Abstract
Introduction
Pancreatic cancer is with the poorest prognosis of all common cancers worldwide. Despite the advances in treatment the results are poor throughout the different methods. Pancreatic resection still yields the best outcome. However only a quarter of the patients present at operable stage. HIFU is a noninvasive technique that can be used to treat pancreatic cancer.
Aim
The aim of this review is to perform a systematic review on the data about the resection rate after HIFU ablation in patients with borderline resectable pancreatic cancer (BRPC) and the impact of this technique over the oncological results.
Materials and methods
The PubMed and Wanfang databases were searched using keywords: pancreatic cancer, HIFU ablation and high-intensity focused ultrasound. All found articles were reviewed. The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard guidelines. This study was financially supported by 2019 ‘Kuan-Ren Elite’ Program of 2nd Affiliated Hospital of Chongqing Medical University, China (Grant no. KY2019G019).
Results
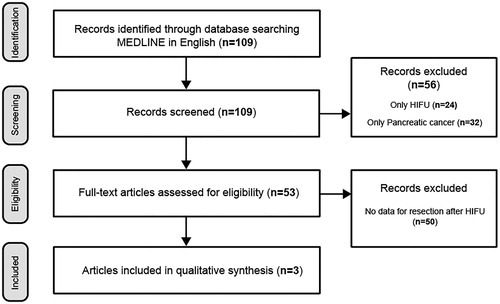
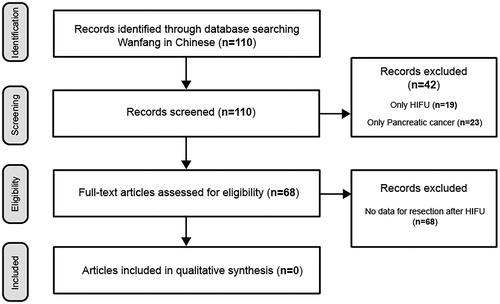
The English database search showed 109 papers, of which 3 met the inclusion criteria. The Wanfang database resulted in 110 papers; however, none met the inclusion criteria of the review. From the included studies 97 patients underwent neoadjuvant HIFU ablation ± chemotherapy. Thirty-four patients reached resection (35.1%). In two patients, residual tumor (R) classification was not reported. R0 resection rate in all reported patients is 30.5% (29/95). R1 resection rate is 3.2% (3/95).
Conclusion
HIFU is found to be safe and feasible in locally advanced and metastatic pancreatic cancer with proven downstaging and downsizing effects. Further research on role of HIFU ablation as a neoadjuvant treatment for borderline resectable pancreatic cancer is needed.
Introduction
Pancreatic cancer is with the poorest prognosis of all common cancers with a 5-year survival rate of less than 8% (range 5–15%). Regardless of the advances in treatment, the 5-year survival has not improved significantly in the last 10 years, except for 3–5% increases in Canada, the USA, Korea, Singapore, and 12 European countries [Citation1]. At diagnosis, around 80% of the patients are at an inoperable stage, either locally advanced or distant metastases presented (III and IV stage). Present-day therapy guidelines for advanced disease include chemotherapy or radiochemotherapy, but the 5-year survival rate is less than 5% in these patients. In the last years, thermal and non-thermal ablation techniques have been presented, including radiofrequency ablation (RFA), microwave ablation, HIFU therapy, cryoablation, irreversible electroporation, and image-guided percutaneous ablation [Citation2]. The resectability status of pancreatic cancer has been classified as resectable, borderline resectable, and locally advanced. Currently, there are no unified criteria for borderline resectable tumors, thus, preventing quality data analysis between different workgroups. The most popular classification systems are the American Hepato-Pancreato-Biliary Association (AHPBA) [Citation3], the National Comprehensive Cancer Network (NCCN) guidelines [Citation4], and MD Anderson [Citation5] classification. Appropriate imaging studies (pancreatic phase contrast-enhanced CT and MRI, transcutaneous and endoscopic ultrasound) and histological assessment are essential for proper therapy regimen decisions. The Standard of care for borderline resectable pancreatic cancer is neoadjuvant therapy with preferred regimens FOLFORINOX ± subsequent chemoradiation or gemcitabine + albumin-bound paclitaxel ± subsequent chemoradiation. For BRCA 1/2 or PALB2 mutations, gemcitabine + cisplatin ± subsequent chemoradiation could be introduced.
