Abstract
Purpose: Oral cancers are one of the commonly diagnosed tumors worldwide in human and veterinary patients. Most oral cancers are surgically resected; however, obtaining an adequate margin of safety in patients without compromising their quality of life is often challenging. Herein, we investigated the ability of non-invasive focused ultrasound (FUS) to thermally ablate a biopsy confirmed canine oral cancer.
Materials and Methods: A male canine patient with a large neurilemmoma (schwannoma) mass on the left maxilla, with evidence of thinning and loss of alveolar bone and pressure necrosis, was treated with FUS ablation instead of the traditional maxillectomy procedure. FUS ablations were performed in three sessions over three weeks. Tumor remission was determined with computed tomography and histopathological examination of the treated site. Additionally, the anti-tumor immune effects of FUS were assessed by flow cytometry analysis of blood and tumor samples.
Results: Complete tumor remission was noted at the treated site. Treatment related adverse events were primarily thermal burns of the buccal mucosa, which were managed with periodic hyperbaric oxygen therapy and surgical coverage of the underlying exposed bones with gingival flaps. Enhanced proliferation of adaptive immunity cells (e.g., T-cells) was observed in tumor and blood samples.
Conclusion: Our limited investigation in a canine oral cancer patient suggests that FUS may avoid the need for large-scale resection of bony tissues, thus potentially improving quality of life.
Introduction
Cancers of the oral cavity have a substantial incidence worldwide, and 3% of cancers diagnosed in the United States are oral cancers [Citation1]. Oral cancers can occur in various regions of the mouth, including the buccal mucosa, tongue, lips, palate, gums, and floor [Citation2]. Depending on the severity and extent of progression, they are treated with a combination of surgery, chemotherapy, and radiotherapy [Citation3,Citation4]. However, oral cancers recur in 18–76% of patients after standard treatment [Citation5]. For example, primary squamous cell carcinomas have high local recurrence rates in patients [Citation6], and the pattern of local invasion and lymph node metastasis significantly affects survival rates. In fact, ∼50% of patients receiving local resection with involved and close margins die within 5 years [Citation7]. In canine and feline species, benign and malignant oral tumors are also common [Citation8], and have an incidence rate similar to that in humans. For example, 6–7% of all cancers are of oral origin in dogs [Citation9]. These oral cancers in veterinary patients mainly include melanomas, squamous cell carcinomas, and fibrosarcomas [Citation10]. Herein, we evaluated the ability of focused ultrasound (FUS) ablation to treat a relatively uncommon oral schwannoma in a canine patient. Schwannomas are peripheral nerve sheath tumors originating from Schwann cells in dogs and cats. Although they are typically benign, malignant cases have been reported [Citation11,Citation12]. Malignant schwannoma cells demonstrate immunoreactivity to glial fibrillary acidic protein (GFAP) [Citation13], laminin, and S100 [Citation14,Citation15], and are devoid of melanoma associated antigens [Citation12]. In humans, as in small animals, schwannomas of neurofibromatosis type I origin in the tongue, palate, floor of the mouth, buccal mucosa, lips, and jaws have been reported [Citation16]. Therefore, we believe that our case report is important because it provides insights into the feasibility of leveraging FUS for the therapeutic management of aggressive oral cancers.
FUS is an emerging noninvasive and non-ionizing clinical modality that uses sonic energy under image guidance to treat target tissue with high spatial precision at various locations in the body. We and others have shown that FUS improves the delivery of both genes and drugs, and enhances the therapeutic clearance of murine tumors [Citation17–20]. A key benefit of FUS is its unique ability to generate both thermal and mechanical effects in tissues without the use of any photoreactive or magnetic agents. FUS parameters are tunable and can elicit ablative, boiling, mild heating, and low-intensity mechanical stress in tumors [Citation18,Citation21,Citation22]. In particular, ablative FUS generates temperatures >60 °C at the focus, thus inducing protein denaturation, coagulative necrosis, tumor cell killing, and antitumor immunity [Citation23]. FUS exposure is generally performed under noninvasive magnetic resonance or ultrasound imaging, and is thus generally considered minimally toxic. Although large randomized clinical trials emphasizing the assessment of normal tissue toxicity of FUS are rare, some breast and liver cancer trials have reported mild to moderate skin burns in some patients [Citation24,Citation25]. This outcome is probably due to damage to tumor adjacent healthy tissues in the absence of reliable real-time thermometry [Citation26]. Therefore, methods to decrease the adverse effects are needed to overcome this limitation of FUS. One approach can be through the application of hyperbaric oxygen therapy (HBOT) following FUS in patients. HBOT allows patients to inspire 100% oxygen for a defined period to increase the oxygen supply, angiogenesis, and fibroblast proliferation in wounds, thereby decreasing tissue edema and infections [Citation27–29]. HBOT has been found to enhance the healing of thermal burns and diabetic foot ulcers [Citation29–31], radiation induced ulcerations of skin [Citation32], and osteoradionecrosis of the jaw [Citation33]. It can also reduce xerostomia in patients with oral or oropharyngeal carcinoma by improving the saliva quality post radiotherapy [Citation34]. Based on this scientific premise, we reasoned that combining HBOT and FUS will similarly reduce the thermal burns and bone necrosis in oral regions, and provide a future motivation for the investigation of this combined approach in large scale trials for ulceration reduction.
