Abstract
Objectives
To compare the ablation margins and safety of microwave ablation (MWA) of perivascular versus non-perivascular liver metastases from colorectal cancer (CRC) and to determine the risk factors for local tumor progression (LTP) after perivascular MWA.
Methods
Between June 2017 and June 2019, 84 metastases were treated: 39 perivascular (<5 mm from a vessel >3 mm), and 46 non-perivascular. Perivascular metastases were treated with either conventional or optimized protocols (maximum power and/or several heating cycles after repositioning the needle regardless of the initial tumor dimensions). The mean diameter of metastases was 15.4 mm (SD: 7.56).
Results
Vascular proximity did not result in a significant difference in ablation margins. The technical success rate, primary efficacy, and secondary efficacy were 90%, 66%, and 83%, respectively. Perivascular location was not a risk factor for time to LTP (p = 0.49), RFS (p = 0.52), or OS (p = 0.54). LTP was statistically related to the presence of a colonic obstruction (p < 0.05), number of metastases at the time of diagnosis (p < 0.05), type of protocol (p < 0.05), ablation margins (p < 0.001) and LTP was proportional to the number of liver resections before MWA (p < 0.05). There was no LTP in tumors ablated with margins over 10 mm. Two grade 4 complications occurred.
Conclusion
MWA is an effective and safe treatment for perivascular liver metastases from CRC, provided that satisfactory margins are achieved. A maximalist attitude could be related to better local control.
Introduction
Colorectal cancer (CRC) is the fourth most frequently diagnosed cancer and the second leading cause of cancer mortality in the United States. Recent improvements in the incidence and mortality are thought to be the result of shifting patterns of the risk factors associated with CRC and improved therapeutic modalities [Citation1]. The liver is one of the most common metastatic sites for CRC and its involvement is a major prognostic factor, particularly in CRC. Treatment of these metastatic tumors improves patients’ survival [Citation2]. A controlled randomized trial recently demonstrated that aggressive local treatment of patients with unresectable colorectal liver metastases could prolong the overall survival [Citation3]. Liver resection is the standard treatment for oligo-metastatic patients. However, liver resection is contraindicated when metastases are close to the major liver vessels, owing to technical difficulties and a high complication rate [Citation4]. For several years, percutaneous and surgical thermoablation (TA) has been a valid therapeutic alternative in the management of small (<3 cm) liver metastases [Citation5,Citation6]. The three- to ten-year survival rates of a large series of patients with liver metastases from CRC treated with TA were found to be equivalent to those reported by most surgical series in literature [Citation7]. During surgery, particular attention is paid to ensure minimal ablation margins. The optimal end point of tumor ablation is a minimal margin of above 10 mm, which is related to local control, similar to hepatectomy, thereby, avoiding surgical morbidity. Recently, a systematic review and meta-analysis argued in favor of ablation over chemotherapy alone by emphasizing the potential for long-term disease control and low complication rates [Citation8]. Microwave ablation (MWA) has developed in recent years and appears to be an effective and safe alternative [Citation9]. However, its efficacy on perivascular metastases remains uncertain, owing to the heat sink effect (HSE) [Citation10], which results in incomplete tumor destruction. The HSE is frequently reported after radiofrequency ablation (RFA), compared to MWA, which may be attributed to the fact that MWA employs different technologies that partially annihilate the HSE [Citation11]. Moreover, RFA protocols seem to depend on the resistivity of the carbonized tissue objectified by the roll-off. One of the advantages of MWA is its ability to eliminate the roll-off and deposit a predefined amount of energy, according to the programmed time and power. Subsequently, optimized protocols are possible, that is the use of higher parameters, compared to those provided by the manufacturers for a given tumor dimension and/or the repetition of ablations after needle repositioning without being limited by the roll-off. Current literature comprises very few studies that focus on MWAs in this context. Qin et al.’s study [Citation12] did not have the same objectives as the current study. However, they observed that tumor progression after ultrasound-guided percutaneous MWA in colorectal liver metastases was more likely in perivascular metastases. Conversely, Shady et al. [Citation13] demonstrated that unlike RFA, the efficiency of MWA was not affected in perivascular tumors, provided that satisfactory ablation margins are achieved.
The objective of the current study was to compare the size of ablation zones, minimal margins, short-term local control, and safety of MWA in the management of perivascular versus non-perivascular metastases and to determine the risk factors for the local tumor progression (LTP) of perivascular metastasis from CRCs after MWA.
Materials and methods
Approval for the present retrospective study was obtained from the Institutional Review Board.
Patient population
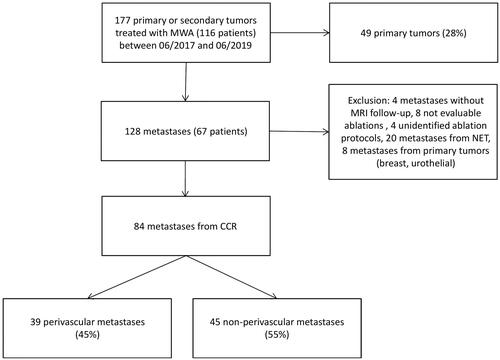
The current study included all consecutive patients who underwent MWA at our institution from June 2017 to June 2019. The inclusion criteria were as follows: patients with liver metastases from CRC, age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status <2, alanine aminotransferase and aspartate aminotransferase levels three times above the upper normal limit, total serum bilirubin <3.0 mg/dL, serum creatinine clearance >60 ml/min, platelet count >50,000/mm3, and international normalized ratio <1.5. The exclusion criteria were as follows: primary liver tumors, owing to a different population in a scenario involving predominant cirrhosis that could increase the dimensions of ablation [Citation14]; patients with metastases from other primitive tumors; lack of data on ablation protocols; inability to undergo medical follow-up due to geographical, social, or psychological factors; patients without adequate baseline imaging, without MRI follow-up after ablation, or poor quality MRI with hardly evaluable ablation. During the course of the study, 116 consecutive patients with 177 metastases underwent monopolar MWA. A flowchart of the patient selection process is shown in .
