Abstract
Background
Multicystic peritoneal mesothelioma (MCPM) is a rare, slowly growing, condition prone to recur after surgery. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) added to complete cytoreductive surgery (CRS) remains controversial and difficult to assess. As patients are mostly reproductive age women, surgical approach, and fertility considerations are important aspects of the management. This observational retrospective review aimed to accurate treatment strategy reflections.
Methods
The RENAPE database (French expert centers network) was analyzed over a 1999–2019 period. MCPM patients treated with CRS were included. A special focus on HIPEC, mini-invasive approach, and fertility considerations was performed.
Results
Overall 60 patients (50 women) were included with a median PCI of 10 (4–14) allowing 97% of complete surgery, followed by HIPEC in 82% of patients. A quarter of patients had a laparoscopic approach. Twelve patients (20%) recurred with a 3-year recurrence free survival of 84.2% (95% confidence interval 74.7–95.0). The hazard of recurrence was numerically reduced among patients receiving HIPEC, however, not statistically significant (hazard ratio 0.41, 0.12–1.42, p = 0.200). A severe post-operative adverse event occurred in 22% of patients with five patients submitted to a subsequent reoperation. Among four patients with a childbearing desire, three were successful (two had a laparoscopic-CRS-HIPEC and one a conventional CRS without HIPEC).
Conclusion
MCPM patients treatment should aim at a complete CRS. The intraoperative treatment options as laparoscopic approach, fertility function sparing and HIPEC should be discussed in expert centers to propose the most appropriate strategy.
Introduction
The term ‘multicystic peritoneal mesothelioma’ (MCPM) was introduced in 1979 by Mennemeyer and Smith [Citation1] after its macroscopic description by Plaut in 1928 [Citation1]. The long delay before the pathologic characterization could be explained by the extremely low incidence of this disease. While peritoneal mesothelioma affects 1–2 cases/million/year, MCPM accounts for 3–5% of these cases and affects mainly women in reproductive age with a sex ratio of 4.7:1 [Citation2–4]. The diagnosis is usually incidental or secondary to unspecific abdominal symptoms, rarely during an infertility work-up [Citation5–7]. Computed tomography scanner and magnetic resonance imaging (MRI), classically allow MCPM diagnosis when pathognomonic multifocal multicystic lesions as bunches of grapes are present [Citation8,Citation9].
Typical MCPM histology harbors border-line signs with a lack of cellular atypia or increased number of mitoses; however, squamous cell metaplasia is possible [Citation10,Citation11]. Lesions are made of small cysts with benign mesothelial epithelium appearing cuboidal cells occasionally forming papillae. Between the cysts, the stroma is characterized by variable degree of inflammation. Sometimes combined endometriosis lesions are found, fueling the theory of a chronic peritoneal irritation pathogenesis [Citation12]. The pathological differential diagnoses include a number of benign and malignant lesions presenting as multicystic abdominal masses and are usually clarified with immunohistochemistry [Citation2,Citation4,Citation13]. The particular epidemiologic characteristics of MCPM patients when compared to malignant mesothelioma patients – young women without history of mineral fiber exposition – suggest an independent pathogenesis. However, nor the hormonal, nor the repetitive peritoneal irritation theories have been decidedly established [Citation11,Citation12,Citation14–16].
Two features of MCPM should guide the therapeutic strategy: the high recurrence rate after surgery, estimated up to 50% [Citation17,Citation18] and the rare eventuality of malignant transformation despite its slow growing course [Citation11,Citation19–22]. It is justified not to classify MCPM as a ‘benign’ disease but as a borderline disease. Several therapeutic strategies have been proposed but comprehensive cytoreductive surgery (CRS), typically followed by an hyperthermic intraperitoneal chemotherapy (HIPEC), has been reported to be associated with the best long-term outcomes [Citation5,Citation23,Citation24]. This invasive treatment has recently been advocated by international clinical guidelines, as a front-line treatment for the Peritoneal Surface Oncology Group International (PSOGI) and after an observation period in case of symptoms or progression for the Chicago Consensus Working Group [Citation4,Citation25]. Therefore, the first paradigm of MCPM is to proceed to an invasive treatment considering its loco-regional recurrence risk, despite its slow growing rate. The second is the confrontation between a childbearing desire and a CRS-HIPEC indication prone to decrease fertility, raising the question of less invasive strategies.
RENAPE is a French network of peritoneal surface malignancies centers that maintains a dedicated database. The present report is an observational retrospective review of a large cohort of MCPM patients treated by CRS followed or not by HIPEC, with a focus on post-operative results, the role of mini-invasive approaches, and fertility considerations, aiming at give insights for tailoring therapeutic strategies.
Materials and methods
The RENAPE observational registry
The French peritoneal surface malignancies centers network RENAPE was set up to harmonize therapeutic strategies and enhance collaborative research projects to overcome the challenge of rare conditions [Citation26]. An annual meeting allows to discuss current issues, to coordinate clinical studies and to find an agreement on main therapeutic strategies. Regional monthly multidisciplinary team meetings are held to decide rare peritoneal disease cases treatment strategies. The cornerstone of that work is the RENAPE database, prospectively collected from 2010 and enriched by retrospective inclusion of cases treated before that date (ClinicalTrials.gov identifier: NCT02834169). All cases reported as MCPM were extracted from the RENAPE registry as of December 2019 [Citation27]. The following inclusion criteria were then applied: having received at least one CRS in a RENAPE center and having a sufficient amount of data for analysis including: diagnosis circumstances, surgery details, HIPEC parameters, post-operative complications description and a postoperative follow-up longer than 6 months. A group of expert pathologists (RENA-PATH group) together with a group of expert radiologists (RENA-RAD group) reviewed these selected cases to validate the diagnosis and, when needed, the recurrence status. The aim was to discriminate differential diagnosis such as isolated mesothelial cyst, endometriosis or postoperative features rather than recurrences. Data quality was assured by pretesting and consistency checks during data entry, when applicable [Citation27]. The RENAPE Observational Registry complies with the ethical principles laid down in the Declaration of Helsinki and has been approved by the Advisory Committee for Data Processing in Health Research at the Research French Ministry (CCTIRS – no. 10.257).
