Abstract
Objective
To evaluate the safety and efficacy of ultrasound (US)-guided percutaneous microwave ablation (UgPMWA) for palliative treatment of advanced head and neck malignancies.
Materials and methods
This study includes 18 consecutive patients with advanced head and neck malignancies (n = 24), who have undergone UgPMWA for palliative treatment at our institution from December 2016 to April 2020. The maximum diameter and volume of the tumor were assessed by US, CT or MRI before microwave ablation (MWA), 1, 3 and 6 months after MWA and every 6 months thereafter. The quality of life was clinically assessed by the University of Washington Head and Neck Quality of Life questionnaire (UW-QOl).
Results
The success rate of tumor-targeting microwave antenna placement was 100%. No nerve injury and serious complications or death occurred during the perioperative period. The follow-up duration varied from 1 month to 38 months (11.56 ± 10.23 months) among patients. By the last follow-up before submission, the value of maximum diameter (5.00 ± 2.90 vs 3.28 ± 2.11 cm. p < 0.05) and tumor volume decreased significantly(74.35 ± 46.88 vs 47.45 ± 24.08 cm3. p < 0.05)respectively after palliative treatment with UgPMWA. UW-QOl of the patients was improved (59.24 ± 11.51 vs 69.84 ± 8.12, p < 0.05).
Conclusion
UgPMWA is safe and effective for the palliative treatment of head and neck malignancies. Ultrasonic guidance can indicate an accurate location of the microwave antenna. It can also monitor the ablation area in real-time during the operation to avoid damage to surrounding normal tissues.
Introduction
Head and neck malignancies are the sixth most common malignancy worldwide. They account for approximately 10% of all malignancies, and more than 600,000 new cases are diagnosed each year globally [Citation1]. The incidence rate ranks sixth in men and the mortality rate ranks seventh in China [Citation2]. The most common pathological type of head and neck malignancies is squamous cell carcinoma (SCC), and the lesions also include some other pathological types of primary and metastatic tumors. Approximately two-thirds of these patients are initially accompanied by local or distant metastases which may cause following symptoms, including intractable pain, compressional airway obstruction, dysphagia, and decreased mobility of the tongue [Citation3].
Surgery with adjuvant radiation therapy has been typically used treating head and neck malignancies. Although the curative effect has been improved, the local recurrence rate of patients in stage III and IV is still as high as 60%, and 3-year survival rate is still lower than 30% [Citation3]. For patients with recurrence, radical surgery is not quite effective for local control and for improvement of survival rate and quality of life [Citation4,Citation5], and the patients can hardly be cured [Citation6]. Besides, some recurrent tumors cannot receive standard salvage treatment due to extensive area of local lesions or risks caused by adjacent important anatomical structures. There are also cases that surgical treatment is limited for physical reasons such as heart or lung dysfunction or liver functional reserve decline. Continuous radiotherapy alone has the disadvantages such as radiation dose limits, damage to surrounding normal tissues and the risk of development of dual cancers [Citation7]. In the past, these inoperable patients were usually given palliative or investigational chemotherapy, or simply provided with the placebos. In recent years, targeted therapy and immunotherapy have emerged, but the average life extension of most patients is still less than 1 year [Citation8–10]. The high recurrence rate and low quality of life of these patients require the development of new and effective palliative treatments.
In recent years, the frequently used thermal ablation techniques for head and neck lesions include microwave ablation (MWA), radiofrequency ablation (RFA), and laser ablation (LA) [Citation11]. Compared with other thermal ablation technologies, MWA can produce a larger ablation area in shorter time and reduce the carbonization of surrounding tissues secondary to the area of heat dissipation. Besides, MWA can reduce the heat sink effect mediated by blood perfusion when treating tumors with rich blood supply or close to the large vessels of the neck [Citation12]. In clinical practice, MWA has increasingly shown tremendous advantages such as small surgical trauma, high safety, rapid recovery, fewer complications, repeatable operation and protection effects for organs and tissues, which leads to a broad application to multiple systems of the body [Citation13–15].