The perivascular location of the tumor was defined as a distance of less than 5 mm between the hepatic metastasis and a major hepatic vessel (portal and/or hepatic veins and/or inferior vena cava). A vessel was considered as large if the diameter was above 3 mm. The current study chose a distance of 5 mm, which corresponds to the estimated ablation minimal margin for satisfactory local tumor control [Citation15,Citation16] and a vessel diameter greater than 3 mm, from which the HSE can be observed [Citation17]. The margins between the metastases and the vessels, the vessel diameters, and the distribution of metastases between the two groups, namely, the perivascular and non-perivascular groups, were determined through consensus after evaluations by three investigators. The exact vessel and the size of the vessels for PV tumors are indicated in .
Table 1. Table detailing the exact vessel, their number and the distances between vessels and metastasis for PV tumors.
The indications for MWA were discussed by a multidisciplinary tumor board including specialists in liver surgery, interventional radiology, and digestive oncology. All metastases eligible for percutaneous thermal ablation treatment (diameter ≤30 mm) were managed with MWA. All metastases with larger diameters were managed surgically. All the patients had histopathological evidence of a primary tumor and/or metastasis and underwent a preoperative MRI or contrast-enhanced CT scan within 30 days prior to the treatment. The patients were verbally informed of the potential complications and treatment alternatives, and verbal consent was obtained. The following data were collected:
Patients: age, sex, WHO (World Health Organization) performance status, American Association of Anesthesia (ASA) score, body mass index (BMI), platelet aggregation inhibitor, and platelet count.
Potential known prognostic factors pertaining to CRC: colonic obstructionor perforation, primary tumor resection; if yes, resection margin clearance, location of the tumor (left colon, right colon, and rectum); history of hepatic metastases surgery; if yes, number of surgical resections; number of chemotherapy lines, time interval between the diagnosis and MWA, mucinous component and percentage, number of liver metastases at the time of diagnosis, TNM classification, vascular or lymphatic invasion, perineural invasion, tumor grade, carcinoembryonic antigen (CEA) at the time of diagnosis and at the time of MWA, meta- or synchronous liver metastases, KRAS mutation, BRAF mutation, microsatellites instability status (since the 2018 updates to the NCCN guidelines, all patients with metastatic disease should have the tumor tissue genotyped for KRAS and BRAF mutations; patients with any known KRAS mutation (exon 2, 3, 4) or NRAS mutation (exon 2, 3, 4) should not be treated with either cetuximab or panitumumab. The response of BRAF V600E mutation to panitumumab or cetuximab is highly unlikely, unless administered with a BRAF inhibitor.4) [Citation1].
Metastases and procedures: proximity to vessels; if yes, vessel type, distance between metastases and vessels, vessel diameter, number of metastases treated in the same session, major diameter of the index ablation metastasis, ablation protocol (conventional or optimized), ablation power (<100 W or 100 W), ablation time (minutes), and minimal ablation margins.
Liver MWA procedure
The MWA interventions were performed by three senior interventional radiologists with more than five years of experience. The procedures were performed under local anesthesia and conscious sedation. Under the guidance of computed tomography (CT) (Somatom Definition AS scanner, Siemens, Munich, Germany), an initial non-contrast CT was performed with an acquisition reported in 0.6-mm thick sections, which was essentially supplemented by the contrast-enhanced CT if the tumor was not visible, with acquisition during the arterial phase (section thickness: 1.5 mm; step: 0, 8), with a delay of 30 s or the portal phase with a delay of 60–90 s after the commencement of the intravenous bolus injection of 100–120 cc of a low osmolality iodine-based contrast agent (Xenetix® 300, Guerbet GmbH, Sulzbach, Germany) at the rate of 3 cc/s. If required, image fusion software was used with the preoperative imaging. The MWA was performed using the Emprint™ microwave (2.45 GHz, 14 G probes) ablation system using the Thermosphere™ technology (Medtronic™, Dublin, Ireland). The needle was placed under the control of several 4 cm sequential acquisition boxes, which facilitated complex pathways. Once the needle was positioned, considering the non-perivascular metastases, the ablation parameters (power: 45/75/100 W; duration: 2.5/5/10 min) were determined according to the dimensions of the ablation index tumor using the manufacturer's charts. In case of perivascular metastases, the manufacturers' recommendations were followed during the initial experiments and the provided ablation charts were applied, which represented the ‘conventional protocol’. Subsequently, considering the feedback from experience, we decided to adopt a maximalist attitude, that is, to choose a maximum power of 100 W, regardless of the initial tumor dimensions, and/or to perform several heating cycles by repositioning the needle, which represented the ‘optimized protocol’. Subsequent to the interventions, a non-contrast CT scan was performed to detect immediate complications. A contrast-enhanced CT scan was not performed after each procedure, in order to preserve the kidney function. The completeness of ablation was assessed using MRI images obtained one month after the MWA. All the patients were closely monitored by an anesthesiologist during the procedure, kept in the recovery room for at least 1 h after the procedure, and hospitalized for one night after the treatment.
