Abstract
Introduction
High-intensity focused ultrasound (HIFU) is an innovative noninvasive procedure for local ablation of different benign and malignant tumors. Preliminary data of animal studies suggest an ablation-associated immune response after HIFU that is induced by cell necrosis and release of intracellular components. The aim of this study is to evaluate if a HIFU-induced early sterile inflammatory reaction is initiated after ablation of uterine fibroids (UF) and pancreatic carcinoma (PaC) which might contribute to the therapeutic effect.
Material and methods
A hundred patients with PaC and 30 patients with UF underwent US-guided HIFU treatment. Serum markers of inflammation (leukocytes, CRP, IL-6) and LDH in both collectives as well as tumor markers CA 19-9, CEA and CYFRA in PaC patients were determined in sub-cohorts before and directly after HIFU (0, 2, 5 and 20 h post-ablation) as well as at 3, 6, 9 and 12 months follow-up. Peri-/post interventional imaging included contrast-enhanced MRI of both cohorts and an additional CT scan of PaC patients.
Results
An early post-ablation inflammatory response was observed in both groups with a significant increase of leukocytes, CRP and LDH within the first 20 h after HIFU. Interestingly, IL-6 was increased at 20 h after HIFU in PaC patients. A significant reduction of tumor volumes was observed during one year follow-up (p < .001) for both tumor entities demonstrating effective treatment outcome.
Conclusion
Tumor ablation with HIFU induces an early sterile inflammation that might serve as a precondition for long-term tumor immunity and a sustainable therapeutic effect.
Introduction
High-Intensity Focused Ultrasound (HIFU) is a noninvasive procedure that allows a targeted local ablation by sonication utilizing acoustic energy, which is generated in an extracorporal transducer, and directed into a target region [Citation1–4]. Different benign tumors such as uterine fibroids, desmoid tumors, benign thyroid nodules or fibroadenomas of the breast as well as malignant tumors such as pancreatic cancer, primary and metastatic liver and bone malignancies, renal cell carcinoma, or breast cancer can be treated with HIFU [Citation1,Citation5–12]. In our HIFU center, therapeutic ultrasound has been mainly used for local ablation of advanced pancreatic cancer (PaC) and symptomatic uterine fibroids (UF). In PaC patients ablation with ultrasound (US)-guided HIFU leads to a significant reduction of cancer-related pain, tumor volume and serum levels of the tumor marker CA 19-9 in approximately 80% of patients, and potentially a benefit of survival [Citation3]. For UF HIFU is used to relieve fibroid-associated symptoms and improve health-associated quality of life.
As the temperature in the target region rises to over 80 °C, the main effect of HIFU is a thermal ablation, which causes coagulation necrosis and as a consequence a volume reduction over time [Citation13–15]. Acoustic cavitation is another important physical effect of HIFU exerted on the target tissue [Citation16]. Furthermore, there is initial evidence for a cell-mediated and ablation-associated immune response induced by the release of intracellular antigens after ablative procedures such as cryoablation, microwave ablation (MWA), radiofrequency ablation (RFA), irreversible electroporation (IRE) and HIFU [Citation17–23]. In animal models it has been shown that cell damage and necrosis after thermal ablation leads to a release of intracellular material and damage-associated molecular patterns (DAMPs) such as DNA, ATP and heat shock proteins (HSP). These are processed by dendritic cells and can lead to a systemic immune response including increased levels of IL-1 and IL-6 [Citation24–27]. Preliminary data of patients with pancreatic and renal cell carcinoma, hepatocellular carcinoma (HCC) or osteosarcoma who underwent ablation with HIFU showed either an increased activity of natural- killer cells (NK-cells) or a raised CD4/CD8-ratio [Citation28,Citation29]. Furthermore, patients with breast cancer were shown to have an increased number of tumor- infiltrating dendritic cells, macrophages and B-lymphocytes after HIFU treatment suggesting an early sterile inflammation reaction [Citation30]. A HIFU-induced early sterile inflammatory reaction might contribute to reinforce the therapeutic effect.
The aim of this study was to therefore to investigate treatment effects of HIFU on tumor volume and ablation-associated laboratory chemical parameters such as lactatdehydrogenase (LDH) and inflammation markers (leukocytes, interleucine 6 (IL-6) and C-reactive protein (CRP) in two different patient cohorts, patients with a malignant (pancreatic carcinoma) and patient with a benign disease (uterine fibroids), both routinely treated in our clinic by US-guided HIFU. Changes in tumor markers (CEA, CA 19-9, CYFRA) were evaluated in pancreatic cancer patients.
