Abstract
Objective
To assess the impact of ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for uterine fibroids on fertility.
Material and methods
A retrospective observational study was conducted of 560 reproductive-age women with symptomatic uterine fibroids who underwent USgHIFU therapy at Mútua Terrassa University Hospital, Spain, between February 2008 and February 2018. We analyzed pregnancy outcomes including time to conception, pregnancy approach, gestational age, delivery mode, neonatal outcomes and complications during pregnancy and delivery.
Results
After USgHIFU treatment, 71 pregnancies were obtained in 55 patients. Of these, 58 (82%) cases were natural pregnancies and 13 (18%) were in vitro fertilization (IVF) pregnancies. The median time to conception was 12 (range 1–72) months. There were 43 (61%) successful deliveries, including a twin gestation, 22 (31%) spontaneous abortions and 6 (8%) therapeutic abortions. The rate of full-term deliveries was 91% (39/43) and the remaining 9% (4/43) were preterm deliveries. Of the 44 live births, 25 (57%) were born vaginally and 19 (43%) by cesarean section. The complications reported included 3 women with retained placenta (7%), 2 with placenta previa (5%) and 1 with severe preeclampsia (2%). The mean birth weight was 3.1 (range: 1.4–4.3) kg, and except for a baby born with a tetralogy of Fallot, all newborns developed well without complications during postpartum and breastfeeding.
Conclusion
Patients undergoing USgHIFU treatment of uterine fibroids can achieve full-term pregnancies with few intrapartum or postpartum complications. More studies are required to compare fertility and perinatal outcomes between patients who underwent or not USgHIFU.
Introduction
Uterine fibroids or leiomyomas are the most common benign tumors of the reproductive system in women of child-bearing age. The prevalence ranges from 5.4% to 77% [Citation1].
The symptoms associated with uterine leiomyomas have a severe impact on women's quality of life ranging from heavy menstrual bleeding, spotting, dysmenorrhea and symptoms related to pressure on adjacent organs, such as a pelvic pain, urinary frequency or constipation [Citation2].
Moreover, uterine fibroids can negatively affect fertility. In modern societies, with the current tendency to delay childbearing and the progressive increase in risk of developing fibroids with age, the number of older patients with fibroids and problems conceiving is increasing [Citation2–4]. There is a consensus that submucosal fibroids of any size and intramural fibroids larger than 4 cm impair fertility and increase the rate of miscarriage up to 20–30% [Citation5–8]. Furthermore, fibroids are associated with obstetric complications such as a preterm delivery, malpresentation, placental abruption, placenta previa, cesarean section and postpartum hemorrhage [Citation9,Citation10].
Regarding the treatment of uterine fibroids, myomectomy is considered the gold standard for women with symptomatic fibroids who wish to conceive [Citation11]. There is evidence that myomectomy performed for submucosal and intramural cavity-distorting myomas significantly improves fertility [Citation12–14]. Nevertheless, it can be associated with surgical complications such as high blood loss and vesical or intestinal injuries, and can cause pelvic adhesions that may increase the risk of infertility [Citation15]. Additionally, surgery increases the risk of uterine rupture in the middle and late period of pregnancy (0.2–10%) [Citation16].
In recent decades, new minimally invasive treatments for uterine fibroids have appeared aimed to shrink the volume of the fibroids to relieve symptoms in women who wish to retain their uterus. These are mostly outpatient procedures, which are less aggressive and with fewer complications than classic surgical treatments. Among them, uterine artery embolization (UAE) has demonstrated efficacy in the treatment of symptomatic uterine fibroids, but it has been suggested that it may impair ovarian function and thus decrease the chances of achieving pregnancy [Citation17]. In addition, UAE can cause pregnancy complications such as increased rate of miscarriage, preterm delivery, intra-uterine growth restriction (IUGR), malpresentation, abnormal placentation, and post-partum hemorrhage [Citation18–23]. Therefore, current guidelines consider future reproductive plans as a contraindication for UAE [Citation24]. Transvaginal radiofrequency ablation (TRFA) is an effective and safe alternative in the treatment of uterine myomas, but it should be restricted to those patients with type II/III small submucosal and intramural cavity-distorting myomas [Citation25–28].