Since the 1960s, with the development of various imaging technologies, image-guided thermal ablation of tumors has developed rapidly, among which US, CT and MRI are the most commonly used guide imaging modalities. In the region of head and neck, US and CT are the most frequently adopted. CT can better show a full view of tumor, more accurate localization and clearer image compared to US. But different than the blind puncture of CT-guided intervention, US can guide the punctures of anesthetic needle and ablation antenna during UgPMWA in real-time, which greatly improves the safety especially in the region of head and neck where blood vessels and nerves are densely packed. CT-guided puncture requires repetitive scanning to confirm the location of the ablation antenna. The treatment process takes a longer time, and patients are at risk of excessive radiation [Citation16]. Besides, US has advanced rapidly in recent years, especially the development of maxillofacial and oral US [Citation17,Citation18], and US has its unique advantages in the diagnosis of cervical metastatic lymph nodes [Citation19]. US provides a new approach to guide the interventional treatment of head and neck malignancies.
The purpose of this study is to evaluate the safety and effectiveness of UgPMWA for palliative treatment of advanced head and neck malignancies.
Materials and methods
Patients
This clinical trial is a retrospective study, which is approved by the ethics committee of Sichuan Cancer Hospital (Ethical batch no. Js-2016-011). Written informed consent was received from each patient before treatment. From December 2016 to April 2020, a total of 18 consecutive patients (11 males, 7 females; age: 60.06 ± 12.95 years, range: 33–80 years) with advanced head and neck malignancies meeting the following criteria were identified and enrolled in our study. Inclusion criteria were as follows: 1) The lesion confirmed as head and neck malignancies by pathology; 2) Patients at stage IV or stage III with severe local symptoms, or with recurrence after surgery; 3) Patients with poor efficacy of chemoradiotherapy, ineligible or refused to undergo repeated neck explorations for high surgical risk or other reasons; 4) lesions with safe puncture pathways (no major vessels or critical nerves were present in the section of the puncture). Exclusion criteria were as follows: 1) Patients are extremely debilitating, or with Eastern Cooperative Oncology Group (ECOG) grade ≥ 3; 2) Patients with serious cardiovascular or cerebrovascular disease; 3) Patients that have experienced any allergic reaction to anesthetic; 4) Patients with coagulation disorder; 5) Patient or family members refuse to implement MWA ().
Table 1. Patient information, tumor characteristics and follow-up information.
Pre-ablation assessment
Clinical assessment
The pre-ablation examination consisted of complete blood count and blood coagulation test (prothrombin time and activated partial thromboplastin time). The auxiliary examinations included pulmonary function, electrocardiogram, echocardiography and fibrolaryngoscopy. Fibrolaryngoscope was performed on all patients before MWA and on patients who complained of hoarseness or other symptoms related to nerve injury possibly caused by MWA.
The UW-QOl [Citation20] was conducted before surgery to assess life quality of the patients.
Ultrasonic evaluation
Equipment and methods
Philips, EPIQ7 (Philips Healthcare, Bothell, WA, USA) ultrasonic diagnostic instrument was used. The L12-5 probe with the frequency of 5–12MHz was used for 2D US and 2D Contrast Enhanced Ultrasound (CEUS). Routine examination, intraoperative guidance and CEUS were performed on the target lesion before, during and after MWA. SonoVue was the ultrasonic contrast agent (Bracco Suisse SA, Italy, approval no. J20130045). The contrast agent, was dissolved in 5 ml saline, and a bolus of 2.4 ml of this solution was injected into the antecubital vein quickly, followed by a 5 ml saline flush.
The size, shape, boundary, location, blood flow and adjacent tissues of the lesion were observed by 2D US. The perfusion, the bearing vessels and the peripheral vessels of the lesions were observed by CEUS. Measurement of the three vertical diameters (maximum diameter and the two other vertical diameters) of the lesion was also carried out under CEUS. Lesion volume was computed by the following formula: volume = The product of the three vertical diameters × π/6. If the lesion had local fluid sonolucent area caused by tumor necrosis, we measured the three vertical diameters of the solid portion and compute its volume. The lesion was reconstructed in three dimensions according to the patients’ CT or MRI images and the results of 2D US and CEUS. Then, the puncture position on the body surface, puncture angle and puncture depth were determined after discussion of the two radiologists who performed the operation. All patients were examined with 2D US and CEUS before MWA by a radiologist specialized in head and neck imaging and surgery.