Methods
Canine patient: case history
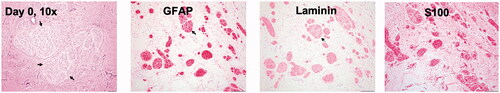
Before the treatment, we obtained owner consent to the terms of the study, including a follow-up postmortem analysis and release from institutional/personal (researcher) liability. All animal related procedures were approved by the Oklahoma State University Animal Care and Use Committee. The canine patient was a 3 year and 8-month-old pit bull mix with an identifiable tumor of 4.3 × 3.8 × 3.8 cm (width × length × height) on the left maxilla. The initial examination of the patient revealed hematological and biochemical parameters in normal ranges. A pretreatment biopsy revealed an un-encapsulated multinodular mass with low cellularity, composed of oval to spindle-shaped neoplastic cells arranged in whorls or bundles supported by a loose fibrovascular stroma (). The neoplastic cells had variably distinct cell borders with moderate amounts of eosinophilic fibrillar cytoplasm and single nuclei with finely stippled chromatin and indistinct nucleoli. Anisocytosis and anisokaryosis were mild. The neoplastic cells showed diffuse, moderate to strong cytoplasmic immunoreactivity toward GFAP, laminin, and S100 proteins—findings indicative of an aggressive neurilemmoma (schwannoma) tumor.
FUS setup and treatments
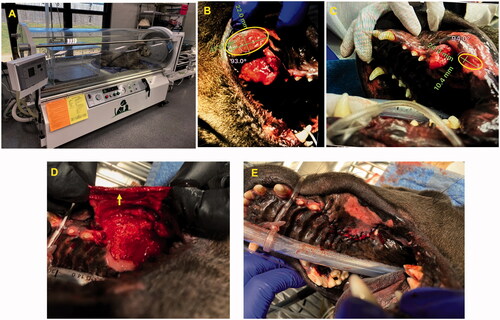
We performed the FUS treatment under ultrasound guidance with an Alpinion dry-type platform with a 1 MHz transducer capable of treating small animals () [Citation17,Citation35–38]. In the dry-type system, the transducer sits inside an acoustically transparent membrane filled with degassed water for easy placement over tumor areas (). The system allowed for mechanical translation of the transducer, with rotation 180° around the Z axis and 0–90° around the X axis. The transducer moved in 3D space with 3 degrees of freedom to adjust to the shape and size of the treated region and allow the sonication to conform to the tumor, avoiding underlying bones as needed. shows the ultrasound image of the oral tumor. Sonication was targeted to the center of the oral tumor in the in the anesthetized patient, by using VIFU planning software under ultrasound guidance to define the target boundary and slice distance in the x, y, and z directions for automatic rastering of the transducer. The FUS parameters used were in the ablation range: 50% duty cycle, 90 W acoustic power, and ∼30 s to 1 min per focal point. Three FUS ablative treatments, each covering ∼50% of the tumor core region, were performed over 1 month. Briefly, computed tomography (CT) scans were used to co-register with real-time ultrasound imaging at the time of FUS to improve ultrasound imaging, and the planning and monitoring of ongoing treatment. Vital signs were monitored with a fiber optic cuff placed around a shaved front paw, and degassed gel was used to provide acoustic coupling. For ultrasound exposure, the tumor was aligned at a fixed focal depth to cover a voxel size of 1 × 1×10 mm. VIFU-2000 software was used to define the target boundary and slice distance in the x, y, and z directions for automatic rastering of the transducer during treatment.
Histopathological and radiological examination
Biopsies were collected before treatment and then after each treatment. Briefly, the tumor region was scrubbed with 2% chlorhexidine solution, and a 6 mm Baker’s punch was used to obtain a sample (4–6 mm in size) from the treated region. The defects in the biopsied regions were closed with one or two simple interrupted sutures. For histopathology, formalin-fixed tissue sections were trimmed and processed, and hematoxylin and eosin (H&E) stained slides were analyzed by a veterinary pathologist at the Oklahoma Animal Disease Diagnostic Laboratory. Immunohistochemistry evaluations of GFAP (Dako, Denmark), S100 (Dako, Denmark), laminin (Cambridge, MA), and Cluster of differentiation 3 (CD3) (Leica, Buffalo Grove, IL) were performed with commercially available laboratory methods. Additionally, CT was performed before treatment, on days 30 and 90 after FUS treatments by a board-certified veterinary radiologist (Vimago, GT 30 Epica International).