Diagnosis and preoperative workup
A tissue biopsy obtained along staging laparoscopy or by interventional radiology allowed the histologic diagnosis. Curative intent treatment was preceding by a morphological assessment including at least an injected computed tomography scanner. The strategy was validated in RENAPE multidisciplinary meeting.
From 2009, a laparoscopic approach was considered for low grade peritoneal disease, depending on following criteria: American Society of Anesthesiologists score of less than 2, limited carcinomatosis on preoperative workup with a Sugarbaker’s Peritoneal Cancer Index (PCI) [Citation28] estimated at 10 or less, and a limited history of abdominal surgery (only two abdominal regions dissected) [Citation29]. If laparoscopic approach was considered, the workup was completed by a peritoneal MRI and a staging laparoscopy to define more accurately the disease extent and confirm the feasibility of the mini-invasive strategy. Of note, in results, a laparoscopic procedure designated only those performed without conversion to laparotomy.
Treatment and operative outcomes
The operation included a comprehensive abdominal exploration, then peritonectomies and organ resections, performed as described previously [Citation30,Citation31]. The PCI and the completeness of cytoreduction score (CC-score) [Citation28] were reported along with all HIPEC parameters (drug(s), concentration, duration, temperature). A CC-score of 0 was scored in the absence of macroscopic residual disease at the end of CRS corresponding to a complete CRS, while CC-1 meant the persistence less than 2.5 mm nodules [Citation28].
Post-operative adverse events were recorded over the 90 postoperative days following CRS and graded according to the CTCAE v5.0 classification [Citation32]. Patients were followed-up at least every 6 months with physical exam and abdomino-pelvic computed tomography scanner or peritoneal MRI. Recurrences were declared in RENAPE multidisciplinary meeting on the basis of histology and/or morphological assessment including a MRI.
Fertility considerations
With the aim to evaluate MCPM treatment impact on fertility, a specific analysis was performed on women of less than 40 years at the time of diagnosis. A survey was built up and those patients were given a phone call to investigate an eventual child bearing desire, assisted reproductive technology intervention, and fertility outcomes after the CRS.
Statistical analysis
Proportions were calculated for categorical data, whereas median and interquartile range (IQR) were calculated for continuous data. Statistical significance for categorical data was assessed using a χ2 test or a Fisher test, as appropriate. Survival probabilities were estimated using the Kaplan–Meier estimator. A comparison of severe postoperative complications (grades 3–5) incidence between patients treated with laparoscopic approach and others was performed. Recurrence-free survival was calculated from the date of surgery to the diagnosis of a recurrence or death. Recurring patients data were independently analyzed.
Results
Study population
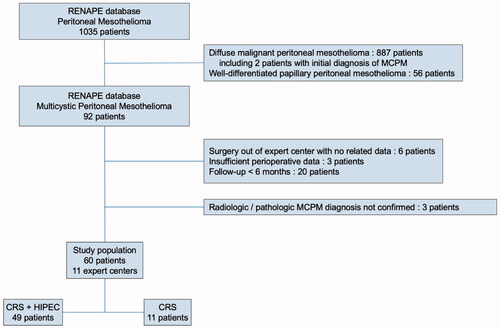
The flowchart is shown in . Over 1035 mesothelioma patients, 92 patients were referenced with MCPM in the RENAPE registry, of which 29 were excluded for insufficient data to perform a comprehensive analysis. Of note, two patients with uncommon pathology, initially diagnosed as MCPM were confirmed as malignant mesothelioma after expert reviewing. After histologic and radiologic reviewing of the remaining 63 patients, 3 patients were further excluded for the impossibility to confirm the MCPM diagnosis. The 60 included patients were treated in 11 RENAPE centers (85% in 5 units). Diagnoses were made between June 1999 and September 2019, 83% of them after June 2011. The median follow-up time was 42.8 months (95%, confidence interval (CI) 35.9–54.3).
Figure 1. Flowchart of the multicystic peritoneal mesothelioma population. MCPM: multicystic peritoneal mesothelioma; CRS: cytoreductive surgery; HIPEC: hyperthermic intraperitoneal chemotherapy.
Main patients characteristics are described in . The population exhibited a majority of women with a sex ratio of 5:1 and a median age at diagnosis of 45 years [31–55]. As expected, no history of mineral fibers exposition was encountered. Infertility was not reported as a revealing modality. The principal diagnostic circumstances were incidental findings or abdominal pain exploration, in 39% and 47% of patients, respectively. Serum tumor markers were normal. Unfortunately, data regarding history of abdominal surgery, endometriosis, and inflammatory bowel disease were insufficiently collected to be analyzed.
Table 1. Patients characteristics.