Ultrasound-guided high-intensity focused ultrasound (USgHIFU) is an emerging noninvasive treatment for solid tumors that has been successfully developed in the last few years. The safety and efficacy of high-intensity focused ultrasound (HIFU) ablation in the treatment of uterine fibroids has been demonstrated in multiple clinical studies [Citation29–34]. It provides targeted volumetric thermal ablation to myomas, leading to myoma volume reduction and symptomatic improvement. Additionally, previous studies have confirmed that HIFU neither damage the surrounding myometrium nor impair ovarian function [Citation35,Citation36]. Although there is insufficient clinical evidence on the real impact of HIFU on fertility and pregnancy, there are many reported cases of pregnancy after HIFU treatment with few obstetric complications [Citation37–39]. However, the only available data on pregnancy outcomes after USgHIFU are currently coming from Asian population. The findings of these studies show that USgHIFU treatment can reduce the pregnancy preparation time, facilitate fertility, and improve pregnancy outcomes [Citation38–39]. Our manuscript provides the opportunity to reveal the clinical and obstetric results of this technique applied to Caucasian patients.
The aim of the study was to evaluate the effect of HIFU ablation on subsequent pregnancies in women who conceived after USgHIFU for conservative treatment of uterine fibroids. We also analyzed the clinical outcome of USgHIFU, focusing on symptom improvement, shrinkage of the fibroids and complications.
Material and methods
Study population
We performed a single-center retrospective analysis of 560 women with symptomatic uterine fibroids who underwent USgHIFU ablation at the Mútua Terrassa University Hospital, Barcelona, Spain, from February 2008 to February 2018. The study was approved by the Institutional Board and Ethics Committee.
The inclusion criteria were: (1) patients older than 18 years; (2) diagnosis of uterine fibroids made by ultrasound or magnetic resonance imaging (MRI); (3) maximum diameter of fibroids smaller than 13 cm; and (4) clinical symptoms attributable to fibroids. The exclusion criteria were: (1) patients with uterine or cervical malignancy or pre-malignancy; and (2) patients with more than three fibroids. Written informed consent was obtained from each patient before every procedure.
Most patients (400 of 560, 71%) declared that they were not intending to conceive and the remainder (160 of 560, 29%) were women with current or future desire to conceive.
Before treatment all patients underwent a contrast-enhanced MRI to establish the number, size, location, tissue characterization and degree of vascularization of the uterine fibroids. They all completed the Uterine Fibroid Symptom and Quality of Life questionnaire (UFS-QOL) to evaluate the severity of symptoms and their impact on health-related quality of life (HRQOL) [Citation40,Citation41].
Patients with large myomas greater than 8 cm in diameter, hyperintense myomas (T2-weighted MRI) and/or highly vascularized myomas (T1-weighted contrast-enhanced MRI) were first treated with GnRH analogs (12 cases) or Ulipristal acetate (3 cases) for 3 months to potentiate the thermoablative effect of USgHIFU [Citation42–44].
HIFU therapy
High-intensity focused ultrasound treatment was performed using the JC Focused Ultrasound Tumor Therapeutic System (Chongqing Haifu Medical Technology Co. Ltd., China) (). The device comprises two parts: a therapeutic transducer with a focal length of 15 cm operating at a frequency of 0.8 MHz, and a convex ultrasound diagnostic transducer operating at 1–4 MHz (Esaote Group, Italy) to provide continuous real-time guidance for targeting. Using a computer-controlled system, the therapeutic transducer can be moved in three dimensions to locate the fibroids and fire energy at different points of the fibroids.
Figure 1. JC Focused Ultrasound Tumor Therapeutic System. (a) Computer-controlled system. (b) Movable HIFU table. (c) Therapeutic transducer with an ultrasound diagnostic transducer integrated. (d) Control monitor image during treatment.
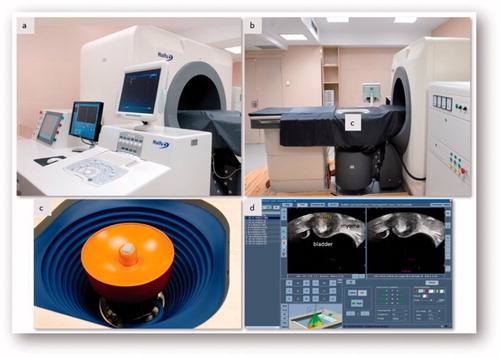
During the procedure, the patient lay prone on a movable HIFU table with the abdominal skin in contact with cold degassed water. HIFU treatment was performed under intravenous conscious sedation (remifentanil and propofol) to reduce discomfort and prevent movement. A urinary catheter was inserted to control the volume of the bladder during treatment. An ultrasound pre-scan was conducted from the sagittal view, obtaining different sections with 5-mm separation. We started treating the deepest area of the central section by applying 1–3 s energy exposures separated by 2–4 s. This starting focal point was placed at a distance of 1 cm of the deep border of the fibroid. The output power was 300–400 W, adjusted according to the patient’s discomfort and the gray-scale changes. When the echogenicity of the target spot changed, the transducer was moved to treat the deep areas of the remaining sections and then the shallow regions. The procedure required 1–3 h depending on the number, size and location of the fibroids. The decision to stop treatment was made according to the intra-operative gray-scale sonographic changes and contrast-enhanced ultrasound (SonoVue) [Citation45–47]. After treatment, women were observed for 3 h before being discharged.