HBOT treatment and post-treatment surgical management
HBOT exposure was performed with a 2800 Sechrist Monoplace Hyperbaric Chamber (Sechrist, USA) pressurized to 1.5 ATA. A total of 16 HBOT sessions were performed after the first FUS treatment between day 8 and day 28 (three times/week, two sessions/day). Each session lasted 45 min. Briefly, the patient was placed in the monoplace hyperbaric oxygen chamber and exposed daily (). After the completion of FUS ablation and healing of thermal burns, the exposed bone tissues were gently debrided once on day 30, and a synthetic dental bone graft (Synergy) and clindamycin (clindoral 2% gel, VEDCO) were placed to encourage bone healing. Vertical diverging incisions were made in the gingiva around the alveolar bone, and a periosteal elevator was used to elevate the HBOT healed gingiva and cover the bony regions. The gingival flap was closed with a 3-0 suture with no tension in a simple interrupted pattern. The patient was administered carprofen (2.2 mg/kg BID for 7 days) for pain control, and follow-up evaluations were performed to assess patient eating and drinking, as well as flap healing.
Flow cytometry analysis of tumors and blood
Blood samples were collected in BD Vacutainer EDTA tubes, and biopsy samples were collected into RPMI supplemented with 2% FBS. Samples were transported and stored on ice or at 4 °C until further analysis. Single-cell suspensions were obtained through mechanical disruption of the tumor biopsy tissues followed by enzymatic digestion with 200 U/ml collagenase IV (Life Technologies, NY, USA). The lysates were filtered through a 70 µm cell strainer (Corning Inc, Corning, NY). Blood samples collected in EDTA tubes were incubated with 1× RBC lysis buffer (multispecies, Invitrogen) for 10–15 min before antibody staining. The following fluorochrome-conjugated antidog antibodies were used to stain cells for 30 min in the dark on ice: anti-CD3+, anti-CD4+, anti-CD-8+ (dog T lymphocyte cocktail, cat. 558699, BD Pharmingen), and APC labeled anti-CD45+ (YKIX716.13, cat. MCA1042, Bio-Rad). For detecting interferon (IFN)-γ and Foxp3 positive T regulatory (Treg) cells, the cells were washed after surface marker staining, fixed, permeabilized with a transcription factor buffer set (BD Biosciences, San Jose, CA), and incubated with Alexa Fluor 700 labeled anti-IFN-γ (CC302, Novus biologicals) and e-fluor 450 labeled anti-Foxp3 (FJK-16s, cat. 5016374, Fisher) for 50 min in the dark on ice. Stained cells were sorted with a FACS Aria II (BD Biosciences) instrument within 24–48 h. Compensation was performed with single-stained UltraComp eBeads (Invitrogen). Datasets were analyzed in FlowJo software v.10.2 (Treestar Inc, Ashland, OR, USA).
Results
FUS induced efficient remission of the treated tumor, and HBOT enhanced wound healing
The efficacy of FUS at the indicated time points was evaluated with longest tumor diameter (mm) measurements and recording of new lesions according to Veterinary Cooperative Oncology Group (VCOG) response evaluation criteria in solid tumors (RECIST) v1.1 guidelines [Citation39]. Complete response involved disappearance of all target lesions, partial response involved a > 30% decrease in the sum of tumor diameters, and stable disease involved a < 30% decrease in tumors or >20% increase in the sum of the diameters of target lesions after FUS treatments. Significant tumor regression after the first FUS exposure, as compared with the pretreatment value, was observed on day 8 by visual examination, and complete remission of the treated tumor was achieved after the 3rd FUS treatment (). The treated regions showed a calcified mass in the tumor, which probably caused thermal burns in the adjoining buccal mucosa, owing to high acoustic absorption (). The calcified mass was debrided on day 10 before the 2nd FUS exposure on day 14. After the completion of FUS treatments, the patient was followed for an additional 3 months. No new lesions were observed during the entire monitoring period ( and ). These findings were also verified by CT examination, which showed an absence of soft tissue mass in the treated regions from day 30 onward (). To aid in the healing of thermal burns, we administered HBOT from day 8 onward (). Compared with the pretreatment burn levels (42 × 22 mm) of the buccal mucosa (), the extent of ulceration decreased progressively by day 20 (24 × 10.4 mm; ) and resolved completely by day 25. After the thermal burns were replaced with healthy granulation tissues in the treated region, HBOT was stopped on day 28, and the exposed bones underlying the tumor were gently debrided on day 30 (); this region was then covered with a gingival flap (). At 2–3 days postsurgery, normal eating, drinking, and other behavioral responses were reported in the treated patient.