MCPM treatment with CRS ± HIPEC
The treatment parameters and outcomes are reported in . Seventeen percent of patients had a history of previous CRS outside of the referral center while one received a systemic pre-operative chemotherapy. The median PCI was 10 (4–14) allowing 97% of complete surgery. The median cytoreduction time was 242 min (210–300). CRS was followed by HIPEC in 82% of patients with cisplatin–doxorubicin being the most frequent intraperitoneal drugs used. From June 2009, a laparoscopic approach was used in 15 patients (25%), who presented a median PCI of 4.0 (3.0–5.0). A majority of these laparoscopic CRS (87%) were performed in one center.
Table 2. Treatment-related data.
Oncologic outcomes
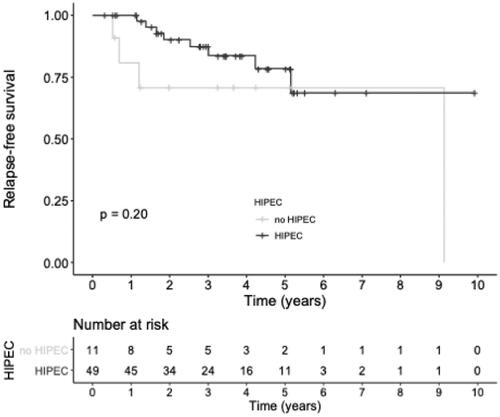
No patients died during the follow-up period while 12 (20%) recurred with a median recurrence-free survival time of 9.1 years (95% CI, 9.1 months – not reached) with a 3-year recurrence-free survival of 84.2% (74.7–95.0) as displays . A focused analysis of those 12 patients is presented in and showed that their median PCI was 11 (6–15.5) and that 2 (17%) had a CC-1 resection but none of them was operated with a laparoscopic approach. Of note, 4 of them (33%) did not receive HIPEC, while HIPEC was omitted in 9 out of the 48 non-recurrent patients (15%). The overall hazard of recurrence was reduced among patients receiving HIPEC (hazard ratio (HR) of recurrence 0.41, 95% CI, 0.12–1.42, p = 0.200). Two patients presented a delayed recurrence, 50.8 and 109.6 months after CRS, respectively.
Table 3. Recurrent patients clinical details.
Post-cytoreduction outcomes
The median length of hospital stay was 14.0 days (11.0–16.2) and 9.0 days (7.2–11.7) for patients treated with a laparotomy or a laparoscopic approach respectively (p < 0.001). No patients died within the 90 postoperative days. Overall 13 patients (22%) presented 15 severe post-operative adverse events. In particular, five patients (8%) were submitted to a subsequent surgery for an intraabdominal abscess, two eviscerations, and one digestive fistula (). All postoperative hemorrhages were manageable medically. One patient treated laparoscopically presented a severe complication (wall abscess), which was proportionally less than for patients treated by laparotomy (7 versus 27%, respectively, p = 0.130).
Fertility considerations
summarizes the outcomes of the 18 MCPM female patients younger than 40 years at diagnosis. Five (28%) had at least one successful pregnancy before surgery and 4 (22%) had a history of gynecologic surgery. One-third of these patients had a pre-operative fertility counseling which was proposed as a systematic center policy that led two patients to proceed to an oocytes cryopreservation. The median PCI of these 18 women was 6 (4–10) with 10 women (56%) presenting with pelvic lesions. The cytoreduction was performed laparoscopically in 8 (44%), including a bilateral adnexectomy in 3, and was followed by HIPEC in 14 (78%). Of the 15 patients with at least one remnant ovary: all had CC-0 CRS, 11 had HIPEC, and 2 had a recurrence at 6.2 and 14.5 months after CRS without HIPEC. Regarding the 10 patients with pelvic disease: 7 kept at least one ovary and the same 2 patients, with both ovaries in place, recurred.
Table 4. Fertility issue in MCPM women of less than 40 years old.
Data regarding fertility were accurate in 15 patients and 4 reported a desire for pregnancy during the postoperative period. Three of them successfully had a child after vaginal delivery, including 1 requiring assisted reproductive intervention. Among those four patients, three had a laparoscopic-CRS-HIPEC (including the one with no pregnancy) and one had a conventional CRS without HIPEC.
Discussion
This RENAPE registry analysis confirmed the favorable long-term outcomes obtained with CRS, usually followed by HIPEC, in MCPM patients with a relatively low recurrence rate and an acceptable safety profile. Based on strict selection criteria, the laparoscopic approach was not followed by any recurrence. All together, these results enhance the role of expert centers to manage such rare peritoneal disease.
The retrospective nature of the data collection over a large period jeopardized relevant statistical comparison but, considering the extreme rarity of the disease, no other method was applicable. It appears clearly that the main issue of MCPM patients is rather avoiding loco-regional recurrences and limiting treatment-related severe toxicities than overall survival.
Thanks to a multicentric national collaboration, RENAPE was able to present a large cohort helping at the assessment of CRS-HIPEC as first-intended treatment of MCPM patients. Thus, the recurrence hazard was numerically reduced among patients receiving HIPEC (HR 0.41, 0.12–1.42, p = 0.200). These outcomes were consistent with the main series from literature [Citation5,Citation23,Citation24]. Historic series, where patients were mainly treated by surgery alone (sometimes also with radiation or systemic chemotherapy) mentioned recurrences in half of patients with a mean interval of 32 months[Citation10,Citation11,Citation17]. Conversely in more recent series using CRS-HIPEC, recurrence rates were around 20% of patients like in the present one [Citation5,Citation24]. Some insights in the literature also suggested that an early radical intervention could decrease the recurrence rate: Baratti et al. [Citation6,Citation7] found that recurrence-free survival following previous debulking surgery was statistically worse than recurrence-free survival after upfront CRS-HIPEC.