Ablation methods
Anesthesia method
Patients enrolled in this study were in older age and in poor physical conditions. To take into account of their high preoperative risk of anesthesia, the method of conscious sedation and cervical plexus block was adopted to reduce the intraoperative pain of patients. The procedure was as follows, all patients were continuously administered Dexmedetomidine hydrochloride at 1 μg/kg.min via the vein passage for 15 min, then the maintenance dose was changed to 0.3 μg/kg.min. The operative region was disinfected and shopped towels routinely, 5 ml mixture of 1% Lidocaine hydrochloride and 0.375% Ropivacaine hydrochloride was used to block the cervical plexus on the surgical side. Patients inhaled oxygen through the nose mask with oxygen flowing through the anesthesia machine, and sufficient oxygen supply was maintained at all times till the end of the operation. During the entire procedure, the anesthesiologist monitored the patients and kept the vital signs stable ().
Table 2. Intraoperative situation and Uw-Qol of the patients.
Ablation process
MWA of the lesion began after the local anesthesia had taken full effect. The MWA instrument (KY-2000; Kangyou Medical, Nanjing, China) was used to administer microwave energy. The generator is capable of producing 1–100 W of power at the frequency of 2450 MHz. A rigid slit needle microwave antenna of a diameter of 1.6 mm was used. The antenna can directly penetrate deep tissue. Coated with Polytetrafluoroethylene and equipped with an internal water cooling system, the antenna can reduce the electrode temperature and prevent tissue adhesion.
Before inserting the antenna, we injected a mixture of 2% lidocaine and physiological saline solution (1:8 dilution) between the lesion and the surrounding tissue under ultrasonic guidance to form a liquid barrier to prevent thermal damage. Then, a small incision of skin about 2 mm was made at the percutaneous site. Under the US-guidance (EPIQ7 equipped with the L12-5 probe), the cooled-shaft needle microwave antenna was inserted into the lesion along the longest axis. The microwave antenna puncture under US-guidance often adopted transverse or oblique transverse scanning to keep the peripheral critical tissues of the lesion in the field view of the image throughout the entire operation. According to the lesion location, the microwave antenna was usually punctured from the medial side of the neck to the lateral side in order to reach the lesion safely without damaging the trachea. If there was local fluid sonolucent area caused by tumor necrosis in the lesion, the fluid was aspirated with a fine needle before ablation. The output power of MWA ranged from 30 to 45 W depending on the position and volume of the lesion. During ablation, the vaporization area expanded to the surrounding area over time, monitored by US in real time. For radical purpose, the ablation area should cover and exceed (diameter ≥ 5 mm) the tumor area. However, in our study, the purpose of ablation is to reduce the lesion volume rather than complete ablation. Therefore, we communicated to the patients and their family before surgery, explaining that the purpose of ablation is to reduce the volume of the lesions and alleviate relative symptoms. If the recurrence occurs, ablation can be repeated. The ablation could be terminated when the vaporization area completely covered and exceeded the boundary of the target ablation area. Next, we administrated CEUS using the same intraoperative US system about 30 min after the cease of MWA to avoid the interference of steam. When CEUS showed no enhancement in the ablation area through scanning in multiple directions and sections, it indicated that the targeted lesion area was fully ablated. At the end of the procedure, all patients remained under observation for 30 min with compression on the site of the percutaneous puncture to prevent bleeding or hematoma formation. Aforementioned ablation procedures were performed by the same 2 radiologists with more than 10-year-experience in musculoskeletal ultrasound and 4-year-experience in US-guided MWA.
Follow-up
The maximum diameter and the volume of the lesion were measured 1, 3 and 6 months after UgPMWA and every 6 months thereafter with 2D US, CEUS and CT or MRI. CT or MRI can improve the subjectivity of measurement of US and monitor the area of head and neck in case there is any new lesion. Meanwhile, the quality of life of the patients was quantitatively evaluated by the UW-QOl. The volume reduction rate was calculated by the following equation: volume reduction rate (VRR) = (initial volume − final volume)×100/initial volume. During the follow-up period, the maximum diameter and the volume of the solid component of the lesion which was aspired liquid was separately measured and the VRR was calculated except for measuring the overall volume.