Follow-up imaging
The patients were examined by interventional radiologists and oncologists using a liver MRI after 1 month and subsequently, every 3 months. All the patients underwent follow-up MRIs using the General Electric MRI 1.5 T SIGNA Artist (GE Healthcare, Milwaukee, WI, USA). The protocol consisted of an axial T2-weighted fat-suppressed spin-echo in respiratory gating imaging (section thickness: 5 mm/1 mm), axial DW echo-planar in a respiratory gated imaging (b400-b800; section thickness: 5 mm/1 mm), axial T1-weighted breath-hold gradient-echo in-phase and out-of-phase imaging (section thickness: 5 mm), axial T2-weighted fast breath-hold spin-echo imaging (section thickness: 6.5 mm/2 mm), and axial unenhanced and dynamic contrast-enhanced T1-weighted 3 D fat-suppressed spoiled breath-hold gradient-echo imaging (section thickness: 5 mm), which was obtained with an initial delay of 35 s (arterial phase) and at two consecutive intervals afterwards, a portal phase (1 min) and a delayed phase (3 min), with an intravenous administration of 20 ml of a contrast agent (Dotarem®, Guerbet, Roissy, France). Additionally, the patients benefited from thoracic-abdominal and pelvic CT scans as part of the systematic follow-up, in agreement with their oncologists.
Evaluation of ablation dimensions, minimal ablation margins, treatment efficacy, and complications
Ideally, for the measurement of the ablation size, an immediate postoperative MRI should have been performed to assess the actual ablation sizes. However, this practice is not included in the follow-up recommendations and appears complex in practice. Hence, the ablation dimensions were measured 1 month after treatment on the T1-weighted 3 D sequence without contrast. The ablation zone appeared as a well-defined scar with a spontaneous high signal, owing to necrosis and hemorrhagic changes. The long and short axes diameters of the ablations were measured on multi-planar reconstructions. In order to evaluate the heterogeneity of the ablation parameters (power and time), these measurements were related to the values provided on the manufacturer's ablation charts for each ablation protocol. The ratios between the dimensions measured on the MRI and the expected dimensions in the manufacturers' charts were estimated. The dimensions of all the ablations at 1 month were smaller, compared to the expected dimensions in the charts, which can be attributed to the tissue contraction, particularly during MWA and secondly to the natural involution due to cicatrization [Citation18]. These changes are assumed to be constant, regardless of the protocol used, and are different from the HSE-induced phenomena [Citation19]. Furthermore, the estimated ablation zone dimensions were determined by the manufacturer’s in-vivo models for classic centro-hepatic ablations and not for perivascular metastases. Computing the ratio of these diameters allowed us to determine whether the dimensions of the ablations obtained were smaller than expected, regardless of the protocol used, and thereby, to determine whether an ablation suffered from HSE. Non- or difficult-to-measure ablations and the metastases treated with ‘optimized protocols’ involving needle repositioning were excluded from this evaluation.
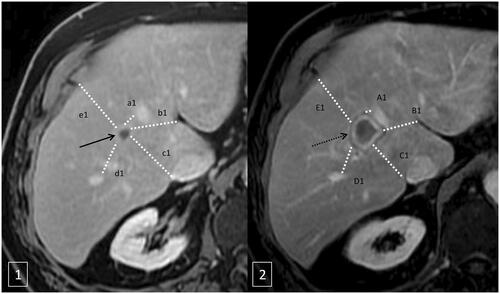
Minimal ablation margins were measured using a method comparing the first pre- and post-ablation MRI after contrast injection in the portal phase. Preoperative MRI or contrast-enhanced CT scan within 30 days prior to the treatment and post-ablation portal venous phase CT or MRI images were reviewed side by side to compare the index tumor and the ablation zone/defect. Multiple distances between the tumors/ablation zone and anatomic landmarks were measured (). Only high level reliability landmarks and nearest landmark to the tumor contour were selected. Anatomic landmarks present on pre- and post-ablation CT images were classified into four categories according to their reliability and reproducibility as previously described by Wang et al. [Citation15]. Only high level reliability landmarks and nearest landmark to the tumor contour were selected. At least 4 landmarks in four directions (medial, lateral, anterior, and posterior) were measured. For each landmark, the pre-ablation distance was subtracted from the post-ablation distance to render the margin at that site. The smallest value was considered as the minimal margin. In order to limit potential errors due to the spatial resolution variations, margin size was classified as less than 1 mm, 2–5 mm, 6–10 mm, and above 10 mm. These assessments were performed in consensus by two radiologists.
Figure 2. Minimal ablation margins measurement method. Axial T1 weighted MRI after intravenous contrast injection with an acquisition during the portal phase before microwave ablation (1) and after ablation (2). The method compared the first pre- and post-ablation MRI after contrast injection in the portal phase measuring multiple distances (a1, b1, c1, d1, e1) between the tumors (black arrow in 1) and anatomic landmarks and ablation zone (black dotted arrow in 2) and anatomic landmarks (A1, B1, C1, D1, E1) (). For each landmark, the pre-ablation distance was subtracted from the post-ablation distance to render the margin at that site (a1-A1, a2-A2…). The smallest value was considered as the minimal margin (a1-A1 in the example).