HIFU is a novel, safe, noninvasive imaging-guided thermal ablation technique that uses focused ultrasound that interferes in the target area resulting in a wave with energy higher than the sum energy of the two waves. This causes cavitation and an immediate increase in the temperature in the very small focal point which thereby spares the surrounding tissue. Safety of conducting ultrasound energy in pancreas tissues [Citation6–8] and good penetration and safety of ultrasonic on surrounding vessels [Citation9,Citation10] have been well established.
Aim
The aim of this review is to perform a systematic review on the data about the resection rate after HIFU ablation in patients with borderline resectable pancreatic cancer (BRPC) and the impact of this technique over the oncological results.
Materials and methods
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard guidelines [Citation11].
Protocol
Search in PubMed on 25 January 2021 for articles in English published in the period between 2010 and 2020 by using keywords: pancreatic cancer, HIFU ablation and high intensity focused ultrasound was done. We also performed literature review on 26 January 2021 in the Wanfang database for articles in Chinese for the period 2015 and 2020 with the same keywords.
Eligibility criteria
The inclusion criteria for the study were papers reporting data about patients with histologically proven pancreatic cancer, categorized as borderline resectable, who have undergone HIFU ablation with or without chemotherapy or radiotherapy. Studies reporting data about less than five patients, data for patients who do not meet the criteria for borderline resectable pancreatic cancer or patients with no histologically proved pancreatic cancer were not included in the review. Studies of patients with distant metastases, local recurrence or synchronous cancer were also excluded from the analysis. Data published in English were investigated by three independent researchers for meeting the inclusion criterias. A single researcher investigated articles in Chinese for meeting the inclusion criterias.
Data extraction
For the selected studies, we extracted the following data from the articles: study characteristics; number of patients; median age; HIFU treatment alone or HIFU + chemotherapy, time from HIFU to surgery, number of resections, residual tumor (R) classification and overall survival (OS).
Evaluation of study quality and risk of bias assessment
The quality of the articles was assessed according to the 2011 Oxford Center for Evidence-Based Medicine (OCEBM) levels of evidence [Citation12]. The bias was assessed by ROBINS-I tool for non-randomized studies of interventions [Citation13].
Endpoints
The primary endpoint of the review is to identify the rate of patients who reached radical surgery after HIFU ablation. The secondary endpoints were the overall survival and rate of R0 resection completion in resected patients.
This study was financially supported by 2019 ‘Kuan-Ren Elite’ Program of 2nd Affiliated Hospital of Chongqing Medical University, China (Grant no. KY2019G019).
Results
Literature search
By following the described criterias, 109 papers in English were identified, and among them 3 were included in the review for further analysis (). The summarized results are shown on . One hundred and ten papers in Chinese were identified in the Wanfang database, using the keywords, but none have met the inclusion criteria ().
Table 1. Studies met the inclusion criterias of the review.
In a prospective cohort study, Zhao et al. analyzed 37 patients treated with Gemcitabine 1000 mg/m2 on 1st, 8th, and 15th day with concurrent HIFU ablation on 1st, 3th, and 15th day every 28 days. Five patients among all reached surgery after four cycles of the treatment. The authors report four R0 resection and one R1 resection. The overall survival (OS) is 12.6 months for all patients. The OS for the patients treated with HIFU and surgery is not reported separately[Citation14].