Statistical analysis
Data analysis was performed with statistical software (SPSS23.0; SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to check normality, then the skewed distribution data were converted to normally distributed data. Lesion maximum diameters and volumes at pre-MWA and each follow-up time point of post-MWA were compared and evaluated by analysis of variance. The UW-QOl of the patients was analyzed by analysis of variance. The level of statistical significance was defined as p values<0.05.
Result
Patient population
Eighteen patients (with 24 lesions) were treated with UgPMWA. There were 10 cases of advanced head and neck squamous cell carcinoma (SCC) or its postoperative metastasis to the cervical lymph nodes, and other carcinomas’ postoperative metastasis to the cervical lymph nodes (thyroid papillary carcinoma, n = 4); left mucoepidermoid carcinoma of parotid gland (n = 1); breast cancer (n = 2); giant follicular carcinoma of thyroid (n = 1). During ablation, all patients were anesthetized with sedative analgesia and nerve block.
Among the 18 patients before submission, 2 were followed up for more than 2 years, 2 for 6–12 months, 5 for 3–6 months, 6 for 1–3 months and 3 were lost contact. The mean follow-up time is (11.56 ± 10.23 months, range 1–38 months). Four patients died, one dying of asphyxiation three months after surgery, the other three passing away as the result of the progress of their disease ().
Figure 1. (A, B) A 79-year-old man had a thyroid papillary carcinomas’ postoperative metastasis to the cervical lymph nodes, before MWA, a huge mass could be seen in the neck, and the lesion (T) consisted of cystic and solid parts. (C, D) Aspirating the fluid in the lesion with a fine needle (arrow), the dark red fluid (arrow) aspirated from the lesion during operation. (E) After aspiration, an microwave atenna was placed in the lesion to ablate the solid portion. (F) During the MWA procedure, multiple echogenic microbubbles (arrow) around the antenna tip were noted. (G) After MWA, CEUS showed that most of the lesion was inactivated and showed no enhancement. (H) Six months after MWA, the lesion almost could not be seen and only a scar and some pigmentation were left on the neck.
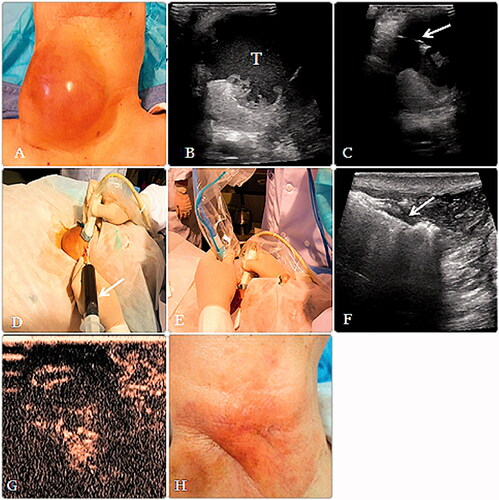
Figure 2. (A, B) A 57-year-old woman had a thyroid papillary carcinomas’ postoperative metastasis to the cervical lymph nodes.A huge mass could be seen in the neck and the lesion is adjacent to the common carotid artery (CCA). (C) Before MWA, axial contrast-enhanced CT scan demonstrated a large tumor (arrow) was located in the right neck of the patient and the airway is compressed. (D) During the procedure, multiple echogenic microbubbles (arrow) around the antenna tip were noted. (E) After MWA, CEUS showed that most of the lesion was inactivated and showed no enhancement. (F) After 8 months, axial CT scan demonstrated that the lesion volume decreased significantly and the airway compression recovered. (G) After 8 months, the volume of the lesion decreased from 139.47 cm3 to 14.01 cm3. (arrow). (H) The lesion almost could not be seen and only a scar and some pigmentation were left on the neck.