Materials and methods
Patient selection and characteristics
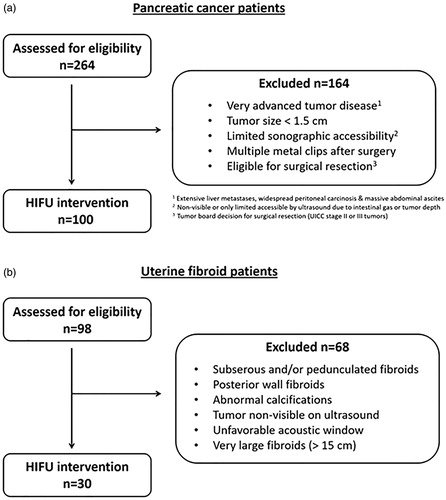
In this study, prospectively collected data of 100 patients with locally advanced inoperable pancreatic cancer and 30 patients with uterine fibroids treated with US-guided HIFU at our center were subsequently analyzed ( and ). Indications for US-guided HIFU treatment of each patient group were confirmed by an interdisciplinary board. The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee (No. 302/12; 307/15; 055/19). All patients provided written informed consent before treatment.
Figure 1. Age distribution of HIFU-treated patients with uterine fibroids (UF) and pancreatic cancer (PaC). The upper horizontal line represents the first quartile, the middle horizontal line the median age (second quartile) and the lower horizontal line the third quartile. The vertical lines show maximum and minimum age. Median age of patients with UF: 42.5 y (min. 28 y, max. 53 y); median age of female patients with PaC: 61.5 y (min. 40 y, max. 79 y); median age of male patients with PaC: 63.5 y (min. 38 y, max. 83 y).
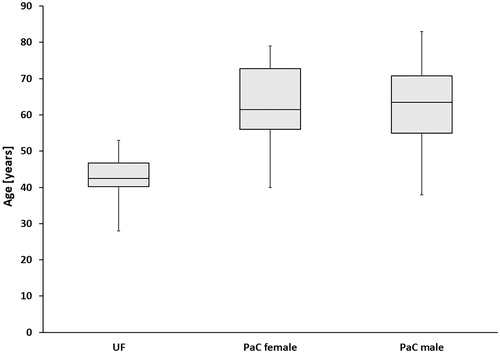
Table 1. Clinical characteristics of 100 patients with pancreatic cancer (PaC) treated with US-guided HIFU at our center.
Table 2. Clinical characteristics of 30 patients with symptomatic uterine fibroids (UF) treated with US-guided HIFU at our center.
Approximately half of the pancreatic tumors were located in the pancreatic head. At presentation all patients suffered from cancer-related pain as a leading symptom (70/100 despite analgesic medication and palliative chemotherapy). Main symptoms of patients with uterine fibroids are referred to hypermenorrhoea and dysmenorrhea, 5 patients (17%) had undergone previous myomectomy, 8 patients (27%) had been previously treated by hormone medication ().
Ablation with US-guided HIFU
Patients were selected according to inclusion criteria for HIFU treatment (). At baseline contrast-enhanced sonography (CEUS) and MRI were performed for both patient groups, additionally a CT scan in patients with pancreatic cancer. Routinely, a preparation of the gastrointestinal tract similar to a preparation prior to colonoscopy was performed and started on the day before the intervention. After skin preparation (shaving, degreasing and degassing) ablation of pancreas was performed under general anesthesia, whereas patients with uterine fibroids were only sedated to minimize the risk of nerve damage. Ablation was performed using the Focused Ultrasound Tumor Therapeutic System (JC TTS, Haifu Medical Technology, Chongqing, China). The system consists of a treatment table with an integrated high power focused US-therapy transducer (diameter 20 cm, focal length 15 cm, frequency 0.8 MHz) placed in a water basin under the treatment table, a high power driving electronic and a control unit. In most patients with pancreatic cancer a nasogastric tube was placed to reduce intragastric gas. Urinary catheterization was performed before ablation in uterine fibroid patients in order to optimize the acoustic pathway by filling the bladder with sterile water. A balloon filled with degassed water was placed in the water basin between the patient´s skin of the anterior abdominal wall and the transducer to compress the bowel away and displace the stomach or bowel parts. Diagnostic ultrasound was used to identify target lesions. A sonication plan was made considering a safety distance of 1 cm from the sonication focus to the lesion margin and surrounding structures at risk (bowel, biliary stents) in order to prevent heat-induced complications. The volume ablation was composed of multiple focal sonications in rows and adjacent layers. The applied power was adjusted individually for every patient. Intervention parameters are shown in . During the intervention the skin was kept in cooled water of 15 °C and examined regularly by palpation.
Table 3. Selection criteria for US-guided HIFU.
Table 4. Intervention parameters in both groups, patients with uterine fibroids and patients with pancreatic cancer.