The efficacy regarding the diagnosis of residual tumors was determined independently by two radiologists. Technical success was confirmed if the ablation zone was completely overgrown or encompassed the target tumor, as observed on the MRI with a contrast injection that was obtained after the first month. LTP was defined as a new, growing tumor () in or abutting the ablation in a zone previously considered to be completely ablated during the follow-up period. The primary efficacy was defined as LTP after one MWA procedure. The secondary efficacy was defined as a successful repeat MWA following identification of the LTP. Time to LTP was calculated from the day of MWA to the day of LTP or the last day of follow-up. RFS was calculated from the day of MWA to the day cancer recurrence was detected or the day of last follow-up. Considering the TLP and RFS, all the patients were censored at the time of death. OS was calculated from the day of MWA to the day of demise or the day of last follow-up.
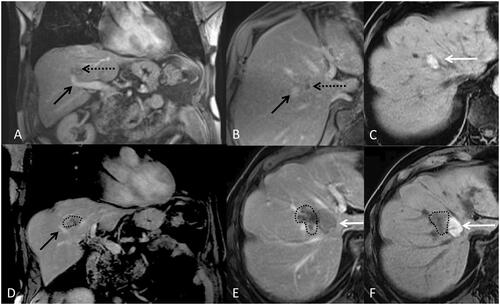
Figure 3. 80-year-old-man with colorectal cancer and a unique liver metastasis (black arrow) close to a segmental portal vein (black doted arrow). A and B represent a coronal and axial T1 weighted MRI after intravenous contrast injection with an acquisition during the portal phase before microwave ablation. C represents the aspect of the ablation zone on T1 weight MRI one month after treatment. Without injection of contrast the ablation zone appears hyper intense (white arrow). D, E and F are a coronal and axial T1 weighted MRI without injection (F) and with intravenous injection of contrast (D and E) during portal phase, three months after microwave ablation representing a growing tumor (dotted line) poorly enhanced abutting the ablation zone and the portal vein.
Adverse events were recorded using the criteria of the Common Terminology for Adverse Events Version 5.0 [Citation20]. Grade 1 corresponds to asymptomatic or mild symptoms not requiring treatment; grade 2 corresponds to moderate symptoms: minimal, local, or noninvasive intervention is indicated; grade 3 corresponds to severe or medically significant but not immediately life-threatening symptoms; hospitalization or prolongation of existing hospitalization is indicated; grade 4 corresponds to life-threatening consequences; urgent intervention is indicated; and grade 5 corresponds to death.
Statistical analyses
Quantitative statistics were presented as means and standard deviations or medians and interquartile ranges and compared with each other using the Mann–Whitney U test. Qualitative data were expressed as frequencies and were compared using the Student’s t and Chi-square tests. Data pertaining to the ablation dimensions were compared using the Wilcoxon rank-sum test with continuity correction. TLP, recurrence-free survival (RFS), and overall survival (OS) were calculated using the Kaplan-Meier method. A log-rank test was performed to compare the TLP, RFS, and OS between the perivascular ablation and non-perivascular ablation groups. Risk factors for LTP were studied using the Cox regression analysis. Statistical significance was assumed for p-values less than 0.05. The statistical analysis was performed using the SPSS software (version 24; SPSS, Chicago, IL).
Results
Baseline characteristics
The present study involved 39 (46%) patients with perivascular metastases who underwent MWA (13 adjacent to portal veins, 18 adjacent to hepatic veins, four adjacent to the inferior vena cava (IVC), and four adjacent to both hepatic veins and IVC). The mean metastases diameter was 15.4 mm (SD: 7.56). In 12 procedures, two metastases were treated during the same session (nine with both perivascular and non-perivascular metastases and three with two non-perivascular metastases). In five procedures, three metastases were treated during the same session (three with two perivascular and one non-perivascular metastases, one with three perivascular metastases, and one with two non-perivascular and one perivascular metastases). The mean distance between the metastases and the vessels was 0.98 mm (SD:1.4) and the mean diameter of the vessels was 10.2 mm (6.7). The characteristics pertaining to the patients and metastases in the two groups were not statistically different ().
Table 2. Table representing patients’ characteristics for the group of perivascular ablations and the group of non-perivascular ablations.
Table 3. Table showing univariate analysis of factors affecting LTP for perivascular metastases.
Ablation dimensions
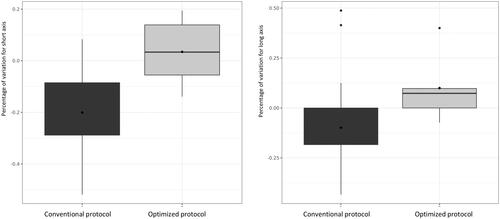
The dimensional variations of the perivascular ablation zones were not significantly different from the non-perivascular ablation zones with regard to either the long axis (−8% ± 1% versus −8% ± 1% respectively; p = 0.92) or the short axis diameters (−17% ± 17% versus −19% ± 16% respectively; p = 0.7). The short axis diameters of perivascular ablations were significantly smaller in the conventional ablation protocols, compared to the optimized protocols (−21% ± 15% versus 3% ± 13% respectively; p < 0.01). The long axis diameters of perivascular ablations were significantly smaller in the conventional ablation protocols, compared to the optimized protocols (−1% ± 16.4% versus 1% ± 18% respectively; p = 0.015) (). There were no significant correlations between the ‘diameter of the vessels’ and ‘the distance between the vessels and metastases,’ on the one hand and the dimensional variations for the long axes (p = 0.5 and p = 0.1 respectively) and short axes (p = 0.7 and p = 0.2, respectively) of the perivascular metastases treated with MWA on the other hand. No significant correlations were observed between the periportal or perihepatic venous tumors and the short (p = 0.6) or long axes (p = 0.8).