In another prospective study, Sofuni et al. report 30 patients that were treated with HIFU plus another neoadjuvant treatment modality such as chemotherapy and/or radiotherapy. Two patients went on for surgery after completion of HIFU ablation; however, it was not reported what type of surgery was performed [Citation7].
Wang et al. in their retrospective study of 30 patients who underwent only HIFU as neoadjuvant treatment reported 27 cases that reached resection and in 25 a R0 resection was achieved [Citation15].
Study quality and risk of bias
Among the included in the review studies, two were prospective cohort studies and one was retrospective studies. Two studies report results from HIFU ablation in combination with chemotherapy and one study report results after HIFU treatment as a single neoadjuvant treatment modality. There were no randomized controlled studies. All studies had no control group. The OCEBM level of evidence was level 3 for all studies. Risk of bias was assessed to be at moderate risk of bias for all articles.
Patient characteristics
A total of 97 patients underwent neoadjuvant HIFU ablation ± chemotherapy. Thirty-four patients reached resection (35.1%). In 2 patients residual tumor (R) classification was not reported. R0 resection rate in all reported patients is 30.5% (29/95). R1 resection rate is 3.2% (3/95).
Discussion
Most studies have yielded similar results confirming the efficacy of HIFU in terms of improved patient pain control, tumor volume reduction, and possible benefits in patient survival. Nevertheless, not all advanced cases of pancreatic cancer are suitable for HIFU. Inclusion criteria reported by most authors are similar [Citation16–23].
Tumors that are not ultrasound visible, distance from the skin to the tumor is more than 12 cm, severe adhesions in the upper abdomen, and scars that obstruct the acoustic pathway are contraindications according to all authors [Citation24].
Despite neoadjuvant therapy (chemotherapy and/or chemoradiotherapy) has been improving outcomes in many other tumor types, the results in pancreatic cancer treatment remain unsatisfactory.
Therefore, NCCN guidelines suggest including patients with locally advanced pancreatic tumors in clinical trials [Citation3]. However, the PubMed data search showed no randomized trials on HIFU but separate small studies.
The resistance of pancreatic tumors to chemotherapy and radiotherapy could be explained by obstructing the vascularization and hypoxia inside the tumor caused by stromal cells. Consequently, hyperthermia, caused by HIFU therapy, results in improved blood flow and a decrease in hypoxia and could act as a sensitizer to chemotherapy and/or radiotherapy [Citation25–29]. Van der Horst et al. in a recent systematic review suggest that hyperthermia in combination with chemotherapy and/or radiotherapy may improve the median OS and response rate of patients with locally advanced or metastatic pancreatic cancer [Citation30].
HIFU is a relatively new technique that is still not fully researched. It can be applied in many different situations and for many different purposes. The up to date data show that HIFU is safe and shows good treatment results in advanced pancreatic cancer. The data reported have very little information regarding the effect of HIFU in BRPC.
The resection rate after HIFU for borderline resectable pancreatic cancer reached 35.1% in this small cohort of 97 patients. Data are sufficient to draw conclusions. Nevertheless, taking into consideration, the proven safety of HIFU around big vessels and reduction of tumor mass, the resection rate results look logical and promising. More research in the area is essential.
Despite the difference in classifications of BRPC, most have something in common – abutment or encasement of adjacent vessels. Therefore, the methods of treatment need to be safe for use and result in retraction of the tumor mass from the vessels. HIFU procedures in unresectable pancreatic cancer patients have proven safe about the integrity of the vessels and vascular adverse events. Guo et al. report 13 patients treated with HIFU with vessels involved by the tumor mass prior to therapy. They recorded the number of involved vessels, observed and followed up vascular adverse events after treatment. No adverse events were reported 1 week after HIFU and in the follow up. No stenosis or occlusion were reported. Hemodynamic parameters of the involved vessels showed no significant change after HIFU treatment. The data from the study indicate that HIFU is safe for use near involved vessels by pancreatic cancer [Citation10]. Furthermore, in one of our studies, we have proven that HIFU is safe for the patency of vessels involved by advanced pancreatic cancer. [Citation31]. Strunk et al. report that in his cohort of 50 consecutive patients HIFU treatment can be safely applied despite major vessel involvement and most patients (94%) did not experience any adverse events [Citation32].
