Abstract
Objectives
To explore the outcomes of CT-guided percutaneous microwave ablation (MWA) in non-small cell lung cancer (NSCLC) patients, and then develop an effective nomogram to predict the survival.
Methods
NSCLC patients treated with MWA were randomly allocated to either the training cohort or the validation cohort (3:1). The primary outcome measurement was overall survival (OS), whose predictors were identified by univariate and multivariate analyses in the training cohort. Then, a predictive nomogram was developed to predict the OS, with the predictive accuracy evaluated by C-statistic and receiver operating characteristic in both the training and validation cohorts.
Results
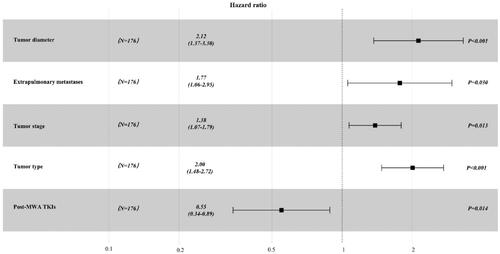
A total of 234 patients (training cohort: n = 176; validation cohort: n = 58) and 271 tumors with a median OS of 17.0 ± 12.2 months were included. The predictors selected into the nomogram included tumor diameter (hazard ratio [HR], 2.12; 95% confidence interval [CI], 1.37-3.30; p < 0.001), extrapulmonary metastases (HR, 1.77; 95% CI, 1.06–2.95; p = 0.030), tumor stage (HR, 1.38; 95% CI, 1.07–1.79; p = 0.013), tumor type (HR, 2.00; 95% CI, 1.48–2.72; p < 0.001) and post-MWA TKIs (HR, 0.55; 95% CI, 0.34–0.89; p < 0.001), based on the results of univariate and multivariate analyses. The C-statistic showed good predictive performance, with a C-statistic of 0.838 (95% CI, 0.779–0.897) internally and 0.808 (95% CI, 0.695–0.920) externally (training cohort and validation cohort, respectively).
Conclusions
The nomogram was effective in predicting the OS in NSCLC patients treated with MWA, and could be applied to identify patients who may benefit most from MWA and be helpful for clinical decision making.
Introduction
Primary lung cancer (PLC) has been reported as the leading cause of cancer incidence and mortality worldwide [Citation1], and non-small cell lung cancer (NSCLC) accounted for 85% of the PLC cases [Citation2]. However, over two-thirds of NSCLC patients were diagnosed at an advanced stage and no longer being best candidates for curative surgery [Citation3]. The overall prognosis of NSCLC remains dismal, with the 5-year survival rate of 16% as reported [Citation4]. In recent decades, thermal ablation has been reported as a primary therapeutic strategy or/and an adjuvant to other treatments for NSCLC, or for the early-stage patients with limited pulmonary reserve who cannot tolerate the surgery [Citation5–7], with a high technical success rate up to 96% according to a meta-analysis [Citation8]. Of these, radiofrequency ablation (RFA) and microwave ablation (MWA) are the two most common types, while the latter has the advantages of a higher intra-tumoral temperatures, a larger ablation scope, decreased ablation duration, and deeper penetration [Citation9].
Although the best candidates for thermal ablation are stage I NSCLC patients who have contraindications to surgery or stereotactic radiotherapy as recommended [Citation10,Citation11], many studies attempted thermal ablation as a salvage therapy or a part of combination therapeutic strategy with other treatments for intermediate and advanced stage patients, which could significantly improve the overall survival (OS) than monotherapy, such as chemotherapy or/and tyrosine kinase inhibitors (TKIs) [Citation12–14]. It was revealed that the OS for NSCLC patients treated with thermal ablation has a wide range of 10.6–71.6 months [Citation15,Citation16]. Although previous studies have reported the predictors of OS after thermal ablation [Citation17–19], these predictors have been investigated generally across different stage and response status to previous therapies. Unfortunately, the ability of these predictors in predicting survival in NSCLC patients treated with MWA is limited and patients with different characteristics who may benefit most from MWA are still ambiguous. A pragmatic and reliable predictive model based on objective measurements is needed for providing information to predict the survival, select target patients and stratify risk. However, as far as we know, no such tool is yet available.
Therefore, a retrospective study was conducted to explore the outcomes of MWA in NSCLC patients, and then develop an effective nomogram to predict the OS.
Methods
Patient criteria
This single center retrospective study included all consecutive PLC patients who received MWA in this institution. The institutional ethics review board approved this study. The study protocol was conducted in accordance with the Declaration of Helsinki. The informed consent was waived owing to the retrospective nature of this study. Confirmed or suspected NSCLC patients treated with MWA between September 2016 and September 2019 at this institution were screened for this study, and were randomly allocated to either the training cohort or the validation cohort at a ratio of 3:1. Randomization allocation was created using the Stata 9.0 (StataCorp., College Station, TX) statistical software. The inclusion criteria for this study were as follows: (a) age older than 18 years; (b) confirmed or suspected NSCLC patients treated with MWA; (c) previous or current histopathological examinations confirming the diagnosis of NSCLC; (d) early-stage patients who cannot tolerate or refuse the surgery receive the MWA, and adequate consent to receive MWA instead of other therapy is underwent; and (e) Eastern Cooperation Oncology Group (ECOG) score of 0–2. The exclusion criteria were as follows: (a) small cell lung cancer patients treated with MWA; (b) without previous and current histopathological examinations; (c) other concomitant therapies performed during the MWA procedure, such as the radioactive seeds implantation; and (d) incomplete data.