Minimal ablation margins
Among all the tumors, 19 (23%) were ablated with a minimal margin of less than 1 mm, 29 (35%) with a minimal margin between 2 and 5 mm, 19 (23%) with a minimal margin between 6 and 10 mm, and 17 (20%) with a minimal margin above 10 mm. Eleven perivascular and eight non-perivascular tumors were ablated with a minimal margin of less than 1 mm. Fourteen perivascular tumors and 15 non-perivascular tumors presented a minimal margin of 2 and 5 mm. Six perivascular tumors and 13 non-perivascular tumors presented a minimal margin of 6 and 10 mm. Eight perivascular tumors and nine non-perivascular tumors presented a minimal margin of above 10 mm. There were no statistically significant differences between the perivascular and non-perivascular tumors with regard to the minimal ablation margins (p = 0.4). Regarding the perivascular MWA, there was a statistically significant association between the ‘optimized protocol’ and minimum ablation margins (p = 0.05) (10 (91%) with a margin below 1 mm, 12 (86%) with a margin between 2 and 5 mm, three (50%) with a margin between 6 and 10 mm, three (38%) with a margin above 10 mm for ‘conventional protocol’ versus one (9.1%) with a margin above 1 mm, two (14%) with a margin between 2 and 5 mm, three (50%) with a margin between 6 and 10 mm, and five (62%) with a margin above 10 mm for the optimized protocol). Minimal margins were not statistically associated with the vessel diameters (p = 0.25) or the distance to vessels (p = 0.14).
Efficacy of liver MWA for perivascular metastases
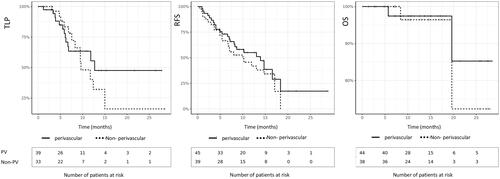
The technical success rate was 90%, with five procedures presenting an incomplete treatment on the follow-up MRI after 1 month (one patient underwent re-treatment with MWA, three patients required chemotherapy, and one patient underwent surgery). The primary efficacy was 66% and the secondary efficacy was 83%. Among the 35 technically successful procedures, 12 ablations had local recurrence. Five recurrences were detected on the follow-up MRI at 6 months, four at 9 months, two at 12 months, and one at 15 months. Five recurrences were successfully treated with a second session of MWA and one was successfully treated with two additional MWAs. In three cases, chemotherapy had to be initiated due to diffuse metastatic progression and surgical resection was performed in three cases. The median follow-up period was 13.3 months. The median TLP was 12.5 months (). The one- and two-year local progression LP rates were 56% and 48%, respectively. The median RFS for perivascular metastases was 14.5 months. The one- and two-year RFS rates were 55% and 15%, respectively. The median overall survival OS was not attained. The one- and two-year OS rates were 97% and 85%, respectively. The perivascular location was not statistically related to the TLP (p = 0.49), RFS (p = 0.52), or OS (p = 0.54).
Factors associated with the LTP of PV metastases after MWA ()
The LTP was statistically related to the presence of a colonic obstruction or a perforation at the time of initial diagnosis (p < 0.05), the number of metastases at the time of diagnosis (p < 0.05), the number of surgical resections of liver metastases before MWA (p < 0.05), the type of protocol (p < 0.05), and ablation margins (p < 0.001) (). No LTP was detected in the tumors ablated with margins above 10 mm. There was no statistical difference between the LTP of tumors close to the portal veins and peri-hepatic venous tumors (9.5 versus 11.7 months, respectively, p = 0.6). A subgroup analysis revealed a statistical significance of the LTP rate, in accordance with the association of the ablation margin and KRAS mutation ().
Figure 6. Patient followed for recto sigmoid adenocarcinoma with synchronous bi-lobar hepatic metastases. Ten FOLFOX cures were performed. The good response to this neoadjuvant treatment made it possible to perform liver surgery by left hepatectomy, on histological examination, two tumors compatible with a Lieberkühnien adenocarcinoma moderately differentiated and tumor regression TRG3 with complete exeresis. A few months later, appearance of two tumors of less than 2 cm in the liver treated by stereotactic radiotherapy. A few months later, recurrence on the hepatectomy slices with MTB decision to perform a percutaneous thermo-ablation. Images A and B are axial (A) and coronal (B) reconstruction scans with intravenous injection of contrast material at portal phase showing metastasis (black arrow) and vascular contact with the right suprahepatic vein (white dotted arrow) and the portal branch of segment VIII (white arrow). Image (C) is a T1-weighted axial MRI acquisition after injection of gadolinium portal phase, showing stereotactic radiotherapy sequellae as a hyper vascularization zone (black asterix). A few weeks later, an alteration in the general state appeared, with evidence of septic thrombosis of the right hepatic vein. Images E and F are scanner acquisitions after intravenous injection of contrast medium at portal phase, showing a probable abcedation of the microwave zone with the presence of a hydroaerous level (black dotted arrow) and fistulization of the right atrium. This case highlights the precautions to be taken in the treatment of CRC metastases close to large vessels by microwave on a weakened hepatic parenchyma. Indeed, the modification related to radiotherapy combined with the effect of microwaves is probably responsible for this complication.