Figure 3. Treatment timeline and response rates. (A) Three partial FUS treatments (∼50% of tumor volume/session) were performed over 3 weeks. Tumor biopsies were collected before and after FUS treatment. HBOT was administered between day 10 to day 28. (B) Caliper measurements of the longest diameter of treated tumor after various FUS sessions. Complete local tumor remission was observed by the end of the 4th week; (C) Therapeutic efficacy evaluated with the response evaluation criteria in solid tumors (RECIST v1.1) suggested complete response at the treated site without emergence of new lesions at 3 months post-treatment (CR: complete response; PR: partial response; SD: stable disease).
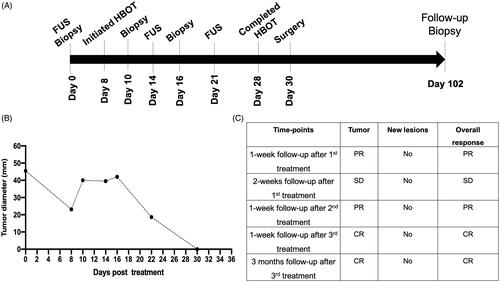
Figure 4. (A) Representative images of the treated tumor regions over 102 days. Complete remission of the treated tumor (arrow) was observed as time progressed. Briefly, necrosis and a calcified mass in the treated tumor were observed 1 week after the first FUS treatment and were associated with thermal burns (ellipse) of the regional buccal mucosa, probably because of high acoustic absorption and heating of the calcified mass during FUS treatment. The calcified mass was debrided on day 10. Additional FUS treatments were administered on days 14 and 21 to induce complete tumor remission. Longitudinal monitoring of the treated regions from days 25 to 102 showed an absence of the tumor in the treated region. (B) CT revealed a central gas filled defect in the left caudal maxilla (green arrow) in the area of FUS focus on days 30 and 102 post-treatment. New bone formation appeared to fill the depth of the osseous defect in the left caudal maxillary crest by day 30. A mild amount of amorphous new bone on the floor of the left orbital dorsal region of the removed soft tissue mass was also observed.
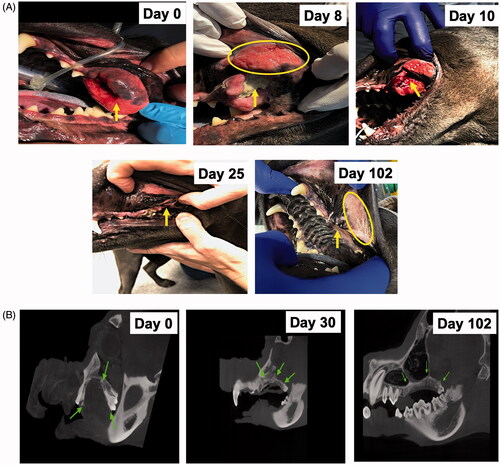
Figure 5. (A) HBOT device and setup. (B) A thermal burn (42 × 22 mm) was observed in the buccal mucosa (ellipse) region close to the treated tumor on day 8. (C) HBOT administration decreased the zone of thermal burns (24 × 10.4 mm; ellipse), as compared with the pretreatment levels. (D, E) The exposed bones underlying the tumors were closed with a gingival flap (arrow) after complete tumor remission and healing of thermal burns on day 30.
Histopathology showed an absence of viable tumor cells in the treated regions
H&E staining of post-treatment biopsy samples on day 10 and day 16 showed minimal areas of necrosis, and tumor cells were surrounded by inflammatory cells. On day 26, coagulative necrosis was observed within the mass, as characterized by hypereosinophilic neoplastic cells lacking nuclear details (). Additionally, moderate numbers of inflammatory cells including CD3+ T-cells surrounding tumor cells were observed ( and ). In general, the tumor periphery showed large numbers of degenerate to non-degenerate neutrophils, low numbers of lymphocytes, and macrophages admixed with a sero-cellular crust. Evaluation of the treated region on day 102 showed an absence of tumor cells, and the submucosa mostly consisted of collagen bundles, a few lymphocytes and melanomacrophages ().
Figure 6. (A–C) Marked areas of coagulative necrosis within the mass, characterized by hyper-eosinophilic neoplastic cells lacking nuclear details. (D, H) Evaluation of treated regions on day 102 showed an absence of tumor cells with submucosa composed of collagen, fibroblasts (arrow head), and melanomomacrophages (arrow). (E–H) The tumor showed infiltrated neutrophils, lymphocytes, and macrophages admixed with tumor cells between day 10 and day 26, and they became rare by day 102.