Post-treatment follow-up
The follow-up imaging included contrast-enhanced MRI (1.5-T. Ingenia MRI, Philips Healthcare, Best, the Netherlands) for both patient groups and an additional CT scan (SOMATOM Force, Siemens Healthineers, Erlangen and 64-slice CT Brilliance, Philips, the Netherlands) for PaC patients. CT was used to exclude early post-interventional complications and for staging and detection of vascular involvement and distant metastases; MRI to evaluate treatment success in detail. Imaging was performed prior to the ablation (baseline) as well as within first post-ablation week, after 6 weeks, 3 months and then in 3-month time intervals.
Laboratory follow-up in both patient groups included evaluation of proinflammatory immunological markers such as leukocytes (UF: n = 30; PaC: n = 99), CRP (UF: n = 24, PaC: n = 100), IL-6 (UF: n = 18, PaC: n = 86) and LDH (UF: n = 15, PaC: n = 44) as a marker of cell destruction. In patients with PaC further laboratory parameters were determined: amylase and lipase as specific pancreatic inflammatory markers, tumor markers CA 19-9 (n = 100), CEA (n = 100) and CYFRA (n = 69). The first follow-up was one week after HIFU, the second one after 6 weeks. Long-term follow-up was performed simultaneously to imaging after 3 months, 6 months, 9 months and 12 months.
In a sub-group of 30 patients (PaC: n = 20, UF: n = 10) the laboratory markers LDH, leukocytes, CRP, IL-6 in all patients and in addition amylase, lipase, CA 19-9, CEA and CYFRA in PaC patients were determined at baseline and at 0, 2, 5 and 20 h after HIFU procedure.
Statistical analysis
Statistical analysis was performed with Stata Version 14.2 (Stata Corp, Lakeway, College Station, Texas, USA) using a mixed linear data model. Primary analysis of tumor volumes and laboratory values was done comparing baseline and follow-up data. Results were considered statistically significant if the p-value was <.05.
Results
HIFU-ablation
Of all presented patients, about 38% of PaC and 30% of UF patients were suitable for HIFU treatment as shown in the consort diagrams (). In the PaC group 83 of 100 patients had been previously treated with standard chemotherapy regimens. HIFU ablation was successfully performed in all patients, even in complex cases with biliary stents and/or tumor cuffing of central abdominal arteries such as the celiac trunk or the superior mesenteric artery. The majority of patients was treated with a single HIFU session (118/130), one UF patient and 11 PaC patients underwent a second HIFU session with a minimum time of 2 months between treatment sessions. The most common reason for a second treatment were tumor recurrence in peripheral localization outside of the ablated area (11/100 pancreatic cancers) or persistence of symptoms (1/30 uterine fibroids).
Follow-up imaging and tumor volume
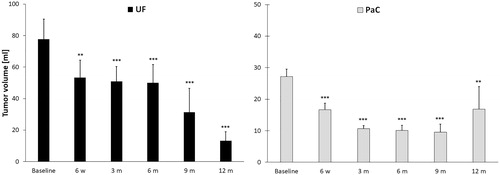
In the first follow-up imaging performed within the first post-ablation week major complications as bleeding, bowel damage or perforation and vessel occlusion were excluded. The tumor volume was evaluated using MRI (UF: n = 29/30, PaC: n = 90/100) and CT imaging (PaC: n = 7/100). A significant reduction of tumor volumes could be observed in both patient groups between 6 weeks and 12 months after ablation (). Taking into account the different tumor stages at 12 months after HIFU (UICC 3: n = 7 patients and UICC 4: n = 6 at 12 months) in the PaC group we observed that patients with locally advanced PaC (UICC 3) had a continuous significant volume reduction from 6 weeks (37.1 ± 10%) to 12 months after ablation (78.2 ± 25.5%). In comparison, patients with distant metastases (UICC 4) had a significant but slower tumor shrinkage at 6 and 9 months after HIFU compared to baseline. The median time to local progression was 16.2 months from initial diagnosis and 8.3 months from HIFU intervention.
Figure 3. A 43-year old premenopausal patient with uterine fibroids and myoma-associated symptoms (leading symptom hypermenorrhea) was treated by US-guided HIFU in our center. The largest fibroid (6 × 6.5 × 6.7 cm, 136.7 mL) was located in the anterior wall of the uterus. A considerable shrinkage of the predominant fibroid was observed during follow-up period leading to a volume reduction of the whole uterus. T2-weighted MR images are shown as follows: (a) A Funaki Typ I fibroid (arrowheads) before HIFU-treatment with an initial volume of 136.7 mL. (b) Immediately after HIFU ablation there was no change in tumor volume. Contrast images (not shown) indicated a large devascularizated zone with a non-perfused volume of >80%. (c) Fibroid volume at 6-week follow-up was 88.2 mL (volume reduction of 35.5%). A considerable reduction in fibroid-associated symptoms was observed at this early time point following HIFU treatment. (d, e) Volume reduction was 48% (tumor volume: 71.2 mL) at 3-month and 51.6% at 6-month follow-up. (f) At 1.5.-year follow-up the fibroid volume reduction was 73.4%. Persistence of the significantly reduced symptoms.