Imaging evaluation
For better assessment of technical success, we performed a gadolinium-contrast MR study at the end of the procedure. We calculated the volume of fibroids treated and their non-perfused volume (NPV) according to the formula of the ellipsoid (V = 0.5233 × D1 × D2 × D3) where D1 is the long diameter, D2 is the left–right diameter and D3 is the anteroposterior diameter.
Post-treatment follow-up and data collection
At 3, 6 and 12 months and annually thereafter we carried out transvaginal sonography to calculate the reduction in volume of ablated fibroids and used the UFS-QOL questionnaire to compare the scores with those at baseline and those at the follow-up intervals. Women with submucosal or intramural fibroids distorting the uterine cavity who wanted to get pregnant immediately after HIFU treatment were advised to wait between 1 and 6 months (depending on the size of the myoma) until the uterine cavity had been completely restored (). When there was no anatomical distortion of the uterine cavity, a waiting time was only recommended in women with bulky fibroids over 7 cm.
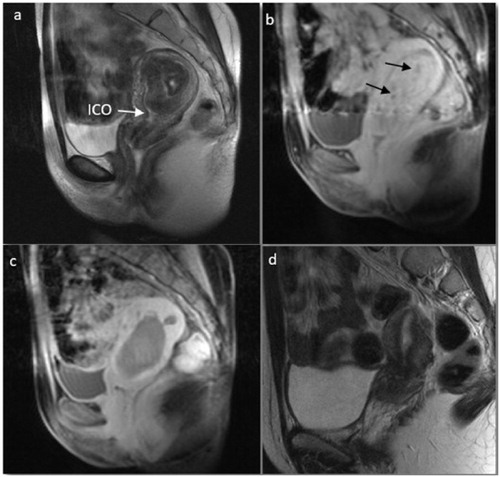
Figure 2. Magnetic resonance images before and after USgHIFU in a 33-year-old nulliparous woman with hypointense submucosal myoma and hypermenorrhea. (a) T2-weighted image before USgHIFU. Myoma volume of 51 cm³ and 6 cm maximum diameter occupying the whole uterine cavity. (b) T1-weighted contrast-enhanced image before USgHIFU. Myoma was highly vascularized with small areas of low vascularization (arrows). (c) T1-weighted contrast-enhanced image immediately after USgHIFU showed a non-perfused volume of 100%. (d) T2-weighted image 6 months after treatment. The myoma had completely disappeared with restoration of normal uterine anatomy. This patient achieved two full-term spontaneous pregnancies that ended in uncomplicated vaginal deliveries at 38.4 and 37 weeks. ICO, internal cervical os.
Afterwards we collected data about clinical and obstetric outcomes in the 55 patients who achieved pregnancy after USgHIFU treatment including age, pregnancy history, characteristics of the fibroids (number, location and size), treatment parameters (power, energy, sonication time, NPV) and complications. Regarding obstetric outcomes, we analyzed the time to conception, pregnancy approach, delivery mode, neonatal outcomes and complications during pregnancy and delivery. Likewise, symptom relief and shrinkage of the fibroids after USgHIFU treatment were also assessed.
Statistical analysis
Qualitative variables were expressed as absolute values and percentages. Quantitative variables were expressed with at least one measurement of central tendency and one of dispersion. The normality of the data was explored using the Kolmogorov-Smirnov and Shapiro-Wilk test. In bivariate analysis, quantitative variables were compared using the paired-samples Wilcoxon test. The level of statistical significance was set to 0.05. Statistical analyses were performed using the Statistical Package for Social Sciences (IBM SPSS®, version 25.0, Armonk, NY, USA).
Results
Characteristics of the cohort
In this study, 55 of the 560 women who underwent USgHIFU for uterine fibroids became pregnant. The mean age of the 560 women was 41 ± 5 (range: 25–54) years, decreasing to 37 ± 3 years among women who declared desire for fertility. The mean age of the 55 women who became pregnant was 35 ± 4 (range: 26–45) years at the time of treatment and 44% (24/55) were at least 36 years old. The mean age at delivery was 37 ± 4 (range: 29–45) years. Among these 55 women, 44 (80%) were nulliparous, 18 (33%) had primary or secondary infertility, 10 (18%) had at least 1 miscarriage, 1 (2%) had an ectopic tube pregnancy and there was a case of preterm birth (2%) before HIFU treatment (). Regarding the fibroids of these patients, the median volume of all fibroids contained in the uterus was 106 (interquartile range [IQR], 49 − 203) cm3. Sixty-five fibroids were treated with a median volume of 82 (IQR, 34 − 165) cm3 and a median maximum diameter of 6.4 (IQR, 4.5 − 8.6) cm (). The type of the fibroids is described in . Most women had more than 1 symptom, menorrhagia being the most common, affecting 58% of women. Based on the results of UFS-QOL, the baseline symptom severity score (SSS) was 59 (IQR, 34 − 72) points ().