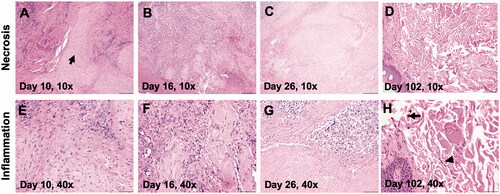
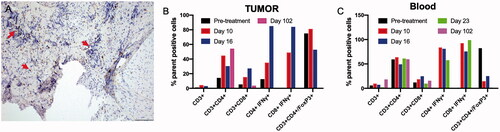
Figure 7. (A) A day 10 tumor biopsy sample showed low to moderate numbers of CD3+ lymphocytes surrounding the tumor cells (arrow, 200× magnification). (B, C) Trends in the treatment effects on T cells in the tumor and blood over time, relative to the pretreatment controls. FUS ablation increased the frequency of CD3+, and IFNγ expressing CD3+ CD4+ T cells and CD3+ CD8+ T cells at 1–3 weeks post-treatment in the tumor, as compared with the pretreatment levels. The population of T-cells became undetectable on day 102 in the tumor region. Similarly, an elevated population of IFNγ expressing CD3+ CD4+ T cells and CD3+ CD8+ T cells was observed in the blood 2–3 weeks post-treatment, and these cells became undetectable by day 102. A consistent decrease in the populations of the immunosuppressive Treg cells in the blood was observed post-treatment, and these cells became undetectable in both the blood and tumor by day 102.
FUS ablation enhanced antitumor immunity
Local and systemic evaluation of the immune responses of harvested tumor biopsy and blood samples after FUS ablation revealed an overall increase in CD3+ T cells from days 10 to 23, relative to the pretreatment levels (). Additional phenotypic characterization suggested an enhancement in CD3+ CD8+ and CD3+ CD4+ cells T cells with high IFNγ expression, thus suggesting an activated cytotoxic phenotype. Interestingly, a concurrent decrease in the population of Foxp3+ CD4+ Tregs, relative to the pretreatment paired control levels, was observed, particularly in the blood samples, and it became undetectable by day 102 ().
Discussion
Oral cancers are primarily treated surgically [Citation3]. However, in advanced cases, resection of tumors is combined with external beam radiotherapy and chemotherapy [Citation40]. The objective of this study was to investigate whether noninvasive FUS ablation might induce remission of a spontaneous oral tumor.
To investigate this possibility, we recruited a canine patient with schwannoma. Schwannoma or neurilemmoma is a rare tumor that can be found in areas such as the lips, jaws, tongue, and mucosa [Citation41,Citation42]. It is treated with transoral excision; however, excision may be challenging to perform in larger tumors requiring sufficient margins [Citation43]. Like human schwannoma, canine schwannoma is a typically benign and solitary lesion; however, malignant cases have also been reported [Citation15]. After enrollment, we performed FUS ablation of the tumor in three sessions over 3 weeks. The premise of this design stemmed from a study in a uterine myoma patient where FUS induced TLS and acute kidney injury [Citation44]. Thus, we reasoned that a single ablative session might similarly induce tumor lysis syndrome (TLS), which occurs when tumor cells rapidly release their contents into the systemic circulation, thereby resulting in hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. These metabolic disturbances can cause cardiac toxicity and neurophysiological abnormalities [Citation45,Citation46]. Our longitudinal assessment indicated that the patient tolerated the treatment, and showed a normal appetite and mild pain at the treated region. Additionally, renal function examination did not indicate any signs of acute toxicity. Studies are currently underway in our laboratory to verify whether TLS is indeed a sequalae of FUS in a larger cohort of patients.
We performed the ablative treatments under ultrasound guidance (). Unlike MR-guided FUS, which provides absolute temperature mapping by proton resonance frequency shifts, ultrasound is limited in providing real-time thermometry of the targeted region [Citation47]. To overcome this barrier, we relied on temperature monitoring with fiber optic temperature sensors [Citation17]. At the selected FUS parameters, we found that the temperatures were in ablative ranges. Progressive tumor remission was observed with each treatment, and the tumor became undetectable after approximately 4 weeks of treatment (). Mechanistically, the FUS treatment induced coagulative necrosis and increased the populations of inflammatory cells in the treated tumor. Prior research has shown that FUS ablation enhances access to the intracellular tumor-associated antigens and the presentation to immune system components, thus improving the immunotherapeutic response [Citation17,Citation23,Citation48,Citation49]. Our findings were in line with those observations. For example, flow cytometry analysis of tumor and blood samples collected during the course of treatment suggested an increase in the populations CD4+ and CD8+ cells with IFN-γ production in tumor and blood samples, as compared with the pretreatment levels () [Citation18,Citation50,Citation51]. We hypothesized that partial ablation of the tumor core generated a heat gradient and moderate hyperthermia in the tumor periphery, thereby releasing heat-shock proteins and tumor antigens and improving antigen presentation. The enhanced T-cell function was also associated with a decrease in the local and systemic populations of Tregs, thereby indicating an activated antitumor immune system [Citation52]. Longitudinal assessment of prophylaxis against local recurrence in more aggressive tumor types (e.g., squamous cell carcinoma) should be investigated in future studies to understand these mechanisms in greater detail.
