?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
To evaluate the feasibility of radiofrequency ablation (RFA) on follicular neoplasm with low standard uptake value (SUV) in a Positron emission tomography (PET/CT) study.
Methods
From January 2018 to July 2019, 86 consecutive patients were diagnosed with follicular neoplasm. Of the patients, 28 with PET/CT scans were enrolled in this study. All patients received ultrasound, fine/core needle aspiration, and PET/CT scan prior to treatment. In accordance with previous studies, we recommended 6 patients who had follicular neoplasm with SUVmax ≥5 undergo surgical resection due to an elevated suspicion of malignancy. For 22 patients SUVmax <5, RFA was performed using the moving shot technique. Ultrasound was performed 6 to 12 months after each procedure.
Results
Statistically significant volume reductions during follow-up between values prior to RFA and 12 months post RFA were demonstrated (12.6 ± 20.9 vs. 2.4 ± 3.0 cm3, p < 0.001). Volume reduction ratios at 6–12 months (mean: 10.1 months) after RFA were 73.3% ± 17.7%. One patient presented with vocal cord palsy and recovered within 3 months after RFA. No postprocedural hypothyroidism occurred in the RFA patients.
Conclusions
By using PET/CT, we can select patients with low SUV follicular neoplasm. RFA offers a safe and feasible alternative treatment option for patients unsuitable or unwilling to undergo surgery.
By using positron emission tomography-computed tomography, we can distinguish low SUV follicular neoplasm for radiofrequency ablation.
For low SUV follicular neoplasm, RF ablation offers a safe and effective alternative treatment option for patients unsuitable or unwilling to undergo surgery.
KEY POINTS
Introduction
Thyroid follicular neoplasms are usually diagnosed following fine-needle aspiration (FNA) of palpable and nonpalpable thyroid nodules [Citation1]. The diagnosis of a follicular neoplasm represents various pathologies, including follicular adenoma, follicular hyperplasia, follicular carcinoma; in addition, Hürthle cell neoplasm may also be classified in this group [Citation2].
The FNA cytology of a follicular neoplasm does not, however, differentiate between a benign and malignant tumor. Consideration should include clinical features such as nodule size, age, and ultrasonographic characteristics including solid, hypoechoic, irregular margin, microcalcifications, tubercle-in-nodule [Citation1,Citation3], and the Bethesda system classification can only be part of the decision-making process when selecting patients for surgery. The Bethesda system classifies thyroid FNA cytology into six categories (category I–VI), while each category is linked to a malignancy risk and indicates a clinical management recommendation [Citation4]. Although controversies indeed exist in clinical practice, especially regarding intermediate thyroid nodules (category III/IV).
A differential diagnosis of carcinoma from adenoma is based on the presence of capsular, vascular, or extrathyroidal tissue invasion, in addition to nodal or distant metastasis [Citation5]. Due to the low sensitivity of FNA, core needle biopsy (CNB) has been introduced as a superior diagnostic tool for the identification of follicular neoplasm [Citation6]. However, the application of CNB to replace the diagnostic lobectomy remains controversial [Citation7–9].
Surgery remains the standard treatment for follicular neoplasm, as the pathologist can detect the presence of vascular and/or capsular invasion to reach a definitive diagnosis. Notably, up to 80% of follicular neoplasms are benign [Citation1]. Moreover, concerns of potential surgical complications, such as vocal cord palsy and hypothyroidism may influence the decision-making process of patients regarding surgical intervention [Citation6]; consequently, patients either unsuitable or unwilling to undergo surgery may opt for more conservative medical treatment. As such, intensive observation could be an alternative; however, the patient may suffer ongoing anxiety with regards to the nature of the tumor, creating a challenge for long-term follow-up, and further decreasing quality of life.
A thermal ablation is a treatment option for application with benign, malignant thyroid diseases and neck metastasis [Citation10–17]; although the use of Radiofrequency ablation (RFA) on Bethesda category III/IV nodules remains controversial. Current RFA guidelines from ATA [Citation8] and Korea [Citation18] do not recommend RFA for the treatment of follicular neoplasm, as there is no evidence of treatment benefit. Furthermore, clinical research into the application of RFA treatment for follicular neoplasm remains limited. One study of 10 patients with follicular neoplasm having received treatment by RFA reported no recurrence in a 5-year follow-up period, indicating that RFA may be effective and safe alternative management for patients presenting with small (<2 cm) follicular neoplasm who refuse surgery [Citation6].