These results plead in favor of adding HIPEC to CRS but there is still a debate regarding the frontline therapeutic strategy for asymptomatic patients with low disease burden. Two alternatives appeared in the recent clinical guidelines produced by the PSOGI and the Chicago Group: either a frontline intervention (without emergency) encouraging the addition of HIPEC, or an intervention at the time of a progression or of symptoms onset with HIPEC as an option [Citation4,Citation25]. For children with MCPM, a retrospective report suggested to reserve HIPEC as a salvage treatment in case of CRS failure [Citation33]. The level of evidence is low and the two strategies are defensible. The comprehensive choice might ideally integrate patients preferences such as a childbearing desire.
Despite a consistent follow-up, no transformation into malignant mesothelioma was found here while several cases were reported in the literature with histologic transition along repetitive CRS [Citation19–22]. Some patients have also been described as MCPM with malignant contingent [Citation34]. Thereby and given the delayed recurrences observed (at 51 and 110 months), a prolonged follow-up is justified for these patients. MRI, accurate to characterize MCPM lesions and avoiding radiation exposure of Computed Tomography scanner, appears as a suitable imaging modality [Citation9,Citation35]. A two-yearly basis from the third postoperative year should probably be recommended to detect treatable recurrences [Citation18].
Severe post-operative adverse events occurred in 22% of the patients in the present cohort, with 8% submitted to a secondary surgery but no postoperative death. These results are consistent with the median PCI of 10 and the corollary resections needed to achieve CC-0 cytoreduction. In the literature, this rate of severe adverse events varied from 7% to 60% of MCPM patients submitted to CRS-HIPEC [Citation4]. Interestingly, the sub-population treated with a laparoscopic approach, selected on the basis of strict criteria, exhibited a lower incidence of severe complications with the absence of recurrence, even if the frame of that study does not allow definitive conclusions. Laparoscopic CRS in the context of peritoneal metastases is a complex topic, rapidly evolving [Citation36–39]. The risk of underestimating the PCI by laparoscopy, and potentially jeopardizing the completeness of CRS justifies a balanced patients selection to take advantage of this approach [Citation38–40]. In this series the median PCI of patients treated laparoscopically was 4.5 (3.0–5.5), considerably lower than the one of the full cohort, which was probably the main reason explaining the excellent postoperative outcomes of this operative strategy.
A major advantage of the laparoscopic approach could be the fertility preservation. Data on peritoneal surface malignancies and fertility are scarce and made of retrospective reports, most of them related to pseudomyxoma peritonei (PMP) [Citation41–44]. In the RENAPE database, the specific survey performed in women of less than 40 years at diagnosis revealed that none had preoperative infertility and four had an assumed postoperative child desire. Three of them had a child, one after in vitro fertilization. The remaining women were expecting pregnancy for less than one year. Looking at the submitted treatment for MCPM showed that three had a laparoscopic-CRS-HIPEC (including the not answered one) and one had a conventional CRS without HIPEC. In literature, two pregnancies in the same patient were reported synchronously to a MCPM diagnosis (and three others in PMP patients) with vaginal delivery of two well children [Citation42,Citation43].
Regarding the impact of CRS-HIPEC on the reproductive function, multiple parameters could presumably interfere. First is the risk of ovarian involvement and the question of the benefit of a systematic bilateral salpingo-oophorectomy which is matter of debate, especially when considering a borderline disease [Citation45]. MCPM was described to follow the redistribution phenomenon [Citation46]. As so, the pelvic region is almost always involved even with low PCI. Regarding the 10 patients of less than 40 years with pelvic disease, 7 kept at least one ovary and 2, with both ovaries in place, recurred after CRS without HIPEC. Only one of them expressed a child desire and had a successful pregnancy.
Different strategies could be proposed to spare the reproductive function [Citation41]. In the case of obvious ovarian invasion, ovarian sparing surgery must include preoperative counseling and informed consent [Citation4]. In the case of extensive peritonectomy, ovarian sparing does not prevent postoperative adhesions potentially responsible for impaired tubal motility and subsequent infertility [Citation47]. The role of HIPEC is difficult to clarify despite several studies showing that pregnancies following CRS-HIPEC is feasible and probably underestimated [Citation41,Citation47]. That suggests that heat and drug toxicities on ovarian function are relatively low. The key component of the proper management of such patients is to deliver balanced information regarding prognosis and fertility in the context of poor retrospective data.
Our study presents several limitations due to its retrospective non comparative nature with diagnosis performed over 20 years. Despite the expertise sharing through multidisciplinary national and regional meetings, a certain degree of variation in treatment strategies depleted the value of outcomes comparisons.
Conclusions
Usually revealed as an incidental finding, the MCPM diagnosis is not difficult. Determining the most appropriate treatment strategy is more challenging considering its high recurrence rate, the potential malignant transformation and the aim of a low-impact treatment in childbearing age women. Complete CRS is the advocated treatment with a potentially lower risk of recurrence when followed by HIPEC. That treatment was feasible by laparoscopic approach in selected patients. In any case, MCPM patients should be referred to expert centers.