Although our treatments were highly conformal, evaluation of the tumor after the first FUS treatment revealed the presence of a calcified mass in the tumor core (). Calcified tissues (e.g., bone) have high acoustic absorption, exceeding the threshold of necrosis by >4-fold. Additionally, the reflected waves from such regions back to the transducer can induce fluctuations of ±15 °C, thus potentially damaging nearby healthy tissues [Citation53]. On this basis, we believe that the unanticipated normal tissue toxicity in the buccal mucosa was probably induced by this process. To enhance healing of thermal wounds close to the tumor, we used HBOT because it is known to decrease tissue hypoxia and infection, and enhance neovascularization [Citation54]. Visual examination of the oral regions suggested improved healing rates in the presence of HBOT (). The ability to approve or disapprove HBOT in burn settings remains questionable, because of the high variability in individual outcomes [Citation55]. However, enhanced microcirculation was found to aid the regeneration and healing of oral mucosal surgical flaps in HBOT group (2.5 bar O2/90 min) relative to the control group on days 7, 9, and 11 in a rabbit model via enhanced neo-angiogenesis in sub-ischemic region [Citation56]. HBOT (28 sessions, 240 kPa for 90 min; n = 14 patients) also improved oxygenation and vascularization of irradiated facial skin and gingival mucosa at 3- and 6-months post treatment compared to HBOT minus irradiated patients (n = 8 patients), and transmucosal oxygen tension from 50 to ∼80% in human oral mucosa of 10 patients [Citation57]. These suggest that adding HBOT may plausibly enhance vascularization in mucosal regions to hypothetically improve the healing of thermal burns. Because the recovery rates were not compared with controls without HBOT, a large veterinary clinical trial will be needed in the future to verify our findings. It may be noted that HBOT alone does not directly induce tumor cell death [Citation58]. However, in mouse models, HBOT has been shown to decrease inflammation by downregulating Toll-like receptor expression, cytokine production, and NF-κB activation [Citation59,Citation60]. A mouse glioma study has also shown that HBOT decreases the populations of CD3+, CD3+/CD8+, CD3+/CD4+, and Treg cells via modulating reactive oxygen species signaling in thymus and tumor cells [Citation61]. Our immunological analysis showed a trend toward enhanced CD8+ and CD4+ populations after FUS and HBOT exposure in the tumor and blood. In contrast, the proliferation of Tregs was markedly diminished in the blood 2–3 weeks post-treatment. On the basis of our findings, we propose that local FUS increased the killing of tumor cells, and its combination with HBOT modulated the tumor microenvironment and hypoxia mediated stress pathways [Citation62], thereby collectively enhancing the activated T-cell populations in the tumor and blood.
This case report has some limitations. The tumor was easily accessible for FUS therapy in the schwannoma patient. However, the ability of FUS to acoustically couple with tumors located in the soft palate region is likely to pose a challenge. Also, additional investigation is needed to determine whether HBOT exposure modifies tumor physiology. In summary, our data suggest that FUS ablation can induce regression of a large schwannoma. Local FUS also generated an inflamed tumor microenvironment that may possibly aid in antitumor immunity. Additional studies in malignant models are needed to shed more light on the therapeutic mechanisms.
Author contributions
A.R. conceived the project, designed the study goals, and wrote the article. D.K., A.S., and H.A. conducted various experiments under the supervision of A.R.; H.A., and A.R. performed FUS, H.A. analyzed flow cytometry data, D.K. performed surgical treatments, T.N. and D.K. performed HBOT, and S.M. led the histopathology efforts.
Acknowledgments
We acknowledge a seed grant from the College of Veterinary Medicine and the Kerr Endowed Chair at Oklahoma State University for supporting this research. We also thank Dr. Valerie for pathology consultation, and McElliott, Korrine Folmar, Carrie Morgan, Hayley Nash and Madison Pfeiffe for clinical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ellington TD, Henley SJ, Senkomago V, et al. Trends in incidence of cancers of the oral cavity and pharynx – United States 2007–2016. MMWR Morb Mortal Wkly Rep. 2020;69:433–438.
- Joo YH, Cho JK, Koo BS, et al. Guidelines for the surgical management of oral cancer: Korean society of thyroid-head and neck surgery. Clin Exp Otorhinolaryngol. 2019;12:107–144.
- Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol. 2014;19:423–430.