In the literature search, we found very few data whether neoadjuvant treatment including HIFU therapy on BRPC can result in transition to resectable stage. In addition, we decided to compare the results of these studies to established methods of neoadjuvant treatment to compare the results and evaluate the applicability of the method. Zhao et al. in 2010 reported a series of 39 patients with BRPC and locally advanced pancreatic cancer treated with HIFU. Of them they report 2 cases of complete response, 15 cases of partial response and 5 cases that reached resection. From them 4 were with R0 resection and 1 with R1. The study reported a median OS of 12.6 months for the whole series. And a 1-year OS of 50.6%. However, they do not report the OS for patients after surgery. The 3rd and the 4th degree toxicity after therapy were reported at 35%. The most experienced hematological toxicity among the patients was neutropenia − 10.8% grade 3 and 5.4% grade 4 [Citation14]. Sofuni et al .in their study of 30 patients in III and IV stage for the safety of HIFU report of 2 cases that have reached operation after the treatment but do not specify what. There is also no detailed information about the type and combinations of pre-HIFU treatment regiments [Citation7]. Wang et al. in 2015 published their research on the topic with 30 cases. All the patients were with BRPC and were treated with HIFU. Of them, 1 refused further treatment after ablation because of pain relief, 1 went on chemotherapy because of left kidney infiltration, 1 underwent palliative bypass surgery, and 27 were resected. In 25 of them, a R0 resection was achieved which corresponds to 92.6% R0 resection rate. The study reports 92.7% 1-year-survival rate but does not report median OS [Citation15]. The Chinese source search found no articles regarding patients with BRPC that reached resection after HIFU treatment. Because of the few data available to assess the effects of HIFU on BRPC, we compared it with standard methods of neoadjuvant treatment. Chuong et al. in their study report 57 patients with BRPC treated with stereotactic body radiotherapy (SBRT). SBRT was delivered in 5 consecutive days with 25–30 Gy delivered to the tumor and 35–50 Gy to the region of vessel abutment/encasement. Of the 57 patients 32 were resected and in 31 was achieved R0. This corresponds to 56.1% resection rate and 96.9% achieved R0 resections. The median OS for resected patients vs not resected after SBRT was 19.3 versus 12.3 months. They also report a low toxicity rate for grade 3 and 4 toxicities at 5.3% [Citation33]. In 2013, Festa et al. published a meta-analysis of prospective studies on neoadjuvant chemo-radiotherapy which includes 10 studies with 182 patients. Of them, 137 received ChRT and 45 only chemotherapy. After completion of therapy 180 were restaged and 128 underwent surgical exploration. One hundred and seven patients were resected (80%) and R0 resection was achieved in 83% of the cases. The data were homogenous among the different trials. The 1-year survival rate was 61% and median OS for resected patients was 22 months. Unresected patients had median OS 9.7 months. The reported estimated toxicity for the meta-analysis was 32%. In the three studies where no radiation was given the toxicity varied from 30 to 46% [Citation34].
The reduction in tumor volume, retraction of tumor from involved vessels and downstaging shows that HIFU potentially will have comparable results to standard neoadjuvant treatment options. Even so we have found very few data in English and Chinese sources. Future areas of research should be aimed at investigating the effects of neoadjuvant treatment with chemotherapy ± HIFU, comparison of median OS of patients, rates of achieved R0 resections and differences in complication rates after surgery by randomized trials and prospective control-cohort study. Multidisciplinary team decisions between surgeons, oncologists, HIFU specialists and radiologists for BRPC could prove useful in the decision making for optimal neoadjuvant treatment.