RENAPE collaborators
Julio Abba, MD (Service de Chirurgie Digestive et de l'Urgence, CHU Grenoble Alpes, Grenoble, France).
Mohammad Alyami, MD (Department of General Surgery and Surgical Oncology, King Khalid Hospital, Najran, Saudi Arabia).
Koceila Amroun, MD (Département de chirurgie Générale, Institut Jean Godinot, Reims, France).
Thierry André, MD, PhD (Service d'Oncologie Médicale, APHP Hôpital Saint Antoine, Paris, France).
Catherine Arvieux, MD, PhD (Service de Chirurgie Digestive et de l'Urgence, CHU Grenoble Alpes, Grenoble, France).
Gerlinde Averous-Lang, MD (Service d'Anatomie et Cytologie Pathologiques, CHRU Hautepierre, Strasbourg, France).
Naoual Bakrin, MD, PhD (Service de Chirurgie Générale et Digestive, Hôpital Lyon Sud, Lyon, France).
Sandrine Barbois, MD (Service de Chirurgie Digestive et de l'Urgence, CHU Grenoble Alpes, Grenoble, France).
Houda Ben Rejeb, MD (Service d'Anatomie Pathologique, Institut Bergonié, Bordeaux, France).
Jean-Marc Bereder, MD (Service de Chirurgie Viscérale, CHU L’Archet II, Nice, France).
Frédéric Bibeau, MD, PhD (Laboratoire d'Anatomie Pathologique, CHU Caen, Caen, France).
Pierre-Emmanuel Bonnot, MD (Service de Chirurgie Générale et Digestive, Hôpital Lyon Sud, Lyon, France).
Olivier Bouché, MD, PhD (Service de Cancérologie Digestive, CHU Robert Debré, Reims, France).
Fatiha Bouhidel, MD (Laboratoire d'Anatomie Pathologique, APHP Hôpital Saint Louis, Paris, France).
Marie-Dominique Bouzard, MD (Service de Chirurgie Digestive, APHP Hôpital Louis Mourier, Colombes, France).
Cécile Brigand, MD, PhD (Service de Chirurgie Générale et Digestive, CHRU Hautepierre, Strasbourg, France);
Sébastien Carrère, MD (Service de Chirurgie Digestive Oncologique, Institut du Cancer de Montpellier – Val d'Aurelle, Montpellier, France).
Bertrand Celerier, MD (Service de Chirurgie Digestive et Endocrinienne, GH Sud Haut-Lévêque Center médico chirurgical Magellan, Bordeaux, France).
Cécilia Ceribelli, MD (Service d'Oncologie Chirurgicale, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France).
Anne Chevallier, MD (Laboratoire d’Anatomie et Cytologie Pathologiques, CHU L’Archet II, Nice, France).
Julien Coget, MD (Service de Chirurgie Digestive et de Physiologie Digestive, CHU Charles-Nicolle, ROUEN, France).
Thomas Courvoisier-Clément, MD (Service de Chirurgie Viscérale, CHU Poitiers, Poitiers, France).
Sabrina Croce, MD, PhD (Service d'Anatomie Pathologique, Institut Bergonié, Bordeaux, France).
Peggy Dartigues, MD (Département d'Anatomie et Cytologie Pathologiques, Institut Gustave Roussy, Villejuif, France).
Cécile de Chaisemartin, MD (Service de Chirurgie Oncologique Digestive, Institut Paoli Calmettes, Marseille, France).
Anthony Dohan, MD, PhD (Service de Radiologie, APHP Hôpital Cochin, Paris, France).
Frédéric Dumont, MD (Département de Chirurgie Oncologique, Institut de Cancérologie de l'Ouest – René Gauducheau, Saint Herblain, France).
Sylvaine Durand-Fontanier, MD, PhD (Service de Chirurgie Digestive, Générale et Endocrinienne, CHU Dupuytren, Limoges, France).
Gwenaël Ferron, MD, PhD (Département de Chirurgie, Institut Universitaire du Cancer Toulouse – Oncopole, Toulouse, France).
Juliette Fontaine, MD (Service d'Anatomie et Cytologie PathologiquesHôpital Lyon Sud, Lyon, France).
Johann Gagnière, MD (Service de Chirurgie Digestive et Hépatobiliaire, CHU Estaing, Clermont-Ferrand, France).
Alexandre Galan, MD (Service de Radiologie, Hôpital Lyon Sud, Lyon, France).
Maximiliano Gelli, MD (Unité de Chirurgie Digestive Oncologique, Institut Gustave Roussy, Villejuif, France).
Laurent Ghouti, MD (Service de Chirurgie Digestive, CHU Rangueil, Toulouse, France).
François-Noël Gilly, MD, PhD (Service de Chirurgie Générale et Digestive, Hôpital Lyon Sud, Lyon, France).
Laurence Gladieff, MD (Service d'Oncologie Médicale, Institut Universitaire du Cancer Toulouse – Oncopole, Toulouse, France).
Jean-Marc Guilloit, MD (Service de Chirurgie Viscérale, Center François Baclesse, Caen, France).
Frédéric Guyon, MD (Service de Chirurgie Oncologique, Institut Bergonié, Bordeaux, France).
Bruno Heyd, MD, PhD (Service de Chirurgie Digestive, CHU Jean Minjoz, Besançon, France).
Christine Hoeffel, MD, PhD (Service de Radiologie, CHU Robert Debré, Reims, France).