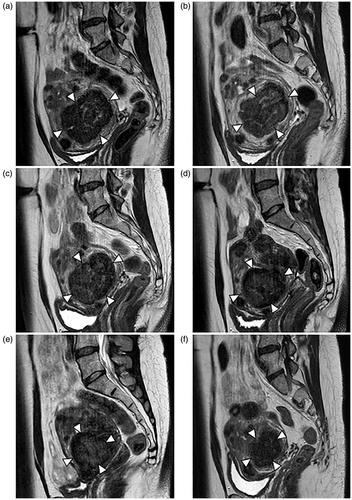
Figure 4. Average reduction of tumor volume (mL) after HIFU in patients with uterine fibroids (UF) and pancreatic cancer (PaC). Significance levels are indicated by the stars attached (* indicating p < .05, ** indicating p < .01, *** indicating p < .001). Standard errors were symmetrical (only positive error bars are shown). Significant volume regression in both patient groups was observed at each time point of follow-up between 6 weeks and 1 year compared to baseline. m: month, w: week.
Markers of inflammation and cell damage
In the early postinterventional period an increase of inflammatory markers and LDH as a marker of cell membrane damage were observed (). LDH was significantly elevated in patients with PaC after 2 and 5 h (p < .01) and 20 h (p < .001) compared to baseline while the cutoff value of 250 U/L was surpassed only at 20 h (255.9 ± 15.3 U/L). Patients with UF also showed a significant increase at 20 h after ablation (270.7 ± 43.9 U/L, p < .001).
Figure 5. Course of leukocytes, CRP, IL-6 and LDH in patients with uterine fibroids (UF) and pancreatic cancer (PaC). Significance levels are indicated by the stars attached (*indicating p < .05, **indicating p < .01, ***indicating p < .001). Standard errors were symmetrical (only positive error bars are shown). A significant increase of leukocytes was observed immediately after HIFU (0 h) and 2 h, 5 h and 20 h after HIFU in UF patients and 2 h and 20 h after HIFU in PaC patients. CRP was significantly elevated after 5 h and 20 h in the PaC group and after 20 h in the UF group whereas a relevant elevation of IL-6 was only observed after 20 h in patients with PaC. LDH increased in PaC patients after 2 h, 5 h and 20 h and in UF patients after 20 h. CRP: C-reactive protein; h: hour; IL-6: interleukin- 6; LDH: lactatdehydrogenase.
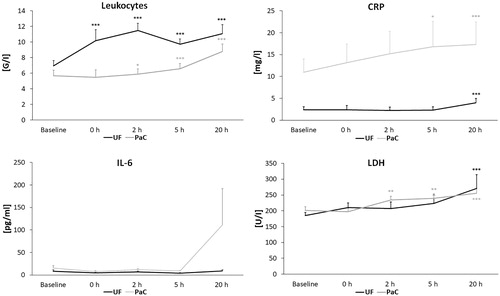
Patients with UF showed a significant increase of white blood cells compared to baseline values immediately after HIFU and at 2 h (11.5 ± 0.9 G/L), 5 h and 20 h (11.1 ± 1.2 G/L) post-procedure (p < .001), with a cutoff value of 10.5 G/L indicating a clinically relevant leukocytosis. In the PaC group leukocytes rose significantly at 2 h (p < .05), at 5 and 20 h (p < .001) compared to baseline but not exceeding 10.5 G/L (6.6 ± 0.7 G/L at 5 h and 8.8 ± 1 G/L at 20 h). CRP was elevated in 61% of PaC patients at baseline (10.9 ± 3 mg/L) indicating a high rate of cancer-related CRP because there was no evidence for infections before HIFU therapy. Postinterventional development of CRP levels showed a continuous increase after HIFU with a significant elevation after 5 h (16.8 ± 5.8 mg/L, p < .05) and 20 h (17.3 ± 5.1 mg/L, p < .001). In patients with UF and normal CRP levels at baseline (2.3 ± 0.7 mg/L) a significant CRP elevation was observed at 20 h (4 ± 1 mg/L, p < .001). IL-6 levels were raised in PaC patients at 20 h (not significant) after ablation (baseline 15.3 ± 5.4 pg/mL vs. 110.6 ± 81.2 pg/mL) but showed no elevation in UF patients.