Table 1. Characteristics of the 55 patients who became pregnant after USgHIFU treatment.
Table 2. Treatment parameters (n = 55).
Table 3. Changes of fibroid volume and symptom severity score (SSS) after HIFU.
Procedure and treatment outcomes
Among the 55 patients who became pregnant, 37 (67%) underwent HIFU treatment only once and 18 (33%) patients twice due to the large volume of the fibroid(s) or due to inadequate treatment based on the NPV in the first session. All patients had an outpatient procedure and were discharged 3–4 h later. The mean follow-up time was 48 (range: 3–120) months. The median sonication time was 28 (IQR, 18 − 46) min. The median NPV after treatment was 56 (IQR, 20 − 95) cm³ according to the contrast-enhanced MRI evaluation, which gave an NPV ratio of 76 (IQR, 40 − 91)% (). The median volume of treated fibroids decreased significantly after HIFU ablation from 102 (IRQ, 49 − 195) cm³ at baseline to 53 (IQR, 18 − 103) cm³, 33 (IQR, 18 − 82) cm³, 28 (IQR, 10 − 72) cm³ and 17 (IQR, 9 − 40) cm³ at 6 months and 1, 2 and 4 years, respectively (p < .001) (). The median SSS decreased significantly from 59 (IQR, 34 − 72) points at baseline to 22 (IQR, 13 − 44), 22 (IQR, 8 − 39), 20 (IQR, 6 − 38) and 18 (IQR, 3 − 33) at 6 months and 1, 2 and 4 years after treatment, respectively (p < .001) (). The volume of fibroids and the severity of symptoms continued decreasing slightly, but significantly, up to the 8-year follow-up (p < .0018) ().
Five (9%) minor complications were observed in 5 patients. In one of them, the 10 cm fibroid treated was almost completely expelled from the vagina 6 months after HIFU ablation. This patient underwent a hysteroscopic resection to remove the remaining 2 cm myoma fragment, resulting in a normal uterine cavity (). Another patient had vaginal discharge of the treated fibroid 2 months after HIFU. One patient had a 1st degree skin burn and two patients had self-limited hematuria. No major complications such as bowel or bladder injury or nerve damage occurred, and no patients developed amenorrhea after HIFU ablation. During the average of 48 months follow-up, 12 (22%) patients required a surgical procedure after pregnancy due to fibroid enlargement with recurrence of symptoms.
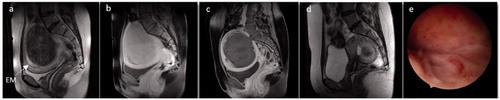
Figure 3. Images from a 30-year-old woman with vaginal delivery after USgHIFU ablation for a myoma. She had menorrhagia, severe anemia (hemoglobin of 9 g/L) and secondary sterility. (a) T2-weighted image before USgHIFU showing an enlarged uterus with a transmural hypointense myoma of 10 cm (463 cm³). (b, c) T1-weighted contrast-enhanced images. (b) Before USgHIFU the myoma had homogenous vascularization lower than that of the surrounding myometrium. (c) After USgHIFU treatment the entire myoma volume was not perfused. (d) T2-weighted image 6 months later showed a reduction in the size of the myoma to 3.9 cm³. The patient underwent a hysteroscopic resection of the remaining myoma fragment. (e) The hysteroscopic image after HIFU and surgery showed a normal uterine cavity. The woman became pregnant spontaneously 3 months later and gave birth to a healthy baby weighing 3.5 kg. EM, endometrium.
Pregnancy outcomes
In the present study among 160 women who had current or future desire to conceive, 55 patients became pregnant a total of 71 times (pregnancy rate: 34%). The median time to conception was 12 (range: 1–72) months. The natural conception rate was 82% (58/71) and the remaining 18% (13/71) were in vitro fertilization (IVF) pregnancies, among whom 4 patients had previously failed attempts at IVF.
Among the 55 patients with 71 pregnancies, there were 43 (61%) successful deliveries, including a natural twin gestation (monochorionic-diamniotic) (44 newborns), 6 (8%) therapeutic abortions for personal reasons and 22 (31%) first-trimester spontaneous abortions.