Charles Honoré, MD, PhD (Unité de Chirurgie Digestive Oncologique, Institut Gustave Roussy, Villejuif, France).
Eve Huart, MD (Service de Chirurgie générale et thoracique, CHU Nord, Saint Etienne, France).
Martin Hübner, MD, PhD (Service de Chirurgie Viscérale, CHUV, Lausanne Switzerland).
Sylvie Isaac, MD (Service d'Anatomie et Cytologie Pathologiques, Hôpital Lyon Sud, Lyon, France).
Rachid Kaci, MD (Service d'Anatomie et Cytologie Pathologiques, APHP Hôpital Lariboisière, Paris, France).
Reza Kianmanesh, MD, PhD (Service de Chirurgie générale digestive et endocrinienne – pôle médico-chirurgical DUNE, CHU Robert Debré, Reims, France).
Marie-Hélène Laverrière, MD (Service d'Anatomie Pathologique, CHU Grenoble Alpes, Grenoble, France).
Valérie Lebrun-Ly, MD (Service d'Oncologie Médicale, CHU Dupuytren, Limoges, France).
Jérémie H. Lefevre, MD, PhD (Service de Chirurgie Générale et Digestive, APHP Hôpital Saint Antoine, Paris, France).
Bernard Lelong, MD (Service de Chirurgie Oncologique Digestive, Institut Paoli-Calmettes, Marseille, France).
Agnès Leroux-Broussier, MD (Service d'Anatomie et Cytologie Pathologiques, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France).
Réa Lo Dico, MD, PhD (Service de Chirurgie Digestive et Cancérologique, APHP Hôpital Saint Louis, Paris, France).
Brice Malgras, MD, PhD (Service de chirurgie viscérale, digestive et endocrinienne, HIA Begin, St Mandé, France).
Frédéric Marchal, MD, PhD (Service d'Oncologie Chirurgicale, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France).
Pascale Mariani, MD (Service d'Oncologie Chirurgicale, Institut Curie, Paris, France).
Pierre Meeus, MD (Service de Chirurgie Oncologique, Center Léon Bérard, Lyon, France).
Eliane Mery, MD (Service d'Anatomie et Cytologie Pathologiques, Institut Universitaire du Cancer Toulouse – Oncopole, Toulouse, France).
Cédric Nadeau, MD (Service de Gynécologie, CHU Poitiers, Poitiers, France).
Cécile Odin (Service de Chirurgie Générale et DigestiveHôpital Lyon Sud, Lyon, France).
Pablo Ortega-Deballon, MD, PhD (Service de Chirurgie Digestive et Cancérologique, CHU Dijon Bourgogne, Dijon, France).
Brice Paquette, MD (Service de Chirurgie Digestive, CHU Jean Minjoz, Besançon, France).
Guillaume Passot, MD, PhD (Service de Chirurgie Générale et Digestive, Hôpital Lyon Sud, Lyon, France).
Patrice Peyrat, MD (Service de Chirurgie Oncologique, Center Léon Bérard, Lyon, France).
Denis Pezet, MD, PhD (Service de Chirurgie Digestive et Hépatobiliaire, CHU Estaing, Clermont-Ferrand, France).
Guillaume Piessen, MD, PhD (Service de Chirurgie Générale et Digestive, CHRU Claude Huriez, Lille, France).
Nicolas Pirro, MD, PhD (Service de Chirurgie Digestive et Générale, CHU La Timône, Marseille, France).
Flora Poizat, MD (Département de Biopathologie, Institut Paoli-Calmettes, Marseille, France).
François Quenet, MD (Service de Chirurgie Digestive Oncologique, Institut du Cancer de Montpellier – Val d'Aurelle, Montpellier, France).
Patrick Rat, MD, PhD (Service de Chirurgie Digestive et Cancérologique, CHU Dijon Bourgogne, Dijon, France).
Pauline Ries, MD (Service de Chirurgie Oncologique Digestive, Institut Paoli-Calmettes, Marseille, France).
Pierre Rousselot, MD (Service d'Anatomie et Cytologie Pathologiques, Center François Baclesse, Caen, France).
Hélène Senellart, MD (Service d'Oncologie Médicale, Institut de Cancérologie de l'Ouest – René Gauducheau, Saint Herblain, France).
Igor Sielezneff, MD, PhD (Service de Chirurgie Digestive et Générale, CHU La Timône, Marseille, France).
Andrea Skanjeti, MD (Service de Médecine Nucléaire, Hôpital Lyon Sud, Lyon, France).
Isabelle Sourrouille, MD (Unité de Chirurgie Digestive Oncologique, Institut Gustave Roussy, Villejuif, France).
Philippe Soyer, MD, PhD (Service de Radiologie, APHP Hôpital Lariboisière, Paris, France).
Magali Svrcek, MD, PhD (Service d'Anatomie Pathologique, APHP Hôpital Saint Antoine, Paris, France).
Abdelkader Taibi, MD (Service de Chirurgie Digestive, Générale et Endocrinienne, CHU Dupuytren, Limoges, France).
Emilie Thibaudeau, MD (Service de Chirurgie Oncologique, Institut de Cancérologie de l'Ouest – René Gauducheau, Saint Herblain, France).
Olivier Tiffet, MD, PhD (Service de Chirurgie générale et thoracique, CHU Nord, Saint Etienne, France).
Jérémie Tordo, MD (Service de Médecine Nucléaire, Hôpital Lyon Sud, Lyon, France).