- Shanti RM, O'Malley BW Jr. Surgical management of oral cancer. Dent Clin North Am. 2018;62:77–86.
- da Silva SD, Hier M, Mlynarek A, et al. Recurrent oral cancer: current and emerging therapeutic approaches. Front Pharmacol. 2012;3:149.
- Wang B, Zhang S, Yue K, et al. The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer. 2013;32:614–618.
- Woolgar JA, Scott J, Vaughan ED, et al. Survival, metastasis and recurrence of oral cancer in relation to pathological features. Ann R Coll Surg Engl. 1995;77:325–331.
- Mikiewicz M, Paździor-Czapula K, Gesek M, et al. Canine and feline oral cavity tumours and tumour-like lesions: a retrospective study of 486 cases (2015–2017). J Comp Pathol. 2019;172:80–87.
- Cray M, Selmic LE, Ruple A. Demographics of dogs and cats with oral tumors presenting to teaching hospitals: 1996–2017. J Vet Sci. 2020;21:e70.
- Dorn CR, Taylor DO, Schneider R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318.
- Silva EO, Goiozo PFI, Pereira LG, et al. Concomitant malignant pulmonary peripheral nerve sheath tumour and benign cutaneous peripheral nerve sheath tumour in a dog. J Comp Pathol. 2017;157:46–50.
- Boonsriroj H, Kimitsuki K, Akagi T, et al. Malignant epithelioid schwannoma of the oral cavity in a cat. J Vet Med Sci. 2014;76:927–930.
- Giangaspero F, Fratamico FC, Ceccarelli C, et al. Malignant peripheral nerve sheath tumors and spindle cell sarcomas: an immunohistochemical analysis of multiple markers. Appl Pathol. 1989;7:134–144.
- Gaitero L, Añor S, Fondevila D, et al. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: a histopathological and immunohistochemical study. J Comp Pathol. 2008;139:16–23.
- Ottinger T, Lindberg R, Ekman S. Malignant acoustic schwannoma in a dog. J Vet Diagn Invest. 2009;21:129–132.
- Lambade PN, Palve D, Lambade D. Schwannoma of the cheek: clinical case and literature review. J Maxillofac Oral Surg. 2015;14:327–331.
- VanOsdol J, Ektate K, Ramasamy S, et al. Sequential HIFU heating and nanobubble encapsulation provide efficient drug penetration from stealth and temperature sensitive liposomes in colon cancer. J Control Release. 2017;247:55–63.
- Sethuraman SN, Singh MP, Patil G, et al. Novel calreticulin-nanoparticle in combination with focused ultrasound induces immunogenic cell death in melanoma to enhance antitumor immunity. Theranostics. 2020;10:3397–3412.
- Stavarache MA, Petersen N, Jurgens EM, et al. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J Neurosurg. 2018;130:989–998.
- Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood–brain barrier with focused ultrasound. J Control Release. 2015;219:61–75.
- Bandyopadhyay S, Quinn TJ, Scandiuzzi L, et al. Low-intensity focused ultrasound induces reversal of tumor-induced T cell tolerance and prevents immune escape. J Immunol. 2016;196:1964–1976.
- Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia. 2007;23:165–171.
- Mauri G, Nicosia L, Xu Z, et al. Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol. 2018;91:20170641.
- Li S, Wu PH. Magnetic resonance image-guided versus ultrasound-guided high-intensity focused ultrasound in the treatment of breast cancer. Chin J Cancer. 2013;32:441–452.
- Leslie T, Ritchie R, Illing R, et al. High-intensity focused ultrasound treatment of liver tumours: post-treatment MRI correlates well with intra-operative estimates of treatment volume. BJR. 2012;85:1363–1370.
- Schmitz AC, Gianfelice D, Daniel BL, et al. Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol. 2008;18:1431–1441.
- Kirby JP, Snyder J, Schuerer DJE, et al. Essentials of hyperbaric oxygen therapy: 2019 review. Mol Med. 2019;116:176–179.
- Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45:316–324.
- Oley MH, Oley MC, Aling DMR, et al. Effects of hyperbaric oxygen therapy on the healing of thermal burns and its relationship with ICAM-1: a case–control study. Ann Med Surg. 2021;61:104–109.
- Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123.
- Cianci P, Slade JB, Jr., Sato RM, et al. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns. Undersea Hyperb Med. 2013;40:89–108.
- Enomoto M, Yagishita K, Okuma K, et al. Hyperbaric oxygen therapy for a refractory skin ulcer after radical mastectomy and radiation therapy: a case report. J Med Case Rep. 2017;11:5–5.
- Sultan A, Hanna GJ, Margalit DN, et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: a Dana-Farber/Brigham and Women's Cancer Center Multidisciplinary Guideline . Oncologist. 2017;22(3):343–350 [published correction appears in Oncologist. 2017 Nov;22(11):1413].