Tumor markers
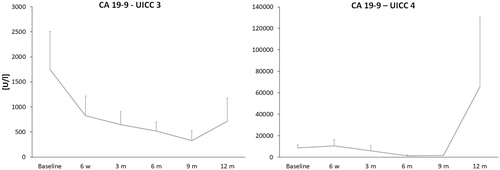
In addition, tumor markers CA 19-9, CEA and CYFRA were evaluated in patients with PaC. At baseline CA19-9 was available in 79% of cases (mean 5762.2 ± 1722.3 U/mL, normal level <34 U/mL), CEA in 51% (38.6 ± 9.4 ng/mL, normal level <5ng/mL) and CYFRA in 21% of patients (8.4 ± 2.3 ng/mL, normal level <3.3 ng/mL). During early post interventional follow-up (0, 2, 5 and 20 h) there were no relevant changes in the courses of CA 19-9, CEA and CYFRA. CA 19-9 in stage UICC 3 patients (1751.6 ± 753.9 U/mL at baseline) showed a reduction of 52.7 ± 46.9% after 6 weeks (829.4 ± 389.3 U/mL) and a continuous decrease at 3, 6 and 9 months after ablation compared to baseline (331.7 ± 194.7 U/mL at 9 months). This was followed by a minor increase between 9 and 12 months (717.1 ± 4569.7 U/mL) with serum levels of CA 19-9 remaining under the levels at 6 weeks (). In UICC stage 4 patients (8755.1 ± 2894.3 U/mL at baseline) we observed a minor CA 19-9 increase after 6 weeks (10691.8 ± 5603.8 U/mL) and a decrease at 3, 6 and 9 months compared to baseline (1575.3 ± 1144.9 U/mL at 9 months); at 12 months CA 19-9 was elevated compared to baseline (65571.5 ± 64976.9 U/mL). CEA has not changed significantly between 6 weeks and 9 months after HIFU in UICC stage 4 patients (58.43 ± 15.5 ng/mL at baseline), but a significant increase was observed after 12 months (1052.1 ± 1050 ng/mL) parallel to the increase of CA 19-9. Patients in stage UICC 3 (CEA 12 ± 4.6 ng/mL at baseline) showed a reduction of 47.2 ± 32.8% after 6 weeks (6.34 ± 2.1 ng/mL) and 70.1 ± 24.8% after 12 months (3.5 ± 0.9 ng/mL). Regarding CYFRA, a decrease of 64.3 ± 31.2% was observed after 3 months in patients with UICC stage 4 (11.6 ± 3.7 ng/mL at baseline vs. 4.1 ± 1.3 ng/mL at 3 months) followed by a reduction of 78.2 ± 42.3% after 6 months (2.5 ± 1.1 ng/mL). There was no relevant change in CYFRA in patients with stage UICC 3 between 6 weeks and 12 months compared to baseline.
Figure 6. Changes of the tumor marker CA 19-9 in pancreatic cancer (PaC) patients (UICC 3 and UICC 4). Standard errors were symmetrical (only positive error bars are shown). Patients in stage UICC 3 showed a considerable decrease of CA 19-9 at 6 weeks compared to baseline (1751.6 at baseline vs. 829.4 U/mL) and a continuous decrease between 3 and 9 months after HIFU followed by a minimal increase between 9 and 12 months after HIFU ablation. Patients in stage UICC 4 had a slight increase after 6 weeks and a decrease at 3, 6 and 9 months after HIFU followed by a considerable increase after 12 months. CA 19-9: Carbohydrate antigen 19-9; h: hour; m: month; UICC: Union for International Cancer Control (tumor classification).
Adverse events
Depending on the organ treated some different minor treatment-associated side effects of a short duration were observed.
Moderate transient abdominal pain was observed as a treatment-associated effect in both patient groups in about 60% of the patients, requiring opioid medication in some cases with PaC. Further post interventional symptoms of UF patients referred minor vaginal bleeding (2/30) that was self-limiting on the day after ablation. Transitory subcutaneous edema (32/100) or edema of the abdominal fat or gastrointestinal organs (25/100) were observed in the acoustic pathway after ablation of pancreatic tumors. Local and superficial skin burn of first grade in the acoustic pathway was observed in one PaC patient resolving without specific therapy within 3 weeks. There were no long-term side effects of treated patients.
Discussion
Induction of a host immune response to tumor antigens after local ablative therapy might play a role in achieving a sustainable therapeutic effect. Animal studies and preliminary clinical data suggest an inflammatory immune activation that might be responsible for delayed tumor growth, reduction of metastases or even increased survival rates after local ablative procedures such as radiofrequency ablation (RFA), cryoablation, microwave ablation (MWA), irreversible electroporation (IRE) or HIFU [Citation17,Citation18,Citation21,Citation23,Citation25,Citation29,Citation31–39].