Positron emission tomography (PET/CT) in combination with ultrasonography to discriminate benign from malignant cytologically indeterminate thyroid nodules has been introduced to avoid unnecessary diagnostic lobectomies. Some studies have reported that PET/CT negative (SUVmax cutoff value < 5) indicates a low risk for thyroid cancers [Citation19].
The aim of the present study was to evaluate the feasibility and safety of RFA for the treatment of follicular neoplasm.
Materials and methods
Patients
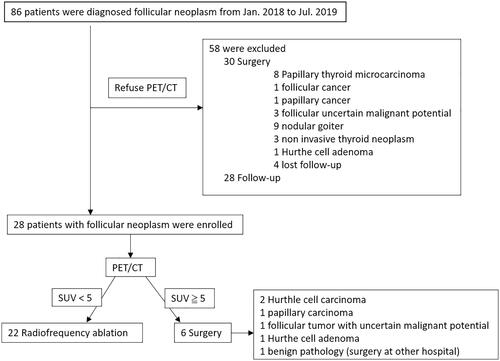
This retrospective study was approved by the institutional review board, and informed consent was waived. Data were retrospectively collected from January 2018 to July 2019, during which time 86 consecutive patients were diagnosed with follicular neoplasm. The 58 patients who refused PET/CT scan were excluded (30 underwent surgery, 28 follow up). Of the 28 patients with PET/CT scan (27 females, 1 male; mean age: 38.8 ± 10.8 years; range: 27–59 years), 6 patients with SUVmax >5 underwent surgical resection due to a high suspicion of malignancy. 22 patients (1 male and 21 females, mean age: 39.6 ± 10.5 years) who had follicular neoplasm with SUVmax <5 were enrolled in this study (). The patient inclusion criteria for this study were as follows: (1) diagnosed follicular neoplasm or suspicion of follicular neoplasm on fine/core needle aspiration; (2) presented with clinical symptom or cosmetic problem; (3) thyroid nodule >1 cm without suspicious malignant features such as spiculated margin, microcalcification, taller than wide or lymph node metastasis on ultrasonography; (4) no clinical evidence of distant metastasis; (5) refusal of primary surgery; (6) SUVmax <5. Ultrasound-guided fine/core needle aspiration was performed prior to treatment. The pathology results showed a follicular neoplasm in all 22 patients (Bethesda cytology IV).
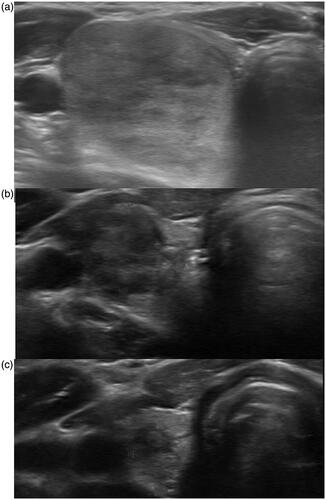
RFA was only performed after a careful explanation to patients of the potential risks of undetected malignancy, and of the need for regular follow-up to detect early tumor regrowth after RFA. The demographic data are shown in . Photographs of pretreatment sonography and PET studies are shown in .
Figure 2. (1a–5a) Sonography of five patients with thyroid follicular neoplasm who underwent surgery. The pathologies were: papillary carcinoma (1a); follicular tumor with uncertain malignant potential (2a); Hurthle cell carcinoma (3a); Hurthle cell carcinoma (4a); and Hurthle cell adenoma (5a). One patient with follicular neoplasm underwent RF ablation (6a). The Bethesda classifications were category IV in all 6 patients. Photographs of PET/CT of six thyroid follicular neoplasms (1b–6b). The SUVmax values are shown.
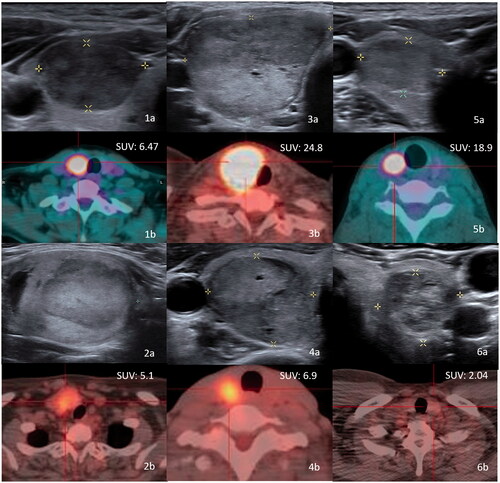
Table 1. Demographic data of patients with follicular neoplasm who underwent ablation.