Of the 19 women who had 22 spontaneous abortion after HIFU ablation, 1 had a monosomy 9 in the cytogenetic analysis of chorionic villi, 1 patient with hematologic alterations had 4 miscarriages but subsequently achieved a full-term pregnancy, 1 patient was found to have an elevated sperm DNA fragmentation of her male partner, 1 patient was diagnosed with fetal hydrops in the first trimester (increased nuchal translucency and massive ascitis) before aborting spontaneously but achieved an uncomplicated full-term pregnancy on her second attempt, 5 patients with a history of primary (4) and secondary (1) infertility had a miscarriage after HIFU ablation but achieved full-term pregnancy at their second attempt (2 IVF, 3 natural), 2 patients aged 40 and 42 years, the first with 3 previous spontaneous abortions, had a miscarriage despite performing a complete ablation of the fibroids with large volume reduction, 3 nulliparous women with complete myoma ablation (intramural and subserous myoma of 8 and 9 cm and submucosal myoma of 3 cm respectively) did not follow the advice given and became pregnant immediately after treatment without allowing time for the fibroids to shrink, and finally 5 patients over 36 years had a spontaneous abortion after a poor response to HIFU treatment with a limited fibroid volume reduction.
Among the 43 pregnancies that ended in successful deliveries, prenatal ultrasound detected 3 (7%) cases of IUGR, 2 (5%) small for gestational age (SGA), 1 (2%) case of tetralogy of Fallot, 1 (2%) of aberrant right subclavian artery (ARSA), 1 (2%) of polyhydramnios and 5 (11%) cases of abnormal fetal position (3 breech and 2 transverse). The average gestational time was 39 ± 2 (range 32–41) weeks. The rate of full-term deliveries was 91% (39/43) and the remaining 9% (4/43) were preterm deliveries at 32.5, 34, 34 and 35 weeks due to preterm premature rupture of membranes (PPROM) of a twin gestation, hemorrhage associated with placenta previa, PPROM and polyhydramnios, respectively (). In these 4 cases of prematurity the women had a small myoma (37, 21, 56 and 82 cc respectively) of intramural and subserous mixed location in 2 cases and intramural and submucous mixed location in the other 2. The NPV after treatment was 89%, 76%, 100% and 91% respectively and there was a progressive reduction of myoma volume in all cases, with complete disappearance in 1 case.
Table 4. Obstetric characteristics of 43 successful deliveries achieved after USgHIFU treatment.
Table 5. Complications during pregnancy and delivery (n = 43).
There were 25 (57%) cases of vaginal delivery and 19 (43%) cases of cesarean section, including a combined vaginal-cesarean delivery of the twin pregnancy. The indications for cesarean sections performed are shown in . The average birth weight of the 44 newborns was 3.1 ± 0.6 (range: 1.4–4.3) Kg, 7 of them were low-birth-weight infants (<2.5 kg) ().
Table 6. Indications for cesarean sections performed (n = 19).
The complications detected during pregnancy (7/43, 16%) were 1 case of first-trimester bleeding, 1 preterm labor, 1 renal colic caused by growth of the myoma up to 15 cm, 2 cases of placenta previa and 2 cases of PPROM ().
The intrapartum and postpartum complications reported (6/43, 14%) included 3 retained placenta with manual removal, 1 of which received a red blood cell transfusion, 1 case of severe preeclampsia that ended in forceps-assisted delivery at 37.4 weeks and 2 cases of emergency cesarean section for fetal bradycardia and for hemorrhage associated with placenta previa at 40.4 and 34 weeks respectively (). In this last case a baby of 1.4 kg was born with a previously diagnosed tetralogy of Fallot and IUGR and was operated 6 months later. The remaining newborns (98%, 43/44) developed well without complications during postpartum and breastfeeding.
Discussion
Our study demonstrates that patients undergoing USgHIFU treatment of uterine fibroids can achieve full-term pregnancies with few intrapartum or postpartum complications. During the follow-up we identified 71 pregnancies in 55 women which is, to our knowledge, the longest series of pregnancies after HIFU treatment in a Western country.
In 2008, Rabinovici et al. reported the longest ever series of pregnancies after MRgHIFU of uterine fibroids at that time [Citation37]. They obtained 54 pregnancies in 51 women from 13 hospitals, with a 41% live birth rate. In another retrospective observational study from 2017, Zou et al. included 406 women with uterine fibroids who wished to become pregnant. After USgHIFU, 80 pregnancies in 78 patients occurred with a total pregnancy rate of 19.2% [Citation38]. In the same year, Li et al. enrolled 189 nulliparous women in a retrospective study reporting 133 pregnancies and 94 newborns after USgHIFU with a 69% pregnancy rate [Citation39].