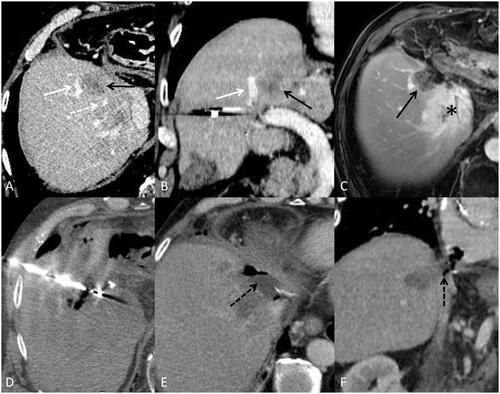
Table 4. Table showing LTP rates according to margin size and Kras mutation.
Safety assessment
Two grade 4 complications involving venous thrombosis occurred in the perivascular metastases group. The first patient developed an abscessed collection in the TA zone, complicated by a septic right supra hepatic venous thrombosis that extended to the IVC, which had to be drained percutaneously. The patient presented with an altered general condition that contraindicated chemotherapy (). The second patient developed vena cava thrombosis less than 6 months after treatment, which was not symptomatic and partially resolved after anticoagulant therapy. One patient presented with a grade 3 complication associated with a perihepatic hemorrhage, with active arterial bleeding from a capsular artery detected on the CT scan, which was treated by embolization. Two patients suffered from grade 2 complications, including one patient with a pneumothorax due to a pleural pathway, which was drained for 2 days, and one patient with bilioma that required extended hospitalization. Five patients suffered from grade 1 complications, including three patients with mild capsular hematomas and two asymptomatic segmental dilatations of the intrahepatic bile ducts.
Discussion
MWA may be an alternative treatment for perivascular metastases from CRCs. A maximalist attitude delivering maximum power with several heating cycles after needle repositioning could be related to larger ablation dimensions, larger minimum ablation margins, and better local control. Risk factors for LTP seem to be related to the patient’s disease history (colonic obstructionor a perforation at the time of initial diagnosis, the number of metastases at the time of diagnosis, and the number of surgical resections of liver metastases before MWA) and to the ablation protocol with the primary focus on controlling minimum ablation margins. There was no LTP with margins above 10 mm, confirming the importance of achieving optimal minimum margins above 10 mm during liver MWA of perivascular metastases from CRC. Particular attention should be paid to the MWA close to the hepatic veins, especially in patients with a history of stereotaxic radiotherapy or hepatectomy, as there is a risk of thrombosis.
The role of percutaneous TA in the treatment of liver metastases from CRC has evolved greatly, owing to the growing knowledge regarding the factors affecting the outcomes and the understanding regarding the importance of minimal margins. In selected patients, liver TA can be considered as a curative treatment similar to surgery, but with less morbidity. MWA is known to have special characteristics, compared to RFA, such as higher intratumoral temperatures, larger ablation zones, shorter ablation time, and lesser dependence on electrical conductivity. MWA is thought to be less sensitive to HSE as it does not propagate by electrical conduction. Theoretically, there is no attenuation of ablative energy in the tissue due to flow within the vessels [Citation21]. Fan et al. [Citation22] compared microwave and monopolar RFA in pig liver in-vivo, demonstrating that the diameters of the long and short axes for all microwave power settings were larger, compared to RFA, and that the time taken for the temperature to reach 60 °C was significantly faster in MWA. Recurrence rates after monopolar RFA are particularly high in metastases adjacent to large vessels [Citation23].
Multipolar RFA has recently been developed to overcome this issue and seems to offer better performance, especially with regard to HSE [Citation24]. However, these results are contradictory to the in-vitro studies. Poch et al. [Citation25] concluded that a vascular cooling effect also occurs, independent of the geometry of multipolar RFA applicators, on in-vitro liver vessel flow simulations. Some studies have attempted to compare the efficacy of multipolar RFA with MWA. Huo et al. [Citation26] reported that MWA and RFA allowed for similar one- and five-year OS, disease-free survival, local recurrence rates, and adverse events. However, comparisons between these techniques require randomized controlled studies. The main theoretical advantage of MWA over multipolar RFA is that MWA is not dependent on tissue electrical conductivity. The advantage highlighted by our study is the possibility of repositioning a single needle in a simple way and to adjust the power and heating time, so as to have maximum values without being limited by roll-off. However, HSE remains the main limitation of MWA.
In practice, the heat dissipation effect must exist, regardless of the TA employed, including MWA [Citation27]. Ringe et al. observed a significant flow-dependent HSE from a radius of 15 mm around the vessels. The current study confirmed this hypothesis and found that the short axis dimensions of perivascular ablations were smaller when the exact manufacturer's charts are applied. In contrast to the present study, Urbanos et al. demonstrated that vascular proximity was a risk factor for local recurrence [Citation28]. This difference could be attributed to the fact that the current study used an ‘optimized protocol’ for the treatment of perivascular tumors. In order to solve this problem that is inherent to any perivascular thermal ablation, some authors have proposed solutions to avoid heat dissipation. Nie et al. [Citation29] proposed a numerical model exploring the HSE effect in MWA and concluded that when the needle is at a distance of less than 10 mm from a vessel, it is essential to take certain effective and reasonable measures to avoid causing an asymmetric shape of the area near the blood vessels. These results are concurrent with those of the current study. Indeed, the implementation of an optimized ablation protocol appears to allow better local control.