F-18 FDG PET/CT acquisition & quantitative analysis
All patients, who had fasted for at least 4 h, were injected intravenously with a single bolus dose of 370–440 MBq of F-18 FDG. The head and neck F-18 FDG PET/CT images were obtained 1 h later using PET/CT scanner (Discovery ST PET/CT system; GE healthcare, Waukesha WI, USA). Unenhanced CT scans were acquired for attenuation correction and imaging fusion, PET scans (5 min/bed) were then performed from vertex to upper chest. The PET images were attenuation-corrected based on the CT images and reconstructed to a resolution of 5.47 × 5.47 × 3.27 mm using an ordered subsets expectation maximization (OSEM) algorithm. Blood glucose levels were checked in all patients prior to F-18 FDG injection, and no patients showed a blood glucose level of >180 mg/dL. For semiquantitative analysis of FDG uptake, region of interest (ROIs) were defined on the target lesion (thyroid nodule) in the transaxial PET images. The maximum standard uptake value (SUV) was calculated by using the following formula:
For each PET dataset, the SUVmax was defined as the highest SUV within a thyroid nodule. A thyroid nodule with SUVmax <5 was considered negative for malignancy; while a thyroid nodule with SUVmax ≥5 was considered suspicious for malignancy.
RFA procedure
RFA was performed under real-time US guidance using the moving shot technique in an outpatient setting for all 22 patients. Each patient was put in a supine position with the patient’s neck extended and head tilting to the non-lesion side. All patients were treated with 2% lidocaine hydrochloride as the local anesthesia at the puncture site. Under the US guidance, an electrode was inserted into the thyroid nodule along the short axis of the nodule using the trans-isthmic approach. Prior to RFA, the nodule was conceptually divided into multiple small conceptual ablation units. The electrode tip was positioned in the deepest and the most remote portion of the nodule. Each unit was sequentially ablated by moving the electrode tip backward and forward, bottom to top. The ablation was terminated when all conceptual units of the nodule had been changed to transient hyperechoic zones. This technique is widely known as the moving shot technique [Citation10]. The active tip (5–10 mm) and appropriate energy power (15–60 W) were selected according to tumor size. All RFA procedures were carried out by one author (15 years of experience in image-guided procedures). The patients were able to tolerate the procedure on an outpatient basis, and each patient was discharged after 1 to 2 h of close observation with ice pack application and compression of the neck for 20 min.
Follow-up
The primary endpoint was defined as achieving tumor volume reduction and cosmetic/symptom scale improvement over a period of 6 to 12 months of follow-up. In addition, 6–12 months after RFA, all patients received fine needle aspiration to reevaluate the neoplasm status. The evaluations of cosmetic and symptom scores were also performed. The cosmetic score was defined as follows: (1) no palpable mass; (2) invisible but palpable mass; (3) visible when swallowing only and (4) easily visible mass. The symptom score recorded five clinical symptoms: compression, cough, difficulty swallowing, voice change, and pain. For each positive symptom, we scored one point, with symptom scores ranging from 0 to 5 [Citation13,Citation18].
The 3-dimensional measurements of each thyroid nodule were estimated on the basis of their maximal lengths in the anterior–posterior, medial–lateral, and cranial–caudal directions. On the post-ablation ultrasound examination, the changes of size, volume, and echogenicity were evaluated. The post-ablation fine needle aspiration was performed after patient consent. The remnant volume of each thyroid tumor was measured at 6–12 months after RFA (). To calculate the volume reduction ratio, the remnant thyroid tumor volume after RFA was divided by the volume prior to RFA. If the follow-up ultrasound showed a viable tumor component, any repeat RFA was performed according to the consensus of the operator and the patient. All measurements were performed by two radiologists with 10–15 years of experience in head and neck imaging. All complications were classified as major complications, minor complications, or side effects, and recorded after RFA [Citation20].