In the present study we therefore evaluated the early changes of white blood cells and proinflammatory serum markers after HIFU treatment in patients with locally advanced and metastatic pancreatic carcinoma and uterine fibroids in order to gain insight into possible ablation-related effects on sterile inflammation that might lead to a HIFU-induced host tumor immunity. An increase of lactate dehydrogenase (LDH) as a marker of permeabilization of plasma membrane and thus cell necrosis was evident in both patient groups. We observed that serum LDH increased after 2 h, 5 h and 20 h in PaC patients and after 20 h in patients with uterine fibroids comparable with previous clinical data of percutaneous RFA of patients with malignant liver tumors that show significant LDH increase at 24 h after treatment [Citation40]. Our results indicate a consecutive early and systemic inflammatory reaction after HIFU as an elevation of white blood cells compared to baseline was observed in both patient groups. In parallel, a delayed increase of CRP was observed after 5 h in PaC patients and in both groups, UF and PaC, after 20 h. Comparable results have been shown after RFA of liver tumors indicating a systemic inflammatory response with a postinterventional increase of white blood cells and a significant increase of CRP after 24 h, whereas lung cancer patients were shown to have a delayed increase of CRP at 3 days after RFA [Citation18,Citation22]. Increase of CRP has also been proven after cryoablation of patients with unresectable liver tumors [Citation17]. Another important inflammatory marker that has been shown to be increased after local ablation is IL-6 as there is clinical evidence for significantly increased serum levels after ablation of different malignant tumors and metastases after RFA, MWA, cryoablation and laser ablation within the first 48 h after therapy [Citation41]. This has been confirmed by further studies that prove elevated serum levels of IL-6 after cryoablation of unresectable liver tumors and during RFA, immediately after RFA and 3 and 24 h after RFA in patients with liver cancer or metastases [Citation17,Citation18,Citation40,Citation42]. Wang et al. demonstrated a marginal increase of IL-6 after HIFU of uterine fibroids 24 h after therapy in a randomized comparison to myomectomy what is compatible with our results showing a marginal elevation of IL-6 at baseline and 20 h after HIFU in the UF group [Citation43]. Serum levels of IL-6 were higher in the PaC group and showed an increase at 20 h that was not statistically significant. Animal studies of pancreatic cancer suggest not only increased serum levels of IL-6, but also increased IL-6 in tumor tissue so that a release of IL-6 into serum due to tumor cell destruction might explain the fact that IL-6 at 20 h after ablation is higher in the PaC group than in the UF group although leucocytes are consistently lower [Citation44].
More data are available referring to cell-mediated immunity after HIFU and other local ablative therapies.
In the past, multiple studies have examined aspects of non-pathogen-associated inflammation after local tumor ablation. It was shown that the release of intracellular components (DAMPs) caused by thermal or mechanical damage to the cell membrane activates cellular and humoral immunological pathways and that ablation-induced tumor debris serves as a source for tumor antigens for immune cells [Citation19,Citation31].
HIFU was shown to be associated with a release of DAMPs as ATP and HSP 60 in vitro as well as in clinical studies showing upregulation of HSP 72 and HSP 73 in border zones of ablated tumors [Citation45,Citation46]. A following increase of CD4+/CD8+ ratios in peripheral blood after HIFU was observed in patients with PaC, osteosarcoma, HCC and RCC and additionally increased natural killer (NK) cells in patients with PaC [Citation28,Citation29]. Further, in vivo animal studies showed an increase of dendritic cell accumulation in tumor draining lymph nodes and increased cytotoxicity of cytotoxic T cells and an elevation of tumor necrosis factor (TNF)-α after HIFU [Citation32,Citation47]. Clinical data also showed a minor elevation of IL-6 between 24 and 72 h after HIFU in patients with uterine fibroids and a reduction of immunosuppressive cytokines in peripheral blood after HIFU of different solid malignant tumors [Citation43,Citation48]. Similar results were observed after RFA as animal studies and clinical data show an upregulation of HSP 70 and an increase of intratumoral T- cell infiltration [Citation31,Citation49]. This is confirmed by clinical data of patients with lung cancer proving increased T-cells in peripheral blood after RFA [Citation33,Citation50]. Increase of serum leukocytes, CRP, IL-6 and LDH of patients after RFA and increased IL-6 and CRP after cryoablation of liver tumors indicate systemic inflammatory response after local ablation [Citation17,Citation18,Citation40]. Further evidence for local ablation-associated inflammation is shown by clinical data with increased CD4+ and CD8+ T cells, NK cells and IL-6 after IRE of locally advanced PaC as well as increased NK cells and macrophages in tumor tissue after MWA of hepatocellular carcinoma [Citation21,Citation23]. An important potential clinical manifestation of ablation-associated systemic inflammation is the abscopal effect referring to systemic antitumor effects after local tumor therapy [Citation51]. In this context, shrinkage of non-target metastases after local therapy of the primary tumor has been described previously, e.g., shrinkage of pulmonary metastases of renal cell carcinoma after RFA, hepatic metastases of squamous cell carcinoma after radioembolization as well as lymph node metastases of breast cancer after HIFU [Citation52–54]. In our patient collective, 17 out of 100 patients with inoperable pancreatic cancer did not receive concurrent palliative chemotherapy. Only 11 of these 17 patients (UICC 4: n = 11, UICC 3: n = 6) presented with distant metastases including 7 patients with metastases in the liver, so that an abscopal effect after HIFU treatment was not observed in this rather small patient subcohort.