- Gerlach NL, Barkhuysen R, Kaanders JHAM, et al. The effect of hyperbaric oxygen therapy on quality of life in oral and oropharyngeal cancer patients treated with radiotherapy. Int J Oral Maxillofac Surg. 2008;37:255–259.
- Wardlow R, Sahoo K, Dugat D, et al. High intensity focused ultrasound (HIFU) heating improves perfusion and antimicrobial efficacy in mouse Staphylococcus abscess. Ultrasound Med Biol. 2018;44:909–914.
- Wardlow R, Bing C, VanOsdol J, et al. Targeted antibiotic delivery using low temperature-sensitive liposomes and magnetic resonance-guided high-intensity focused ultrasound hyperthermia. Int J Hypertherm. 2016;32:254–264.
- Ranjan A, Jacobs GC, Woods DL, et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Controlled Release. 2012;158:487–494.
- Maples D, et al. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int J Hypertherm. 2015;31:674–685.
- Nguyen SM, Thamm DH, Vail DM, et al. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13:176–183.
- Kim D, Li R. Contemporary treatment of locally advanced oral cancer. Curr Treat Options Oncol. 2019;20:32.
- Martins MD, Anunciato de Jesus L, Fernandes KPS, et al. Intra-oral schwannoma: case report and literature review. Indian J Dent Res. 2009;20:121–125.
- Santos PP, Freitas VS, Pinto LP, et al. Clinicopathologic analysis of 7 cases of oral schwannoma and review of the literature. Ann Diagn Pathol. 2010;14:235–239.
- Cohen M, Wang MB. Schwannoma of the tongue: two case reports and review of the literature. Eur Arch Otorhinolaryngol. 2009;266:1823–1829.
- Park J, Lee JS, Cho JH, et al. Effects of high-intensity-focused ultrasound treatment on benign uterine tumor. J Korean Med Sci. 2016;31:1279–1283.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854.
- Strauss PZ, Hamlin SK, Dang J. Tumor lysis syndrome: a unique solute disturbance. Nurs Clin North Am. 2017;52:309–320.
- Fernando R, Downs J, Maples D, et al. MRI-guided monitoring of thermal dose and targeted drug delivery for cancer therapy. Pharm Res. 2013;30:2709–2717.
- D'Souza AL, Chevillet JR, Ghanouni P, et al. Tumor characterization by ultrasound-release of multiple protein and microRNA biomarkers, preclinical and clinical evidence. PLoS One. 2018;13:e0194268.
- Xu ZL, Zhu XQ, Lu P, et al. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol. 2009;35:50–57.
- Castro F, Cardoso AP, Gonçalves RM, et al. Interferon-gamma at the crossroads of tumor immune surveillance or Evasion. Front Immunol. 2018;9:847.
- Ektate K, Munteanu MC, Ashar H, et al. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8:13062.
- Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171.
- Nell DM, Myers MR. Thermal effects generated by high-intensity focused ultrasound beams at normal incidence to a bone surface. J Acoust Soc Am. 2010;127:549–559.
- Weitgasser L, Ihra G, Schäfer B, et al. Update on hyperbaric oxygen therapy in burn treatment. Wien Klin Wochenschr. 2021;133(3-4):137–143.
- Weitgasser L, Ihra G, Schäfer B, et al. Update on hyperbaric oxygen therapy in burn treatment. Wien Klin Wochenschr. 2021;133:137–143.
- Helmers R, Milstein DM, van Hulst RA, et al. Hyperbaric oxygen therapy accelerates vascularization in keratinized oral mucosal surgical flaps. Head Neck. 2014;36:1241–1247.
- Thorn JJ, Kallehave F, Westergaard P, et al. The effect of hyperbaric oxygen on irradiated oral tissues: transmucosal oxygen tension measurements. J Oral Maxillofac Surg. 1997;55:1103–1107.
- Schönmeyr BH, Wong AK, Reid VJ, et al. The effect of hyperbaric oxygen treatment on squamous cell cancer growth and tumor hypoxia. Ann Plast Surg. 2008;60:81–88.
- Rinaldi B, Cuzzocrea S, Donniacuo M, et al. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37:1110–1119.
- Lu Z, Ma J, Liu B, et al. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med. 2016;5:3147–3155.
- Wang YG, Long J, Shao DC, et al. Hyperbaric oxygen inhibits production of CD3+ T cells in the thymus and facilitates malignant glioma cell growth. J Int Med Res. 2018;46:2780–2791.
- Li Y, Patel SP, Roszik J, et al. Hypoxia-driven immunosuppressive metabolites in the tumor microenvironment: new approaches for combinational immunotherapy. Front Immunol. 2018;9:1591–1591.