Statistical analysis
Statistical analysis was performed using SPSS 16. All data were expressed as mean ± standard deviation. Variables at the time of each patient’s enrollment and the last follow-up examination were evaluated using the Wilcoxon rank-sum test (non-parametric statistics). A p-value <0.05 was considered significant.
Results
The clinical data of patients are shown in . The SUVmax value of PET/CT RFA patient was 2.6 ± 0.9 (range: 1.4–5.0). The maximal tumor diameter and tumor volume before RFA were 3.5 ± 2.2 cm (range, 1.1–8.7 cm) and 12.6 ± 20.9 (range, 0.4–96.7 cm3), respectively. There was statistically significant volume reduction during follow-up between values before RFA and 3 months after RFA (5.5 ± 9.6 cm3), 6 months after RFA (3.5 ± 6.0 cm3), and 12 months after RFA (2.4 ± 3.0 cm3, p < 0.001). While volume reduction ratios at 3 months, 6 months, and 12 months after RFA were 49.5 ± 21.0%, 65.4% ± 24.2%, and 73.7% ± 17.7%, respectively. In addition, the mean cosmetic (2.3 ± 1.3 → 1.3 ± 0.6, p = 0.001) and symptom (1.0 ± 1.3 → 0.2 ± 0.4, p = 0.002) scores also demonstrated significant improvements from the pre-RFA evaluations to 6–12 months after RFA.
A total of 26 RFA sessions were performed in 22 patients. All thyroid follicular neoplasm ablation procedures were uneventful, with no periprocedural complications noted. However, one patient had a postprocedural minor complication. The patient presented with vocal cord palsy and recovered within 3 months post RFA. No post-procedural hypothyroidism occurred in the RFA patients. Thus, the incidence rate of complications was 4.5% (1/22). Patients received post-ablation fine needle aspiration within 6–12 months, with every cytology in accordance with Bethesda category I. There was no incident of local tumor recurrence, lymph node metastasis or distant metastasis, and none of the patients underwent surgery during the follow-up period.
For the 6 patients with high SUV follicular neoplasms who underwent thyroidectomy, the pathology report showed two Hurthle cell carcinomas, two follicular carcinomas, one Hurthle cell adenoma, and one benign tumor (surgery at other hospitals).
Discussion
We herein demonstrate that RFA can be a safe and feasible treatment for low-risk follicular neoplasm (SUVmax value < 5) in a 1-year follow-up period. None of the patients presented local tumor recurrence or lymph node metastasis. The patients received FNA post-ablation in 6–12 months, with each cytology showing Bethesda category I. Among the patients in this study, RFA achieved effective volume reduction, with cosmetic and symptom recovery. The complication incidence rate was 4.5% (1/22, right vocal cord palsy, recovery within 3 months after ablation), while no patients presented with hypothyroidism. The results of this study are comparable with ablation for thyroid nodules findings in previous reports [Citation10,Citation21].
Many studies have identified a high negative predictive value (83–96%) of 18 F-FDG PET/CT to rule out malignant thyroid nodules [Citation22–25]. Superior diagnostic performance values of 18 F-FDG PET/CT are expected in patients presenting thyroid nodules with a diameter of >1 cm [Citation19,Citation22,Citation24,Citation25]. However, insufficient data exist to perform a subgroup analysis that excludes thyroid nodules with a diameter of <1 cm [Citation19]. In recent years, PET/CT in combination with ultrasonography to discriminate benign from malignant cytologically indeterminate thyroid nodules has been introduced to avoid unnecessary diagnostic lobectomies, resulting in lower costs incurred by healthcare systems and a reported increase in health-related quality of life [Citation19,Citation26].
Furthermore, low SUVmax values in PET/CT (a cutoff value ranging from 2 to 5) have a low reported risk for thyroid cancers [Citation19,Citation23]. In our study, only two patients with high SUV follicular neoplasm underwent thyroidectomy (false positive: 23%, 2/6) with benign pathologies, which is consistent with previous studies (0–39%) [Citation8,Citation23]. One of the benign pathologies was Hurthle cell adenoma, with an SUVmax value of 18.9. Several studies have reported that the hypermetabolic Hurthle cell adenoma could show a high SUVmax value in PET/CT. This phenomenon may have led to the false-positive result of follicular neoplasm in our surgical group [Citation2,Citation5,Citation23].