However, when interpreting pregnancy results, it is important to consider the age factor, as it is well known that the fertility of women begins to gradually, but significantly, decline at around age 32 [Citation48]. It is noteworthy that although our pregnancy rate was much lower than that reported by Li et al. (34% and 69% respectively), the average age of patients with gestational desire in our series was markedly higher than that in the Li series (37 ± 3 and 31 ± 4 years respectively). Comparing with standard surgical procedures, our pregnancy rate is equal to or better than that after laparotomic (10–46%) or laparoscopic myomectomy (33–58%) [Citation49–52] and higher or lower than that after hysteroscopic myomectomy depending on the study (8–76%) [Citation53,Citation54].
In the same way, there is evidence that advanced maternal age at conception is a strong risk factor for spontaneous abortion [Citation55]. That could justify the 31% spontaneous abortion rate of our series since the average age of the patients who had a miscarriage was 37.6 years and 53% (10/19) were at least 38 years old. Furthermore, other causes and risk factors for spontaneous abortion were detected in these patients, such as numerical chromosomal abnormalities in embryo, sperm DNA damage, hematological alterations, and fetal hydrops. Nonetheless, in our study the spontaneous abortion rate after HIFU was similar to or lower than that in pregnancies with untreated fibroids present (20–46.7%) [Citation56] and markedly lower than those reported for UAE (56%) [Citation23].
According to the current literature, uterine fibroids exert a negative impact on fertility and increase the risk of miscarriage [Citation5–8]. Consistent with this, in our series, 33% (18/55) of women who became pregnant after HIFU treatment had a previous history of primary or secondary infertility and 18% (10/55) had at least one miscarriage. It is reasonable to think that HIFU could help to improve fertility in this type of patient.
On the other hand, some reviews have suggested that noncavity-distorting intramural fibroids, even those of small size [Citation57], would significantly reduce the implantation rate, clinical pregnancy rate and live birth rate and significantly increase the miscarriage rate after IVF treatment [Citation58,Citation59]. There is agreement that surgery would be a totally disproportionate and inadvisable treatment for patients with this type of fibroid [Citation14]. Based on previous studies demonstrating the safety of USgHIFU for the surrounding myometrial tissue, ovarian and endometrial function [Citation35,Citation36], we strongly believe that HIFU therapy could be a good treatment option for these patients.
It should be noted that our series had a lower preterm delivery rate (4/43, 9%) compared with series of pregnancies with untreated fibroids (20–33.3%) or pregnancies following UAE (20%) [Citation19]. In these 4 cases of prematurity the women had a small fibroid that was completely ablated with USgHIFU and had progressively shrunk before pregnancy. Given these good clinical results, we really believe that myomas could have had a minimal or no negative impact on these pregnancies, in which there were other risk factors for preterm delivery such as polyhydramnios, twin gestation, hemorrhage associated with placenta previa and PPROM.
Additionally, the incidence of cesarean section in our series was 43%, which was much lower than that in patients undergoing myomectomy (59%) or UAE (63–80%) [Citation20] or in those reported by other authors after USgHIFU (50–81%) [Citation38,Citation39]. The mean birth weight of the newborns in our study (3.1 kg) slightly exceeded that reported in a UAE series (2.9 kg) [Citation21,Citation22].
The low incidence of placenta previa (4.6%) and the nonoccurrence of cases of placenta accreta or uterine rupture during pregnancy or delivery were notable in our series.
Regarding the clinical outcomes, HIFU ablation was followed by substantial shrinkage of the ablated myomas with significant symptom improvement. We must highlight that these results were achieved in women with a median maximum diameter of treated fibroids of 6.4 cm, which is higher than that reported in most series of HIFU treatment [Citation37–39]. Minor complications (9%) and major complications (0%) rate was much lower compared with series of patients undergoing UAE (52%, 3%), hysterectomy (28%, 6%) or myomectomy (14%, 5%) [Citation24]. Additionally, in our series all patients underwent ambulatory surgery while the mean length of hospital stay reported for UAE, hysterectomy and myomectomy is 2, 5 and 6 days respectively. In short, HIFU allowed us to treat uterine fibroids with good clinical results and without the surgical morbidity of myomectomy and minimally invasive procedures.
Limitations of this study include its retrospective nature causing some probable bias, a relatively small sample size and the lack of comparison of HIFU results with any other treatment, which calls for further studies. Another limitation could be that we used an MR study for evaluation for the first time after treatment and yet a transvaginal sonography for post-treatment follow-up. It was done in such a way because we are a public hospital, MRI is an expensive test, and the study did not have any financial support.