Conclusion
HIFU is found to be safe and feasible in locally advanced and metastatic pancreatic cancer with proven downstaging and downsizing effects. Further research on role of HIFU ablation as a neoadjuvant treatment for borderline resectable pancreatic cancer is needed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Linecker M, Pfammatter T, Kambakamba P, et al. Ablation strategies for locally advanced pancreatic cancer. Dig Surg. 2016;33(4):351–359.
- Vauthey JN, Dixon E. AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16(7):1725–1726.
- National Comprehensive Cancer Network (NCCN) guidelines. Accessed January 3, 2021; Available from: http://www.nccn.org/professionals/physician_gls/recently_updated.asp.
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–1046.
- Goldberg SN, Mallery S, Gazelle GS, et al. EUS radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50(3):392–401.
- Sofuni A, Moriyasu F, Sano T, et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol. 2014;20(28):9570–9577.
- Ning Z, Xie J, Chen Q, et al. HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients. OTT. 2019;12:1021–1029.
- Dorr LN, Hynynen K. The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia. 1992;8(1):45–59.
- Guo X, Zhu H, Zhou K, et al. Effects of high‑intensity focused ultrasound treatment on peripancreatic arterial and venous blood vessels in pancreatic cancer. Oncol Lett. 2020;19:3839–3850.
- Moher D, Liberati A, Tetzlaff J, The PRISMA Group, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
- Oxford Levels of Evidence Working Group. The Oxford 2011 levels of evidence; 2011. Available from: http://www.cebm.net/index.aspx?o¼5653.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452.
- Wang G, Zhou D. Preoperative ultrasound ablation for borderline resectable pancreatic cancer: report of 30 cases. Ultrason Sonochem. 2015;27:694–702.
- Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2(3):175–184.
- Li PZ, Zhu SH, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2012;11(6):655–660.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2019;40(5):625–637.
- Marinova M, Rauch M, Mücke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26(11):4047–4056.
- Strunk HM, Henseler J, Rauch M, et al. Clinical use of high-intensity focused ultrasound (HIFU) for tumor and pain reduction in advanced pancreatic cancer. Rofo. 2016;188(7):662–670.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086.
- Vidal-Jove J, Perich E, del Castillo MA. Ultrasound guided high intensity focused ultrasound for malignant tumors: the Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem. 2015;27:703–706.
- Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20(44):16480–16488.
- Marinova M, Wilhelm-Buchstab T, Strunk H. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden [Advanced pancreatic cancer: high-intensity focused ultrasound (HIFU) and other local ablative therapies]. Rofo. 2019;191(3):216–227.
- Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(S1):i90–i98.
- Datta NR, Gomez Ordonez S, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753.
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol. 2011;100(1):22–32.
- Kirui DK, Koay EJ, Guo X, et al. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine. 2014;10(7):1487–1496.
- (a) van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184. (b) Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17(1):1–18.
- van der Horst A, Versteijne E, Besselink MGH, et al. The clinical benefit of hyperthermia in pancreatic cancer: a systematic review. Int J Hyperthermia. 2018;34(7):969–979.
- Dimitrov D, Stanislavova N, Angelov D. Influence of the Focussed ultrasound surgery at the patency of abdominal vessels in patients with locally advanced pancreatic cancer. Rentgenologiya i Radiologiya. 2019;3(58):212–219.
- Strunk HM, Lützow C, Henseler J, et al. Auswirkungen der US-gesteuerten HIFU-Ablation auf die großen Oberbauchgefäße beim lokal infiltrierenden [Mesenteric vessel patency following HIFU therapy in patients with locally invasive pancreatic cancer]. Ultraschall Med. 2018;6(39):650–658.
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86(3):516–522.
- Festa V, Andriulli A, Valvano MR, et al. Neoadjuvant chemo-radiotherapy for patients with borderline resectable pancreatic cancer: meta-analytical evaluation of prospective studies. J Pancreas. 2013;14(6):618–625.