According to the current guidelines of the American Thyroid Association (ATA), surgery is the standard treatment for follicular neoplasm [Citation8]. The advantages of surgery are confirmation of tumor pathology and resolution of symptoms. However, the use of general anesthesia and the risk of complications, such as permanent hypothyroidism, thyroid hormone dependence, permanent recurrent laryngeal nerve paralysis, hypertrophic or keloid scarring [Citation27,Citation28] are major concerns that may affect patients’ quality of life.
Of note, up to 70–80% of thyroid nodules diagnosed as a follicular neoplasm turn out to be a benign pathology [Citation29,Citation30]. Many studies have attempted to determine predictive factors, including male gender, and a nodule size of >4 cm; however, controversy over the accurate identification of malignancy remains [Citation1,Citation30–33].
Minimally invasive RFA is not only effective treatment for benign thyroid nodules [Citation10] but also a safe option for primary [Citation34] and recurrent [Citation11] thyroid cancers, especially in patients unsuitable for surgery. One 5-year follow-up study notably suggests that RFA could be an effective and safe treatment for patients with small (<2 cm) follicular neoplasm who refuse surgery [Citation6].
However, current RFA guidelines from the ATA [Citation8] and Korea [Citation18] do not recommend RFA for the treatment of follicular neoplasm, citing no evidence of any treatment benefit from RFA. One study has reported on RFA in six patients with follicular neoplasms and found recurrence in two patients with nodule volume >20 ml. After surgery, the pathology showed minimally invasive follicular carcinoma and follicular neoplasm of indeterminate malignant behavior [Citation35]. The incidence of malignancy in follicular neoplasm is reportedly higher with a nodule size >4 cm [Citation33,Citation36], with larger nodules possibly at risk of incomplete ablation, resulting in recurrence.
In our study, we found that a thyroid nodule of >20ml or size of >4cm may not be an accurate predictor for tumor malignancy, as 2 out of 4 (50%) malignant follicular neoplasms did not meet these criteria. Both of those tumors demonstrated high SUV values (6.4, 6.9), and indicated a high possibility of malignancy by PET/CT. Studies have also suggested that tumor volume and size are not accurate factors to distinguish malignant tumors, thus PET/CT could be a valuable tool in this regard [Citation23].
Current studies of the indication value of minimally invasive treatments such as RFA for follicular neoplasm are insufficient. Studies have suggested that a follicular neoplasm can be managed by close follow-up and repeated FNA if patients are willing to accept a relatively low risk of cancer [Citation3]. However, by using PET/CT, clinicians may more accurately distinguish high-risk follicular neoplasms and consequently recommend surgical intervention [Citation19,Citation23,Citation37]. Meanwhile, for low-risk follicular neoplasms on PET/CT, RFA may offer a safe and feasible treatment option for patients unsuitable or unwilling to undergo surgery.
The main limitation of this study is the small number of cases observed within the 1-year follow-up period. In addition, this was a retrospective study in which uncontrolled bias could have been introduced. Furthermore, there was non-uniformity in the patient follow-up intervals and short-term follow-up after RFA. Therefore, the risk of recurrence or the requirement for additional RFA sessions must be considered with nodules of a larger volume, while careful pre-procedural evaluations and long-term follow-ups are required to eliminate the possibility of metastasis.
Acknowledgments
The authors wish to thank all subjects who participated in this study. The authors would also like to thank their colleagues at Kaohsiung Chang Gung Memorial Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. The raw data are available from the corresponding author, upon reasonable request.
References
- Baloch ZW, Fleisher S, LiVolsi VA, et al. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26(1):41–44.
- Ahmadi S, Stang M, Jiang X, et al. Hürthle cell carcinoma: current perspectives. OncoTargets Ther. 2016;9:6873–6884.
- Gulcelik NE, Gulcelik MA, Kuru B. Risk of malignancy in patients with follicular neoplasm: predictive value of clinical and ultrasonographic features. Arch Otolaryngol Head Neck Surg. 2008;134(12):1312–1315.
- Cibas ES, Ali SZ. The 2017 Bethesda System for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346.
- Shawky M, Sakr M. Hurthle cell lesion: controversies, challenges, and debates. Indian J Surg. 2016;78(1):41–48.
- Ha SM, Sung JY, Baek JH, et al. Radiofrequency ablation of small follicular neoplasms: initial clinical outcomes. Int J Hyperthermia. 2017;33(8):1–937.