The safety and efficacy of MWA for perivascular metastases remains an active area for research. The efficacy of MWA in the treatment of perivascular tumors has already been studied through a surgical approach, but not through a percutaneous approach. Rhaiem et al. [Citation30] recently published a study in this context, but combined MWA with a pringle maneuver. Ren et al. [Citation31] studied the efficacy of ethanol injection along with MWA for liver tumors adjacent to the hilum. Unfortunately, the patient group only included three patients with metastases. Shady et al. [Citation13] performed a retrospective review that compared MWA with RFA in the treatment of perivascular metastases from CRC. The present study obtained the same results as the study by Shady et al. pertaining to the perivascular data regarding MWA. Significant predictors of shorter LTP free survival on univariate analysis for MWA were ablation margins of 5 mm or less (p < 0.001). No LTP was observed in the tumors ablated with margins over 10 mm and perivascular tumors were not predictive of LTP (p = 0.43), which was concurrent with the current study. One difference between the results of the two studies is that the absence of history of prior liver resection was a significant predictor of shorter LTP (p < 0.013) in the study by Shady et al. In the current study, the mean number of surgical resections of liver metastases before MWA was 0.500 (±0.673) in the group without LTP and 1.00 (±0.791) in the group with LTP (p = 0.044). Regardless of the TA technique employed, a major predictor of local response reported by several studies is the ablation margin [Citation32]. A recent study has emphasized the relation of a margin of 10 mm or larger with complete tumor control [Citation33]. Our study obtained quite similar results, without LTP for minimal margins above 10 mm, and a very high rate of LTP (around 90% in our study) for margins less than 1 mm. In the study by Kurilova et al., minimal margins above 10 mm offered optimal tumor control with no LTP, compared with the LTP rates of 26% for margins of 6 and 10 mm (p = 0.0001), 60% for margins of 1 and 5 mm (p = 0.0001), and 79% for no margin or 0 mm (p = 0.0001). This study also described factors associated with complications according to the ablation margins. Prior hepatic arterial infusion therapy was associated with increased major complications (p = 0.004), especially biliary complications. These findings were not observed in the present study.
No significant association between KRAS mutation and LTP was observed in the current study, which is concurrent with some previous studies [Citation34]. However, the subgroup analysis associating KRAS mutation and ablation margins revealed a significant association with LTP. These results are not totally consistent with other study results demonstrating that mutant RAS is associated with an earlier and higher rate of LTP [Citation35]. In the study by Odisio et al. [Citation35], involving 92 patients, 39% had mutant RAS. The rates of LTP were 14% for patients with the wild-type RAS and 39% for patients with mutant RAS (p = 0.007). The actuarial 3-year LTP-free survival rate was worse in patients with mutant RAS than in those with the wild-type RAS. Shady et al. [Citation34] and Calandri et al. [Citation36] further investigated the interaction between RAS status and minimal ablation margins. Shady et al. found that KRAS mutation was a significant predictor of overall survival, and new liver and peritoneal metastases after RFA and recommended a minimal ablation margin of at least 6 mm especially for the RAS mutant. Calandri et al. found that the minimal ablation margin and RAS status interacted as independent predictors of LTP-free survival. They concluded that minimal ablation margins over 10 mm should always be the procedural goal and this became especially critical for mutant RAS. The general point to be retained considering RAS status and ablation margins is that the major objective of any liver thermal ablation is to achieve minimum ablation margins of at least 10 mm and that particular attention should be paid to patients with mutant RAS for whom a minimal ablation margin of 10 mm is critical. Unlike the study by Yamashita et al., the embryonic origin of primary colon cancer was not observed to be a predictor of LTP in the present study [Citation37]. Other predictors, such as Ki 67, have not been investigated here [Citation38].
The current study has several limitations. First, it was a retrospective study. Consequently, an inherent selection bias was inevitable. Second, only a small number of patients underwent MWA with an optimized protocol. Contrast-enhanced CT scans were not performed after each procedure, in order to preserve the kidney function. The completeness of ablation was assessed using MRI images obtained 1 month after MWA, which may have artificially underestimated the technical efficiency. This is non-compliant with the guidelines and represents an important limitation of the study. The evaluation of ablation zones with immediate post-RFA triple-phase CT is associated with a significantly lower LTP rate [Citation32]. Given the importance of ablation zone assessment immediately after liver thermal ablation, some studies have developed innovative techniques for tissue characterization. A potential real-time biomarker of complete tumor ablation has been found using a rapid fluorescent tissue examination [Citation39]. Diffuse reflectance spectroscopy may also allow accurate quantification of thermal tissue damage after RFA, improving the accuracy and quality of the procedures by lowering incomplete ablation rates [Citation40]. Ablation margins were assessed using a simple two-dimensional method, the same method used in prior papers [Citation15]. A more recent, accurate method could have been used [Citation41]. The present study did involve long-term patient follow-up. No detailed data regarding further treatment after disease recurrence were available to comprehend the survival data, owing to the heterogeneity of subsequent management and missing data. A prospective study is necessary to compare the conventional and optimized ablation of perivascular metastases.