Yann Touchefeu, MD (Unité de Gastroentérologie, CHU Nantes Hôtel-Dieu, Nantes, France).
Bertrand Trilling, MD (Service de Chirurgie Digestive et de l'Urgence, CHU Grenoble Alpes, Grenoble, France).
Jean-Jacques Tuech, MD, PhD (Service de Chirurgie Digestive et de Physiologie Digestive, CHU Charles-Nicolle, Rouen, France).
Dimitri Tzanis, MD (Service d'Oncologie Chirurgicale, Institut Curie, Paris, France).
Séverine Valmary-Degano, MD, PhD (Service d'Anatomie Pathologique, CHU Grenoble Alpes, Grenoble, France).
Sharmini Varatharajah, MD (Service de Chirurgie Viscérale, Center François Baclesse, Caen, France).
Delphine Vaudoyer, MD (Service de Chirurgie Générale et Digestive, Hôpital Lyon Sud, Lyon, France).
Sophie Vermersch, MD (Service de Chirurgie Infantile, CHU Nord, Saint Etienne, France).
Véronique Verriele-Beurrier, MD (Service d'Anatomie Pathologique, Institut de Cancérologie de l'Ouest – Paul Papin, Angers, France).
Guillaume Vogin, MD, PhD (Service de Radiothérapie, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France).
Romuald Wernert, MD, Service de Chirurgie Digestive, Institut de Cancérologie de l'Ouest – Paul Papin, Angers, France).
Benoit You, MD, PhD (Service d'Oncologie Médicale, Hôpital Lyon Sud, Lyon, France).
Author contributions
Kepenekian: study design, data recording, results analysis, drafting manuscript; Péron: study design, results analysis, statistical analysis, drafting manuscript; Goéré: data acquisition, results analysis, manuscript revision; Sgarbura: data acquisition, results analysis, manuscript revision; Delhorme: data acquisition, results analysis, manuscript revision; Eveno: data acquisition, results analysis, manuscript revision; Benzerdjeb: pathologic analysis reviewing, results analysis, manuscript revision; Bonnefoy: data acquisition (fertility issues survey), results analysis, manuscript revision; Villeneuve: study design, data acquisition, results analysis, manuscript revision; Rousset: radiologic reviewing, study design, results analysis, manuscript revision; Abboud: data acquisition, results analysis, manuscript revision; Pocard: data acquisition, results analysis, manuscript revision; Glehen: study design, results analysis, drafting manuscript; All coauthors approved the final version of the manuscript.
Disclosure statement
Drs Kepenekian, Péron, Goéré, Sgarbura, Delhorme, Eveno, Benzerdjeb, Bonnefoy, Villeneuve, Rousset, Abboud, and Pocard report no conflict of interest or financial ties to disclose. Olivier Glehen is consultant for Gamida.
Data availability statement
Original article not based on a previous communication to a society or meeting. The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Mennemeyer R, Smith M. Multicystic, peritoneal mesothelioma: a report with electron microscopy of a case mimicking intra-abdominal cystic hygroma (lymphangioma). Cancer. 1979;44:692–698.
- Noiret B, Renaud F, Piessen G, et al. Multicystic peritoneal mesothelioma: a systematic review of the literature. Pleura Peritoneum. 2019;4:20190024.
- Zhang C-H, Yu J-W, Luo M. Multicystic peritoneal mesothelioma: a short review. Curr Probl Cancer. 2017;41:340–348.
- Kusamura S, Kepenekian V, Villeneuve L, et al., PSOGI. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2020;47:36–59.
- Nizri E, Baratti D, Guaglio M, et al. Multicystic mesothelioma: operative and long-term outcomes with cytoreductive surgery and hyperthermic intra peritoneal chemotherapy. Eur J Surg Oncol. 2018;44:1100–1104.
- Baratti D, Kusamura S, Nonaka D, et al. Multicystic and well-differentiated papillary peritoneal mesothelioma treated by surgical cytoreduction and hyperthermic intra-peritoneal chemotherapy (HIPEC). Ann Surg Oncol. 2007;14:2790–2797.
- Baratti D, Vaira M, Kusamura S, et al. Multicystic peritoneal mesothelioma: outcomes and patho-biological features in a multi-institutional series treated by cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Eur J Surg Oncol. 2010;36:1047–1053.
- Wong WL, Johns TA, Herlihy WG, et al. Best cases from the AFIP: multicystic mesothelioma. Radiographics. 2004;24:247–250.
- Jouvin I, Dohan A, Gergi P, et al. Intra-abdominal benign multicystic peritoneal mesothelioma. J Visc Surg. 2014;151:155–157.
- Ross MJ, Welch WR, Scully RE. Multilocular peritoneal inclusion cysts (so-called cystic mesotheliomas). Cancer. 1989;64:1336–1346.
- Weiss SW, Tavassoli FA. Multicystic mesothelioma. An analysis of pathologic findings and biologic behavior in 37 cases. Am J Surg Pathol. 1988;12:737–746.
- Kurisu Y, Tsuji M, Shibayama Y, et al. Multicystic mesothelioma caused by endometriosis: 2 case reports and review of the literature. Int J Gynecol Pathol. 2011;30:163–166.
- Safioleas MC, Constantinos K, Michael S, et al. Benign multicystic peritoneal mesothelioma: a case report and review of the literature. World J Gastroenterol. 2006;12:5739–5742.