- Chung SR, Baek JH, Choi YJ, et al. The role of core needle biopsy for the evaluation of thyroid nodules with suspicious ultrasound features. Korean J Radiol. 2019;20(1):158–165.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Yoon JH, Kwak JY, Moon HJ, et al. Ultrasonography-guided core needle biopsy did not reduce diagnostic lobectomy for thyroid nodules diagnosed as atypia of undetermined significance/follicular lesion of undetermined significance. Ultrasound Q. 2019;35(3):253–258.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Kim J-h, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label Trial (Lara Trial). Thyroid. 2020;30(6):847–856.
- Lin W-C, Kan N-N, Chen H-L, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Mauri G, Cova L, Ierace T, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016;39(7):1023–1030.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Xiao J, Zhang Y, Lan Y, et al. Ultrasonography-guided radiofrequency ablation vs. surgery for the treatment of solitary T1bN0M0 papillary thyroid carcinoma: a comparative study. Clin Endocrinol. 2021;94(4):684–691.
- Kim J-H, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Castellana M, Trimboli P, Piccardo A, et al. Performance of 18F-FDG PET/CT in selecting thyroid nodules with indeterminate fine-needle aspiration cytology for surgery. A systematic review and a meta-analysis. J Clin Med. 2019;8(9):1333.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian Minimally Invasive Treatments of the Thyroid Group. Thyroid. 2020;30(12):1759–1770.
- Merten MM, Castro MR, Zhang J, et al. Examining the role of preoperative positron emission tomography/computerized tomography in combination with ultrasonography in discriminating benign from malignant cytologically indeterminate thyroid nodules. Thyroid. 2017;27(1):95–102.
- Pathak KA, Goertzen AL, Nason RW, et al. A prospective cohort study to assess the role of FDG-PET in differentiating benign and malignant follicular neoplasms. Ann Med Surg. 2016;12:27–31.
- Piccardo A, Puntoni M, Treglia G, et al. Thyroid nodules with indeterminate cytology: prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur J Endocrinol. 2016;174(5):693–703.
- Rager O, Radojewski P, Dumont RA, et al. Radioisotope imaging for discriminating benign from malignant cytologically indeterminate thyroid nodules. Gland Surg. 2019;8(2):S118–S125.
- Vriens D, Adang EMM, Netea-Maier RT, et al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodules: a decision analytic approach. J Clin Endocrinol Metab. 2014;99(9):3263–3274.
- Antunes CM, Taveira-Gomes A. Lobectomy in follicular thyroid neoplasms’ treatment. Int J Surg. 2013;11(9):919–922.
- Soni N, Gedam B, Akhtar M. Thyroidectomy: post-operative complications and management. Int Surg J. 2019;6(5):1659.
- Mihai R, Parker AJC, Roskell D, et al. One in four patients with follicular thyroid cytology (THY3) has a thyroid carcinoma. Thyroid. 2009;19(1):33–37.
- Trimboli P, Treglia G, Guidobaldi L, et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: a meta-analysis. Endocrine. 2014;46(1):52–59.
- Lee KH, Shin JH, Ko ES, et al. Predictive factors of malignancy in patients with cytologically suspicious for Hurthle cell neoplasm of thyroid nodules. Int J Surg. 2013;11(9):898–902.
- Kuo T-C, Wu M-H, Chen K-Y, et al. Ultrasonographic features for differentiating follicular thyroid carcinoma and follicular adenoma. Asian J Surg. 2020;43(1):339–346.
- Paramo J, Mesko T. Age, tumor size, and in-office ultrasonography are predictive parameters of malignancy in follicular neoplasms of the thyroid. Endocrine Pract. 2008;14(4):447–451.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Dobrinja C, Bernardi S, Fabris B, et al. Surgical and pathological changes after radiofrequency ablation of thyroid nodules. Int J Endocrinol. 2015. 2015;2015:576576.
- Zoric G, Paunovic I, Diklić A, et al. Analysis of malignancy predictors for follicular thyroid tumors. Vojnosanitetski Pregled. 2018;77:79.
- Munoz Perez N, Villar del Moral JM, Muros Fuentes MA, et al. Could 18F-FDG-PET/CT avoid unnecessary thyroidectomies in patients with cytological diagnosis of follicular neoplasm? Langenbecks Arch Surg. 2013;398(5):709–716.