The results of our study indicate that USgHIFU may be an approach to treat fibroids in women with gestational desire, including those who have a history of infertility, as they can achieve full-term pregnancies with few perinatal complications and no additional obstetric risks. We also demonstrated with a long-term follow-up that USgHIFU is safe and effective in treating symptomatic uterine fibroids and has unique advantages because of its noninvasive nature.
Acknowledgments
We would like to thank Pr. Philippe Descamps and Pr. Hervé Fernandez for their useful advice in the preparation of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lethaby A, Vollenhoven B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin Evid. 2015;2015:0814.
- Sparic R, Mirkovic L, Malvasi A, et al. Epidemiology of uterine Myomas: a review. Int J Fertil Steril. 2016;9(4):424–435.
- Mills M, Rindfuss RR, McDonald P, et al. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860.
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Fetal Diagn Ther. 2011;29(4):263–273.
- Somigliana E, De Benedictis S, Vercellini P, et al. Fibroids not encroaching the endometrial cavity and IVF success rate: a prospective study. Hum Reprod. 2011;26(4):834–839.
- Galliano D, Bellver J, Díaz-García C, et al. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update. 2015;21(1):13–38.
- Zepiridis LI, Grimbizis GF, Tarlatzis BC. Infertility and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:66–73.
- McWilliams MM, Chennathukuzhi VM. Recent advances in uterine fibroid etiology. Semin Reprod Med. 2017;35(2):181–189.
- Cook H, Ezzati M, Segars JH, et al. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62(3):225–236.
- Klatsky PC, Tran ND, Caughey AB, et al. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198(4):357–366.
- Van der Kooij SM, Ankum WM, Hehenkamp WJ. Review of nonsurgical/minimally invasive treatments for uterine fibroids. Curr Opin Obstet Gynecol. 2012;24(6):368–375.
- Casini ML, Rossi F, Agostini R, et al. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006;22(2):106–109.
- Olive DL. The surgical treatment of fibroids for infertility. Semin Reprod Med. 2011;29(2):113–123.
- Practice Committee of the American Society for Reproductive Medicine. Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertil Steril. 2017;108(3):416–425.
- Kumakiri J, Kikuchi I, Kitade M, et al. Association between uterine repair at laparoscopic myomectomy and postoperative adhesions. Acta Obstet Gynecol Scand. 2012;91(3):331–337.
- Gambacorti-Passerini Z, Gimovsky AC, Locatelli A, et al. Trial of labor after myomectomy and uterine rupture: a systematic review. Acta Obstet Gynecol Scand. 2016;95(7):724–734.
- Jacob GP, Oraif A, Power S. When helping hurts: the effect of surgical interventions on ovarian reserve. Hum Fertil. 2016;19(1):3–8.
- Czuczwar P, Stępniak A, Wrona W, et al. The influence of uterine artery embolisation on ovarian reserve, fertility, and pregnancy outcomes – a review of literature. Prz Menopauzalny. 2016;15(4):205–209.
- Holub Z, Mara M, Kuzel D, et al. Pregnancy outcomes after uterine artery occlusion: prospective multicentric study. Fertil Steril. 2008;90(5):1886–1891.
- Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191(1):18–21.
- Ravina JH, Vigneron NC, Aymard A, et al. Pregnancy after embolization of uterine myoma: report of 12 cases. Fertil Steril. 2000;73(6):1241–1243.
- Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105(1):67–76.
- Homer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril. 2010;94(1):324–330.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
- Cho HH, Kim JH, Kim MR. Transvaginal radiofrequency thermal ablation: a day-care approach to symptomatic uterine myomas. Aust NZ J Obstet Gynaecol. 2008;48(3):296–301.
- Jiang X, Thapa A, Lu J, et al. Ultrasound-guided transvaginal radiofrequency myolysis for symptomatic uterine myomas. Eur J Obstet Gynecol Reprod Biol. 2014;177:38–43.
- Brölmann H, Bongers M, Garza-Leal JG, et al. The FAST-EU trial: 12-month clinical outcomes of women after intrauterine sonography-guided transcervical radiofrequency ablation of uterine fibroids. Gynecol Surg. 2016;13(1):27–35.
- Rey VE, Labrador R, Falcon M, et al. Transvaginal radiofrequency ablation of myomas: technique, outcomes, and complications. J Laparoendosc Adv Surg Tech A. 2019;29(1):24–28.
- Wang F, Tang L, Wang L, et al. Ultrasound-guided high-intensity focused ultrasound vs laparoscopic myomectomy for symptomatic uterine myomas. J Minim Invasive Gynecol. 2014;21(2):279–284.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Liu Y, Ran W, Shen Y, et al. High-intensity focused ultrasound and laparoscopic myomectomy in the treatment of uterine fibroids: a comparative study. BJOG: Int J Obstet Gy. 2017;124(Suppl. 3):36–39.