Furthermore, we evaluated changes of tumor markers CA 19-9, CEA and CYFRA after tumor ablation as parameters of treatment response of advanced pancreatic cancer. CA 19-9 has not only been proven to be associated with a prolonged survival if declined after surgical resection or chemotherapy, but can also indicate local tumor control of PaC after local ablation with HIFU or RFA [Citation55–57]. CEA as another common marker was shown to be increased in 30–60% of patients with PaC and to drop after local ablation, e.g., RFA of liver metastases of colorectal carcinoma [Citation58,Citation59]. In parallel, our results showed UICC 3 PaC patients to have not significantly decreased serum levels of CA 19-9 and CEA and stable levels of CYFRA suggesting local tumor control. This is confirmed by a continuous and significant decrease of tumor volumes between 6 weeks and 12 months after ablation in stage UICC 3. Analogous to this, UICC 4 PaC patients had decreased levels of CA 19-9 and stable serum levels of CEA between 3 and 9 months after HIFU. In contrast, a significant increase of CEA and a not significant increase of CA 19-9 at 12 months after HIFU in patients with metastatic PaC (UICC 4) indicate tumor progression, presumably of distant metastases (hepatic metastases or peritoneal carcinosis).
In conclusion, our findings suggest an early subclinical systemic inflammatory response after HIFU represented by increased serum levels of LDH, leukocytes, CRP and IL-6 in patients with benign and malignant abdominal tumors. An increase of serum LDH indicates a release of intracellular proteins and thus tumor cell necrosis as a consequence of thermal and mechanical tumor cell destruction being a precondition for following inflammatory cascades. The following increase of white blood cells and CRP indicates an inflammatory response with an earlier initiation in patients with pancreatic cancer than in patients with uterine fibroids, which is comparable to postinterventional clinical data after RFA of liver tumors [Citation18]. Although not statistically significant, increase of IL-6 after HIFU of PaC might point to an increased acute-phase activity of immune cells as macrophages or T-cells due to cell necrosis.
Our data indicate an early immune response following local ablation by therapeutic ultrasound. The HIFU-induced proinflammatory environment might be a precondition for tumor immunity based on immune activation by antigens in tumor debris after local ablation. As there is a very low rate of mostly self-limiting complications HIFU can be performed as a complementary treatment allowing a safe ablation, local tumor control and symptom relief in patients with locally advanced PaC and uterine fibroids.
Acknowledgments
The authors acknowledge Kathrin Bird, Irene Zender and Olga Ramig for their continuous support in clinical management of HIFU-treated patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wu F, Wang Z-B, Chen W-Z, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11(3–4):149–154.
- Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10(2):123–129.
- Marinova M, Huxold H, Henseler J, et al. Clinical effectiveness and potential survival benefit of us-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall in Der Medizin – Euro J Ultrasound. 2019;40(05):625–637.
- Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced pancreatic cancer: high-intensity Focused Ultrasound (HIFU) and other local ablative therapies. Rofo. 2019;191(3):216–227.
- Lyon PC, Rai V, Price N, et al. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschall Med. 2020;41(05):550–556.
- Trimboli P, Pelloni F, Bini F, et al. High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65(2):312–317.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589.
- Wu F, Wang Z-B, Cao Y-D, et al. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J Surg Oncol. 2007;96(2):130–136.
- Li Y-Y, Sha W-H, Zhou Y-J, et al. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22(12):2148–2154.
- Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol. 2009;72(1):160–166.
- Klatte T, Kroeger N, Zimmermann U, et al. The contemporary role of ablative treatment approaches in the management of renal cell carcinoma (RCC): focus on radiofrequency ablation (RFA), high-intensity focused ultrasound (HIFU), and cryoablation. World J Urol. 2014;32(3):597–605.
- Hsiao Y-H, Kuo S-J, Tsai H-D, et al. Clinical application of high-intensity focused ultrasound in cancer therapy. J Cancer. 2016;7(3):225–231.
- Hwang JH, Wang Y-N, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35(6):967–975.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–52.
- Marinova M, Rauch M, Mücke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26(11):4047–4056.
- Elhelf IAS, Albahar H, Shah U, et al. High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018;99(6):349–359.
- Osada S, Imai H, Tomita H, et al. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol. 2007;95(6):491–498.
- Jansen MC, van Wanrooy S, van Hillegersberg R, et al. Assessment of systemic inflammatory response (SIR) in patients undergoing radiofrequency ablation or partial liver resection for liver tumors. Eur J Surg Oncol. 2008;34(6):662–667.
- van den Bijgaart RJE, Eikelenboom DC, Hoogenboom M, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–258.
- Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72(2):430–439.