- Sawh RN, Malpica A, Deavers MT, et al. Benign cystic mesothelioma of the peritoneum: a clinicopathologic study of 17 cases and immunohistochemical analysis of estrogen and progesterone receptor status. Hum Pathol. 2003;34:369–374.
- Kannerstein M, Churg J. Peritoneal mesothelioma. Hum Pathol. 1977;8:83–94.
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology. 1997;30:403–418.
- van Ruth S, Bronkhorst MW, Van Coevorden F, et al. Peritoneal benign cystic mesothelioma: a case report and review of the literature. Eur J Surg Oncol. 2002;28:192–195.
- Lee CE, Agrawal A. Remote recurrence of benign multicystic peritoneal mesothelioma. J Obstet Gynaecol Can. 2017;39:1042–1045.
- DeStephano DB, Wesley JR, Heidelberger KP, et al. Primitive cystic hepatic neoplasm of infancy with mesothelial differentiation: report of a case. Pediatr Pathol. 1985;4:291–302.
- González-Moreno S, Yan H, Alcorn KW, et al. Malignant transformation of “benign” cystic mesothelioma of the peritoneum. J Surg Oncol. 2002;79:243–251.
- Sethna K, Mohamed F, Marchettini P, et al. Peritoneal cystic mesothelioma: a case series. Tumori J. 2003;89:31–35.
- Mino JS, Monteiro R, Pigalarga R, et al. Diffuse malignant epithelioid mesothelioma in a background of benign multicystic peritoneal mesothelioma: a case report and review of the literature. BMJ Case Rep. 2014;2014:bcr2013200212.
- Chua TC, Yan TD, Deraco M, et al., Peritoneal Surface Oncology Group. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg. 2011;98:60–64.
- Gilani SNS, Mehta A, García-Fadrique A, et al. Outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma and predictors of survival. Int J Hyperthermia. 2018;58:1–7.
- Chicago Consensus Working Group. The Chicago consensus on peritoneal surface malignancies: management of peritoneal mesothelioma. Ann Surg Oncol. 2020;19:1–6.
- Villeneuve L, Passot G, Glehen O, et al., RENAPE Network. The RENAPE observational registry: rationale and framework of the rare peritoneal tumors French patient registry. Orphanet J Rare Dis. 2017;12:37–39.
- Villeneuve L, Isaac S, Glehen O, et al. The RENAPE network: towards a new healthcare organization for the treatment of rare tumors of the peritoneum. Description of the network and role of the pathologists. Ann Pathol. 2014;34:4–8.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
- Passot G, Bakrin N, Isaac S, et al. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur J Surg Oncol. 2014;40:957–962.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42.
- Mercier F, Mohamed F, Cazauran J-B, et al. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int J Hyperthermia. 2019;36:744–752.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. [accessed 2020 May 19]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Vermersch S, Arnaud A, Orbach D, et al. Multicystic and diffuse malignant peritoneal mesothelioma in children. Pediatr Blood Cancer. 2020;67:571.
- Cashin PH, Jansson Palmer G, Asplund D, et al. Peritoneal mesothelioma in Sweden: a population-based study. Cancer Med. 2019;8:6468–6475.
- Low RN, Barone RM, Rousset P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: history, clinical applications, and implementation. Eur J Surg Oncol. 2019;47:65–74.
- Ricci F, Borzellino G, Ghimenton C, et al. Benign cystic mesothelioma in a male patient: surgical treatment by the laparoscopic route. Surg Laparosc Endosc. 1995;5:157–160.
- Esquivel J, Averbach A. Combined laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a patient with peritoneal mesothelioma. J Laparoendosc Adv Surg Tech A. 2009;19:505–507.
- Mercier F, Jeremie G, Alyami M, et al. Long-term results of laparoscopic cytoreductive surgery and HIPEC for the curative treatment of low-grade pseudomyxoma peritonei and multicystic mesothelioma. Surg Endosc. 2020;34:4916–4918.
- Rodríguez-Ortiz L, Arjona-Sánchez Á, Ibañez-Rubio M, et al. Laparoscopic cytoreductive surgery and HIPEC: a comparative matched analysis. World J Surg. 2018;42:3120–3128.
- Passot G, Dumont F, Goéré D, et al., BIG-RENAPE Surgery Working Group. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg. 2018;105:663–667.
- Ortega-Deballon P, Glehen O, Levine E, et al. Childbearing after hyperthermic intraperitoneal chemotherapy: results from an international survey. Ann Surg Oncol. 2011;18:2297–2301.
- van Bijsterveldt C, Willemsen W, Bulten J. Peritoneal benign mesothelioma during and after two pregnancies. Eur J Obstet Gynecol Reprod Biol. 2006;127:265–266.
- Haase E, Yoo D, Sugarbaker PH. Management of appendiceal pseudomyxoma peritonei diagnosed during pregnancy. World J Surg Oncol. 2009;7:48.
- Sheehan LA, Mehta AM, Sawan S, et al. Preserving fertility in pseudomyxoma peritonei, a novel approach. Pleura Peritoneum. 2017;2:33–36.
- Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. 2011;98:287–292.
- Huang Y, Alzahrani NA, Liauw W, et al. Effects of sex hormones on survival of peritoneal mesothelioma. World J Surg Oncol. 2015;13:210.
- Kyser K, Bidus MA, Rodriguez M, et al. Spontaneous pregnancy following cytoreduction with peritonectomy and hyperthermic intraperitoneal chemotherapy. Gynecol Oncol. 2006;100:198–200.