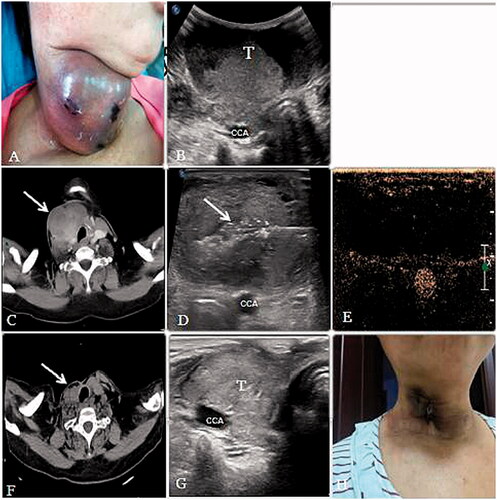
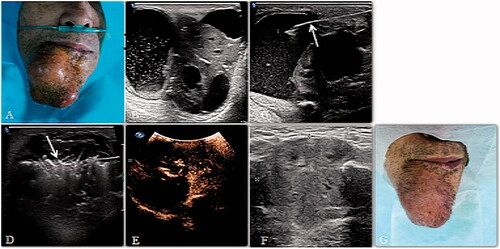
Figure 3. (A, B) A 69-year-old man had a primary SCC on the right mentum region. An irregularly shaped tumor could be seen and the lesion consisted of cystic and solid parts. (C) Aspirating the fluid in the lesion with a fine needle (arrow). (D) After aspiration, an microwave atenna (arrow) was placed in the lesion to ablate the solid portion. (E) After MWA, CEUS showed that most of the tumor was inactivated and showed no enhancement. (F, G) Sonogram of the lesion 4 months after MWA, the tumor shrank visibly.
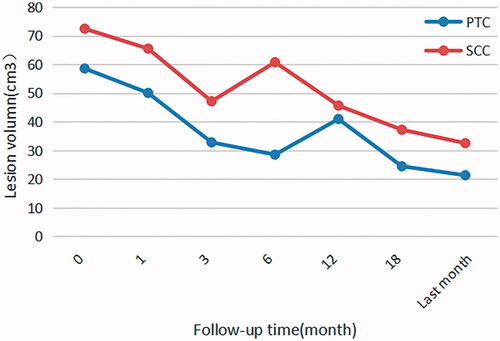
Maximum diameter and tumor volume
Since our study is just palliative treatment for the advanced head and neck malignancies and the lesions near important tissues could not be completely ablated, some of the patients’ tumors might grow again. In our study, the most common pathological types of lesions were papillary thyroid carcinoma (PTC) and SCC. During the follow-up period, in the first 6 months, the volume of the ablated area gradually decreased. After about 6 months, proximal vascular residue in patients with SCC would regrow. Then, after about 12 months, proximal vascular residue in patients with PTC would regrow. So UgPMWA was repeated, for 12 patients with 1 session, 4 patients with 2 sessions, 1 patient with 3 sessions and 1 patient with 4 sessions. The mean number of session is (1.44 ± 0.78, range 1–4). At the last follow-up the mean value of maximum diameter of the lesion post-ablation was significantly smaller than that pre-ablation (3.28 ± 2.11 vs 5.00 ± 2.90 cm. p < 0.05). The volume of the lesions post-ablation was also significantly smaller than that pre-ablation (47.45 ± 24.08 vs 74.35 ± 46.88 cm3. p < 0.05). The VRR was (62.76 ± 20.45, range, 21.90–94.50) % at the last follow-up. The mean value of maximum diameter of the solid part of the lesions which had more liquid component post-ablation was significantly lower than that pre-ablation (1.72 ± 1.42 vs 3.72 ± 1.42 cm. p < 0.05). And the post-ablation volume was also notably smaller than pre-ablation volume (4.32 ± 3.40 vs 10.07 ± 5.42 cm3. p < 0.05). The VRR was (57.22 ± 24.08, range, 23.58–83.57) % at the last follow-up.
University of Washington Head and Neck Quality of Life (UW-QOl) questionnaire
At the last follow-up, the average UW-QOl post-ablation was significantly higher than pre-MWA value (69.84 ± 8.12 vs 59.24 ± 11.51, p < 0.05). Our study suggests that patients’ quality of life is negatively correlated with the volume of lesions. With the reduction of lesion volume, UW-QOl of patients increased steadily.