Conclusion
MWA is an effective and safe treatment for perivascular liver metastases from CRC, provided that satisfactory margins are achieved. An optimal attitude delivering maximum power with several heating cycles after needle repositioning could be related to larger ablation dimensions, larger minimum ablation margins, and better local control. Minimum ablation margins are essential for ensuring local control. A minimal margin of above 10 mm should be targeted. Particular attention should be paid to the risk of hepatic vein thrombosis.
Guarantor
The scientific guarantor of this publication is J. Izaaryene.
Statistics and biometry
No complex statistical methods were necessary for this study.
| Abbreviations | ||
| CRC | = | Colorectal cancer |
| ECOG | = | Eastern Cooperative Oncology Group |
| HSE | = | Heat sink effect |
| INR | = | International Normalized Ratio |
| LTP | = | Local tumor progression |
| MWA | = | Microwave ablation |
| NET | = | Neuro endocrine tumor |
| OS | = | Overall survival |
| RFS | = | Recurrence-free survival |
| RFA | = | Radiofrequency ablation |
Disclosure statement
The authors report no conflict of interest: The authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
References
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Cancer Netw. 2018;16(4):359–369.
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990; 77(11):1241–1246.
- Ruers T, Van Coevorden F, Punt CJA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109:djx015.
- Azoulay D, Andreani P, Maggi U, et al. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244(1):80–88.
- Management of Patients with Metastatic Colorectal Cancer | ESMO. https://www.esmo.org/guidelines/gastrointestinal-cancers/metastatic-colorectal-cancer/management-of-patients-with-metastatic-colorectal-cancer.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Meijerink MR, Puijk RS, van Tilborg AAJM, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Interv Radiol. 2018;41(8):1189–1204.
- Yang G, Xiong Y, Sun J, et al. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Int J Surg. 2020;77:85–93.
- Goldberg S, Hahn P, Tanabe K, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9(1):101–111.
- Ruiter S, Heerink W, de Jong K. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol. 2019;29(8):4026–4035.
- Qin S, Liu GJ, Huang M, et al. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int J Hyperthermia. 2019;36(1):36–43.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.e1.
- Deshazer G, Merck D, Hagmann M, et al. Physical modeling of microwave ablation zone clinical margin variance. Med Phys. 2016;43(4):1764–1776.
- Wang X, Sofocleous C, Erinjeri J, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Interv Radiol. 2013;36(1):166–175.
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Yu N, Raman S, Jun Kim Y, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19(7):1087–1092.
- Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol. 2010;21(8):1280–1286.
- Amabile C, Farina L, Lopresto V, et al. Tissue shrinkage in microwave ablation of liver: an ex vivo predictive model. Int J Hyperthermia. 2017;33(1):101–109.
- Muneeb M. A image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Nan Q, Zheng W, Fan Z, et al. Analysis to a critical state of thermal field in microwave ablation of liver cancer influenced by large vessels. Int J Hyperthermia. 2010;26(1):34–38.
- Fan W, Li X, Zhang L, et al. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol. 2012;198(1):W46–W50.
- Lu DS, Raman SS, Vodopich DJ, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002;178(1):47–51.
- Snoeren N, Nijkamp MW, Berendsen T, et al. Multipolar radiofrequency ablation for colorectal liver metastases close to major hepatic vessels. Surgeon. 2015;13(2):77–82.
- Poch FM, Rieder C, Ballhausen H, et al. The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex-vivo. Int J Hyperthermia. 2016;32(7):749–756.
- Huo YR, Eslick GD. Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis. J Vasc Interv Radiol. 2015;26(8):1139–1146.e2.
- Ringe KI, Lutat C, Rieder C, et al. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS One. 2015;10(7):e0134301.
- Urbonas T, Anderson EM, Gordon-Weeks AN, et al. Factors predicting ablation site recurrence following percutaneous microwave ablation of colorectal hepatic metastases. HPB 2019;21(9):1175–1184.
- Nie X, Nan Q, Guo X, et al. Numerical study of the effect of blood vessel on the microwave ablation shape. BME. 2015;26(s1):S265–S270.
- Rhaiem R, Kianmanesh R, Minon M, et al. Microwave thermoablation of colorectal liver metastases close to large hepatic vessels under pringle maneuver minimizes the "heat sink effect". World J Surg. 2020;44(5):1595–1603.
- Ren H, Liang P, Yu X, et al. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: a pilot study. Int J Hyperthermia. 2011;27(3):249–254.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2020:S1533-0028(20)30134-1.
- Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8(39):66117–66127.
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768.
- Calandri M, Yamashita S, Gazzera C. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28:2727–2734.
- Yamashita S, Odisio BC, Huang SY, et al. Embryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastases. Eur J Surg Oncol. 2017;43(6):1040–1049.
- Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–4269.
- Sotirchos VS, Fujisawa S, Vakiani E, et al. Fluorescent tissue assessment of colorectal cancer liver metastases ablation zone: a potential real-time biomarker of complete tumor ablation. Ann Surg Oncol. 2019;26(6):1833–1840.
- Tanis E, Spliethoff JW, Evers DJ, et al. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur J Surg Oncol. 2016;42(2):251–259.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2018;29(5):2698–2705.