citation33]. The incidence of LNM after thermal ablation ranged from 0.6% to 3.0% [Citation33,Citation36]. Moreover, comparative studies have suggested that there were no significant differences between patients treated with thermal ablation and surgery in terms of recurrence and LNM [Citation24,Citation30]. Despite of this, concerns remain about incomplete ablation. Ma et al. [Citation37] reported 12 patients with PTCs who were initially treated by thermal ablation and subsequently underwent surgical therapy. Surgical pathology demonstrated residual cancers and LNM. However, their inclusion for RFA was not low risk PTCs, as seven patients in their study had multiple tumors, and two patients had LNM before thermal ablation. Therefore, cautious consideration should be made before thermal ablation. In addition, careful evaluation using US or CT prior to RFA is of great importance because of its tendency toward the multiplicity of PTC and high incidence of LNM [Citation38]. We propose that low risk PTC should be suitable for the treatment of thermal ablation.
Several limitations should be mentioned in our study. Due to the retrospective design and its performance at a single center, selection bias existed. As multifocality in PTC more commonly occurs incidentally as microscopic foci diagnosed on histology report, occult tumors may be missed when performing RFA. However, patients with unifocal and multifocal disease had similar outcomes [Citation39]. Regardless of the fact that CNB was performed in all patients prior to RFA, it cannot eliminate aggressive variants of PTC. The number of patients was small, and the follow-up period was relatively short considering such an indolent disease process. Thus, the general results on long-term effectiveness cannot be derived from our research. Prospective studies with larger samples are required to further evaluate the therapeutic effect of RFA on T2N0M0 PTC.
In conclusion, US-guided RFA appears to be effective in the management of T2N0M0 PTC. RFA may be a potential, minimally invasive alternative to surgical resection for patients who have high risk of surgery or decline surgery. Further studies are needed to address this issue.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29.
- Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–1348.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(Suppl. 1):1–122.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Endocr Pract. 2016;22(5):622–639.
- Linos D, Economopoulos KP, Kiriakopoulos A, et al. Scar perceptions after thyroid and parathyroid surgery: comparison of minimal and conventional approaches. Surgery. 2013;153(3):400–407.
- Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer – recent advances and future directions. Nat Rev Endocrinol. 2018;14(11):670–683.
- Sakai T, Sugitani I, Ebina A, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019;29(1):59–63.
- Jeon MJ, Lee Y, Sung T, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: a cross-sectional study. Thyroid. 2019;29(7):956–962.
- Mauri G, Sconfienza LM. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int J Hyperthermia. 2017;33(4):489–490.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian Minimally Invasive Treatments of the Thyroid Group. Thyroid. 2020;30(12):1759–1770.
- Shi Y, Zhou P, Zhao Y, et al. Microwave ablation compared with laser ablation for treating benign thyroid nodules in a propensity-score matching study. Front Endocrinol. 2019;10:874.
- Yan L, Lan Y, Xiao J, et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol. 2021;31(2):685–694.
- Han Z, Dou J, Cheng Z, et al. Efficacy and safety of percutaneous ultrasound-guided microwave ablation for cervical metastatic lymph nodes from papillary thyroid carcinoma. Int J Hyperthermia. 2020;37(1):971–975.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol. 2021;11:575152.
- Kim J, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian Minimally-Invasive Treatments of the Thyroid (MITT) Group. Int J Hyperther. 2019;36(1):375–381.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Zhang M, Tufano RP, Russell J, et al. Ultrasound-guided radiofrequency ablation versus surgery for low risk papillary thyroid micro-carcinoma: results of over 5 years follow-up. Thyroid. 2020;30(3):408–417.
- Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–595.
- Zhang Y, Zhou P, Tian S, et al. Usefulness of combined use of contrast-enhanced ultrasound and TI-RADS classification for the differentiation of benign from malignant lesions of thyroid nodules. Eur Radiol. 2017;27(4):1527–1536.
- Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European Thyroid Association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2(3):147–159.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology. 2014;273(1):241–260.
- Xiao J, Zhang Y, Zhang M, et al. Ultrasonography-guided radiofrequency ablation vs. surgery for the treatment of solitary T1bN0M0 papillary thyroid carcinoma: a comparative study. Clin Endocrinol. 2020;1–8.
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214.
- Barney BM, Hitchcock YJ, Sharma P, et al. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011;33(5):645–649.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperther. 2019;36(1):1277–1285.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Ji L, Wu Q, Gu J, et al. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19(1):16.
- Ma B, Wei W, Xu W, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: a retrospective case review and literature review. Thyroid. 2018;28(9):1134–1142.
- Yeh MW, Bauer AJ, Bernet VA, et al. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid. 2015;25(1):3–14.
- Harries V, Wang LY, McGill M, et al. Should multifocality be an indication for completion thyroidectomy in papillary thyroid carcinoma? Surgery. 2020;167(1):10–17.