Complication
There were no major complications and deaths during the perioperative period. The most common complications include postoperative edema of ablation site (n = 18), pain (n = 18), mild fever after ablation (n = 10), and cellulitis (n = 2). All these complications were improved within 3 to 7 days after anti-edema, anti-pain and anti-inflammatory symptomatic treatment. No peripheral nerve injury and vessel rupture or thrombosis occurred in this study. Some patients had pigmentation of the skin at the ablation site, likely resulted from the effect of high temperature on the skin during ablation.
Discussion
Patients with inoperable head and neck malignancies often have short survival time and poor quality of life. Chemoradiotherapy is the standard treatment. However, chemotherapy alone has no curative potential [Citation21,Citation22], alternating chemotherapy and radiotherapy also may not prevent persistent or recurrent disease [Citation23,Citation24]. Chemotherapy has some toxic nature. Irradiating the same region repeatedly has the risk of complications and increases toxicity in the organs near the lesion [Citation25,Citation26]. Standard supportive care does not significantly improve the quality of life and prolong the survival time of patients. A minimally invasive treatment is urgently needed for these patients. Most patients with advanced head and neck malignancies undergo surgery and/or radiation prior to MWA, whose head and neck anatomy is often distorted, and difficult to reach surgically. Any approach must be undertaken with great care and consideration of the proximities of multiple cranial and cervical nerves, major vessels, and aerodigestive structure, so image guidance is important to improve the safety. At present, image-guided thermal ablation of head and neck lesions is principally described in the treatment of benign and malignant thyroid nodules [Citation27–30], which includes RFA, MWA, LA and High-Intensity Focused Ultrasound (HIFU). Some studies have employed RFA guided by CT or US in primary and recurrent head and neck malignancies and in cervical lymph nodal metastasis (LNMs) [Citation31–35], which had invariably demonstrated that CT or US guided RFA for head and neck malignancies were feasible, efficacious, and safe. In clinical practice, MWA has increasingly shown advantages such as small surgical trauma, high safety, rapid recovery, fewer complications, repeatable operation and protection effects for organs and tissues. Zhou W et al. [Citation36] studied the effectiveness and safety of UPgMWA for LNMs from PTC, the average maximum diameter and volume of the tumors were reduced, neither progression of treated tumors nor new suspicious LNMs were detected after treatment. This research demonstrated that UgPMWA is feasible and safe in head and neck malignancies. To the best of our knowledge, relatively few reports about UgPMWA for primary and recurrent head and neck malignances have been published.
During the ablation in this study, microwave antenna was put into the target site of the lesion, which was equivalent to core needle puncture of tumors, and the safety of the US-guided core needle biopsy of head and neck tumors had been verified [Citation37,Citation38]. The success rate of the placement of the microwave antenna was 100% in our study.
In 10 out of 18 patients in our study, the maximum tumor diameter and tumor volume decreased significantly. Oppressive symptoms related to the lesion volume such as pain, airway obstruction and dysphagia have also substantially relieved. The symptom relief improved patients’ quality of life, correlated with quantitative results of UW-QOl analysis (post-ablation UW-QOl was significantly higher than pre-ablation counterpart). Thus, our study proved that MWA is an effective palliative treatment in reducing the volume of tumors, alleviating symptoms and improving quality of life in head and neck malignancies patients.
Our study showed that patients in the advanced stage of disease all had a clear intention to ablation, and their quality of life was improved after the treatment. According to follow-up and statistical analysis results, the lesion volume after ablation decreased steadily in the first follow-up 6 months, and the symptoms were relieved. The purpose of our study is to relieve symptoms by palliative ablation, inevitablely residual tumor tissue left untreated close to critical vessels and nerves, as a result the residual tumor would regrow in different degrees after a period of time. This situation was closely monitored in patients based on their pathologic types. The situation of patients with cervical LNMs after thyroid papillary carcinoma was stable, it took about 1 year for the disease to grow again. 2 patients were followed up for more than 2 years, and their disease states and quality of life are stable after going through UgPMWA several times. However, the remission of lesions after palliative ablation was not ideal in patients with postoperative SCC recurrence or cervical LNMs, the lesion usually increased within 6 months, and still grew rapidly after re-ablation. Situation above is illustrated in . This may be related to the change of microenvironment and some immune cell subsets after ablation, or some specific cells in the body of patients with relapse are higher than those of patients without relapse. The study by Zhou Y et al. [Citation39] confirmed that the dynamic changes of T-cell subsets in hepatocellular carcinoma patients were closely related to tumor recurrence after MWA. Thus, we have recorded the survival rate of patients and studied the effect of microenvironment changes on survival of patients with advanced head and neck malignancies after MWA, yet all of which will require more research in the future.