- Cheung VYT. High-intensity focused ultrasound therapy. Best Pract Res Clin Obstet Gynaecol. 2018;46:74–83.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251.
- Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol. 2014;26(3):151–161.
- Cheung VY, Lam TP, Jenkins CR, et al. Ovarian reserve after ultrasound-guided high-intensity focused ultrasound for uterine fibroids: preliminary experience. J Obstet Gynaecol Can. 2016;38(4):357–361.
- Rabinovici J, David M, Fukunishi H, et al. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93(1):199–209.
- Zou M, Chen L, Wu C, et al. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG: Int J Obstet Gy. 2017;124(Suppl. 3):30–35.
- Li JS, Wang Y, Chen JY, et al. Pregnancy outcomes in nulliparous women after ultrasound ablation of uterine fibroids: a single-central retrospective study. Sci Rep. 2017;7(1):3977.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Harding G, Coyne KS, Thompson CL, et al. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes. 2008;6(1):99.
- Smart OC, Hindley JT, Regan L, et al. Magnetic resonance guided focused ultrasound surgery of uterine fibroids–the tissue effects of GnRH agonist pre-treatment. Eur J Radiol. 2006;59(2):163–167.
- Zhao WP, Chen JY, Chen WZ. Dynamic contrast-enhanced MRI serves as a predictor of HIFU treatment outcome for uterine fibroids with hyperintensity in T2-weighted images. Exp Ther Med. 2016;11(1):328–334.
- Yang S, Kong F, Hou R, et al. Ultrasound guided high-intensity focused ultrasound combined with gonadotropin releasing hormone analogue (GnRHa) ablating uterine leiomyoma with homogeneous hyperintensity on T2 weighted MR imaging. Br J Radiol. 2017;90(1073):20160760.
- Peng S, Xiong Y, Li K, et al. Clinical utility of a microbubble-enhancing contrast (“SonoVue”) in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur J Radiol. 2012;81(12):3832–3838.
- Wang YJ, Zhang PH, Zhang R, et al. Predictive value of quantitative uterine fibroid perfusion parameters from contrast-enhanced ultrasound for the therapeutic effect of High-Intensity Focused Ultrasound ablation. J Ultrasound Med. 2019;38(6):1511–1517.
- Isern J, Pessarrodona A, Rodriguez J, et al. Using microbubble sonographic contrast agent to enhance the effect of high intensity focused ultrasound for the treatment of uterine fibroids. Ultrason Sonochem. 2015;27:688–693.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–634.
- Sudik R, Hüsch K, Steller J, et al. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Dubuisson JB, Fauconnier A, Deffarges JV, et al. Pregnancy outcome and deliveries following laparoscopic myomectomy. Hum Reprod. 2000;15(4):869–873.
- Soriano D, Dessolle L, Poncelet C, et al. Pregnancy outcome after laparoscopic and laparoconverted myomectomy. Eur J Obstet Gynecol Reprod Biol. 2003;108(2):194–198.
- Kameda S, Toyoshima M, Tanaka K, et al. Utility of laparoscopic uterine myomectomy as a treatment for infertility with no obvious cause except for uterine fibroids. Gynecol Minim Invasive Ther. 2018;7(4):152–155.
- Bernard G, Darai E, Poncelet C, et al. Fertility after hysteroscopic myomectomy: effect of intramural myomas associated. Eur J Obstet Gynecol Reprod Biol. 2000;88(1):85–90.
- Donnez J, Donnez O, Dolmans MM. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice? Fertil Steril. 2014;102(3):640–648.
- Nybo Andersen AM, Wohlfahrt J, Christens P, et al. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320(7251):1708–1712.
- Radhika BH, Naik K, Shreelatha S, et al. Case series: pregnancy outcome in patients with uterine fibroids. J Clin Diagn Res. 2015;9(10):QR01–QR4.
- Behbehani S, Polesello S, Hasson J, et al. The effect of intramural myomas without an intracavity component on in vitro fertilization outcomes in single fresh blastocyst transfer cycles. J Minim Invasive Gynecol. 2018;25(7):1241–1248.
- Wang X, Chen L, Wang H, et al. The impact of noncavity-distorting intramural fibroids on the efficacy of in vitro fertilization-embryo transfer: an updated meta-analysis. Biomed Res Int. 2018;2018:8924703.
- Lisiecki M, Paszkowski M, Woźniak S. Fertility impairment associated with uterine fibroids – a review of literature. Prz Menopauzalny. 2017;16(4):137–140.