- Dong BW, Zhang J, Liang P, et al. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia. 2003;19(2):119–133.
- Fietta AM, Morosini M, Passadore I, et al. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum Immunol. 2009;70(7):477–486.
- He C, Wang J, Sun S, et al. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J Oncol. 2019;2019:9346017.
- Slovak R, Ludwig JM, Gettinger SN, et al. Immuno-thermal ablations – boosting the anticancer immune response. j Immunotherapy Cancer. 2017;5(1):78.
- Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58(1):1–11.
- Ahmad F, Gravante G, Bhardwaj N, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg. 2010;200(4):500–506.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J. 2002;115(9):1332–1335.
- Wu F, Wang Z-B, Lu P, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30(9):1217–1222.
- Xu Z-L, Zhu X-Q, Lu P, et al. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol. 2009;35(1):50–57.
- Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250.
- Deng J, Zhang Y, Feng J, et al. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound Med Biol. 2010;36(3):441–448.
- Ito F, Ku AW, Bucsek MJ, et al. Immune adjuvant activity of pre-resectional radiofrequency ablation protects against local and systemic recurrence in aggressive murine colorectal cancer. PLoS One. 2015;10(11):e0143370.
- Sabel MS, Su G, Griffith KA, et al. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol. 2010;17(4):1187–1193. Apr
- Alteber Z, Azulay M, Cafri G, et al. Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specific antitumor immunity. Cancer Immunol Immunother. 2014;63(4):369–380.
- Chen Z, Shen S, Peng B, et al. Intratumoural GM-CSF microspheres and CTLA-4 blockade enhance the antitumour immunity induced by thermal ablation in a subcutaneous murine hepatoma model. Int J Hyperthermia. 2009;25(5):374–382.
- Ringel-Scaia VM, Beitel-White N, Lorenzo MF, et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity. EBioMedicine. 2019;44:112–125.
- Kim H, Park BK, Kim CK. Spontaneous regression of pulmonary and adrenal metastases following percutaneous radiofrequency ablation of a recurrent renal cell carcinoma. Korean J Radiol. 2008;9(5):470–472.
- Mauri G, Nicosia L, Xu Z, et al. Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol. 2018;91(1083):20170641
- Schälte G, Henzler D, Waning C, et al. Case study of hepatic radiofrequency ablation causing a systemic inflammatory response under total intravenous anesthesia. Korean J Radiol. 2010;11(6):640–647.
- Erinjeri JP, Thomas CT, Samoilia A, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol. 2013;24(8):1105–1112.
- Evrard S, Menetrier-Caux C, Biota C, et al. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31(2):141–145.
- Wang X, Qin J, Chen J, et al. The effect of high-intensity focused ultrasound treatment on immune function in patients with uterine fibroids. Int J Hyperthermia. 2013;29(3):225–233.
- Long KB, Tooker G, Tooker E, et al. IL6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(9):1898–1908.
- Hu Z, Yang XY, Liu Y, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs. Biochem Biophys Res Commun. 2005;335(1):124–131.
- Kramer G, Steiner GE, Gröbl M, et al. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate. 2004;58(2):109–120.
- Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34.
- Zhou Q, Zhu X-Q, Zhang J, et al. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34(1):81–87.
- Schueller G, Kettenbach J, Sedivy R, et al. Expression of heat shock proteins in human hepatocellular carcinoma after radiofrequency ablation in an animal model. Oncol Rep. 2004;12(3):495–499.
- Schneider T, Hoffmann H, Dienemann H, et al. Immune response after radiofrequency ablation and surgical resection in nonsmall cell lung cancer. Semin Thorac Cardiovasc Surg. 2016;28(2):585–592.
- Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9(1):45–51.
- Rao P, Escudier B, de Baere T. Spontaneous regression of multiple pulmonary metastases after radiofrequency ablation of a single metastasis. Cardiovasc Intervent Radiol. 2011;34(2):424–430.
- Ghodadra A, Bhatt S, Camacho JC, et al. Abscopal effects and yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2016;39(7):1076–1080.
- Hans-Christian K, Cornelia K-L. High focused ultrasound in a case of previously untreated breast cancer. Clin Med Rev Case Rep. 2019;6(2):28.
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105–119.
- Li Y-J, Huang G-L, Sun X-L, et al. The combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinoma. World J Surg Oncol. 2016;14:60.
- D’Onofrio M, Barbi E, Girelli R, et al. Variation of tumoral marker after radiofrequency ablation of pancreatic adenocarcinoma. J Gastrointest Oncol. 2016;7(2):213–220.
- Meng Q, Shi S, Liang C, et al. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:4591–4598.
- Ghanamah M, Berber E, Siperstein A. Pattern of carcinoembryonic antigen drop after laparoscopic radiofrequency ablation of liver metastasis from colorectal carcinoma. Cancer. 2006;107(1):149–153.