It has been reported that during the thermal ablation of head and neck lesions, thrombosis and vascular rupture may occur [Citation31]. In our study, lesions in the neck were mostly located near the major vessels. Besides water isolation, the lesions close to the major vessels were ablated at a lower power level than the average of MWA or were just injected with Ethanol to avoid potential damage during the treatment. Therefore, no vessel rupture or thrombosis occurred. Our anesthesia method was also safe and effective for MWA patients. The average age of the patients involved in this study was older and the physical condition of them was poor, the risk of general anesthesia was higher. Therefore, conscious sedation and cervical plexus block were adopted in our study. The distribution of vessels and important nerves in the region of head and neck is dense. Our anesthetic method controlled the pain in the ablation area meanwhile retaining the patient’s consciousness, this prevented peripheral nerve damage caused by excessive ablation. Nerve injury caused by the MWA of head and neck malignancies had been reported in the previous study [Citation40], but in our study, no peripheral nerve injury ever occurred. The efficacy of this type anesthesia is quite obvious, and no patient interrupted surgery because he or she could not tolerate the pain. This has a unique advantage for the elderly, especially those at higher risk of anesthesia.
There are several limitations in our study. First, the study had a heterogeneous sample, although all patients had advanced head and neck malignancies. Secondly, the number of patients was relatively small given that the application of MWA in the treatment of head and neck malignancies is still in the development stage. Finally, because the patients enrolled were in the advanced stage of the disease, the follow-up was not very regular.
Conclusion
In conclusion, MWA is feasible and effective for palliative treatment in advanced head and neck malignancies. US-guided MWA can accurately guide the targeted placement of the microwave antenna and ensure the safety of the percutaneous puncture. For patients with advanced head and neck malignancies, the related symptoms have been effectively alleviated, the quality of life has been significantly improved. Currently, MWA is not a substitute for surgery or chemoradiotherapy, nevertheless the MWA under US-guidance is a valuable method in the palliative treatment for advanced head and neck malignancies.
Disclosure statement
The authors report no conflicts of interest
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Pan R, Zhu M, Yu C, et al., China Kadoorie Biobank Collaborative Group. Cancer incidence and mortality: a cohort study in China, 2008–2013. Int J Cancer. 2017;141(7):1315–1323.
- Vokes EE, Weichselbaum RR, Lippman SM, et al. Head and neck cancer. N Engl J Med. 1993;328(3):184–194.
- Maruo T, Zenda S, Shinozaki T, et al. Comparison of salvage surgery for recurrent or residual head and neck squamous cell carcinoma. Jpn J Clin Oncol. 2020;50(3):288–295.
- Hamoir M, Schmitz S, Suarez C, et al. The current role of salvage surgery in recurrent head and neck squamous cell carcinoma. Cancers. 2018;10(8):267.
- Tupchong L, Scott CB, Blitzer PH, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long-term follow-up of RTOG study 73-03. Int J Radiat Oncol Biol Phys. 1991;20(1):21–28.
- Kao J, Garofalo MC, Milano MT, et al. Reirradiation of recurrent and second primary head and neck malignancies: a comprehensive review. Cancer Treat Rev. 2003;29(1):21–30.
- Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697–710.
- Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20(2):1033–1043.
- Echarri M, Lopez-Martin A, Hitt R. Targeted therapy in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (LA-R/M HNSCC). Cancers. 2016;8(3):27.
- Bredlau AL, Mccrackin MA, Motamarry A, et al. Thermal therapy approaches for treatment of brain tumors in animals and humans. Crit Rev Biomed Eng. 2016;44(6):443–457.
- Webb H, Lubner MG, Hinshaw JL. Thermal ablation. Semin Roentgenol. 2011;46(2):133–141.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014;30(2):150–157.
- Olaitan A, Mould T. Successful microwave ablation of endometrial carcinoma. Bjog. 2003;110(8):1410–1412.
- Yang W, Ping L, Xiaoling Y, et al. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25(6):455–461.
- Leng S, Christner JA, Carlson SK, et al. Radiation dose levels for interventional CT procedures. AJR Am J Roentgenol. 2011;197(1):W97–W103.
- Jong RJBD, Rongen RJ, Laméris JS, et al. Metastatic neck disease. Palpation vs ultrasound examination. Arch Otolaryngol Head Neck Surg. 1989;115(6):689–690.
- Senchenkov A, Staren ED. Ultrasound in head and neck surgery: thyroid, parathyroid, and cervical lymph nodes. Surg Clin North Am. 2004;84(4):973–1000.
- Yeh MW, Bauer AJ, Bernet VA, et al., American Thyroid Association Surgical Affairs Committee Writing Task Force. American Thyroid Association Statement on Preoperative Imaging for Thyroid Cancer Surgery. Thyroid. 2015;25(1):3–14.
- Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15(6):485–496.
- Nwizu T, Adelstein D. Pharmacotherapy of head and neck cancer. Expert Opin Pharmacother. 2015;16(16):1–14.
- Chen JH, Yen YC, Liu SH, et al. Outcomes of induction chemotherapy for head and neck cancer patients: a combined study of two national cohorts in Taiwan. Medicine. 2016;95(7):e2845.
- Wong SJ, Machtay M, Li Y. Locally recurrent, previously irradiated head and neck cancer: concurrent re-irradiation and chemotherapy, or chemotherapy alone? J Clin Oncol. 2006;24(17):2653–2658.
- Singh NY, Shalini S, Ranjan DN. Chemo-reirradiation in persistent/recurrent head and neck cancers. Jpn J Clin Oncol. 2004;34(2):61–68.
- Cacicedo J, Navarro A, Alongi F, et al. The role of re-irradiation of secondary and recurrent head and neck carcinomas. Is it a potentially curative treatment? A practical approach. Cancer Treat Rev. 2014;40(1):178–189.
- Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):731–740.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Biamonte E, Solbiati L, Ierace T, et al. Medullary thyroid carcinoma treated with percutaneous ultrasound-guided radiofrequency ablation. Endocrine. 2019;65(3):515–519.
- Trimboli P, Pelloni F, Bini F, et al. High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65(2):312–317.
- Roberto N, Gabriele G. Percutaneous ethanol injection in combination with laser ablation for a 100 ml partially cystic thyroid nodule. Case Rep Endocrinol. 2018;2018:8046378.
- Brook AL, Gold MM, Miller TS, et al. CT-guided radiofrequency ablation in the palliative treatment of recurrent advanced head and neck malignancies. J Vasc Interv Radiol. 2008;19(5):725–735.
- Belfiore MP, Sciandra M, Romano F, et al. Preliminary results in unresectable head and neck cancer treated by radiofrequency and microwave ablation: feasibility, efficacy, and safety. J Vasc Interv Radiol. 2015;26(8):1189–1196.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10(7):144–149.
- Owen RP, Lee JS, Silver CE, et al. Radiofrequency ablation in advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271(2):207–210.
- Bäck LJJ, Mäkitie AA, O'Malley BW, et al. When is radiofrequency ablation not indicated in head and neck squamous cell carcinoma management? Eur Arch Otorhinolaryngol. 2015;272(5):1045–1046.
- Zhou W, Chen Y, Zhang L, et al. Percutaneous microwave ablation of metastatic lymph nodes from papillary thyroid carcinoma: preliminary results. World J Surgery. 2019;434(4):1029–1037.
- Kalra A, Prucher G, Hodges S. The role of core needle biopsies in the management of neck lumps. Ann R Coll Surg Engl. 2019;101(3):193–196.
- Han F, Xu M, Xie T, et al. Efficacy of ultrasound-guided core needle biopsy in cervical lymphadenopathy: a retrospective study of 6,695 cases. Eur Radiol. 2018;28(5):1809–1817.
- Zhou Y, Xu X, Ding J, et al. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J Cancer Res Ther. 2018;14(1):40–45.
- Chung SR, Baek JH, Choi YJ, et al. Management strategy for nerve damage during radiofrequency ablation of thyroid nodules. Int J Hyperthermia. 2019;36(1):203–209.