The NSCLC tumor stage was identified via the clinical TNM staging system of the Union for International Cancer Control (8th edition) [Citation20]. All NSCLC patients underwent chest CT (GE Healthcare) before the MWA procedure to evaluate the location, quantity and size of tumors. PET/CT or contrast-enhanced CT was performed to evaluate the lymph node and distant metastases. All laboratory examinations were conducted 1–4 days before MWA.
Assessments
The primary outcome measurement was OS, which was defined as the interval from the start of MWA to death or the last follow-up (30 November 2020). For patients who had mortality in the follow-up, OS was calculated as the interval from the MWA procedure to the death. For patients who had survival or lost to follow-up, OS was calculated as the interval from the MWA procedure to the last follow-up. The secondary outcome measurement was progression-free survival (PFS). PFS was defined as the interval from the MWA procedures to the time of objective progression, including local progression or/and extrapulmonary progression, which was evaluated by two independent interventional radiologists. In patients who did not die or progress, the censoring date was defined as the last clinical assessment date. Other secondary outcome measurement was the complications of MWA, which were classified as side effects, intraprocedural minor complications, intraprocedural major complications, delayed major complications and mortality according to the criteria of Cardiovascular and Interventional Radiology Society of Europe (CIRSE) [Citation21].
MWA procedure and peri-MWA management
The MWA indications and procedures followed the standards of the CIRSE [Citation21] and were performed by several experienced interventional radiologists (S.X. and B.L. with >5 years of experience, and X.-G. L with >20 years of experience). An MTC-3C MWA system (Vison Medicine) or an ECO-100A1 MWA system (ECO Medical Instrument) were used, with a microwave emission frequency of 2450 ± 50 MHz and an adjustable continuous wave output power of 20–80 W. The MWA needles (Vison Medicine or ECO Medical Instrument) were 10–18 cm in effective length and 15–17 G in outside diameter, with a 15 mm active tip. Pre-procedural CT was performed to inform the treatment plan to clarify the suitable position, puncture sites location, optimal puncture trajectory and number of MWA needles. Local anesthesia or intravenous anesthesia were performed as needed. Needles were introduced into the planned site, and the position was confirmed by CT. Subsequently, MWA was performed at the planed power and duration, with adjustments being carried out as needed. The procedure was terminated when the ablation zone presented a 5–10mm rim beyond the lesion boundary. The MWA parameters were selected according to the productive ablation zone recommended by MWA systems. Finally, a repeat chest CT scan was performed to evaluate the ablation zone and detect possible complications. For patients without a histopathological examination, a synchronous coaxial-cannula biopsy was performed if necessary. A 15 G coaxial introducer needle (Argon Medical Devices) was first advanced into the tumor, and then the stylet was replaced by a 16 G full‑core biopsy needle (Argon Medical Devices) through the cannula. The biopsy was performed for histopathological examination. A 17 G MWA needle was introduced into the tumor through the cannula [Citation22]. Short-term follow-up with CT reexaminations was conducted 1–5 days after MWA during hospitalization and 3–4 weeks after MWA at an outpatient visit to detect delayed complications, including pneumothorax and pleural effusion. Intercostal tube placement was performed for patients with moderate and severe pneumothorax or pleural effusion. Long-term follow-up with CT reexaminations was conducted every 1–4 months after MWA.
For patients with epidermal growth factor receptor (EGFR) mutants according to the specimen genetic testing, the TKIs were orally administrated before or after MWA procedures, including erlotinib, gefitinib, icotinib, oxitinib and afatinib. For patients with anaplastic lymphoma kinase (ALK) mutants, the TKIs were orally administrated, including crizotinib, lorlatinib and aletinib. For advanced stage patients, the subsequent chemotherapy was performed on demand for patients with ECOG score below 2.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 for Windows (IBM, Somers, NY. USA). Categorical variables are described as frequencies and percentages, and continuous variables are described as the median/mean ± SDs. The data of the two cohorts were compared by Student’s t-test or Mann–Whitney U tests for continuous variables and by Chi-square tests for categorical variables. The OS were estimated using the Kaplan–Meier method. The potential predictors for OS were analyzed by the univariate analyses in the training cohort, including 19 parameters on demographics, treatment history, ablation factors and radiological features. Variables with p < 0.05 in the univariate analyses were entered as candidate variables into the multivariate Cox regression analyses. Model discrimination was assessed using C-statistic. A p < 0.05 was considered to indicate statistical significance in the multivariate analyses. A forest plot was constructed using R (Version 3.0.2; R Project for Statistical Computing; www.rproject.org) to present the results of multivariate analyses.
As described previously [Citation23], the establishment of the nomogram was based on the results of multivariable Cox regression analyses of the training cohort. The nomogram was constructed with the regression modeling strategies package in R package. After establishing the predictive nomogram, C-statistics and the receiver operating characteristic (ROC) curve were used to validate the accuracy and discriminative capacity of the nomogram both internally (training cohort) and externally (validation cohort). The C-statistic was calculated as the area under the ROC curve and used to evaluate the predictive ability of the risk model. The larger the C-statistic is, the more accurate the prognostic prediction is. In addition, the performance of the nomogram was assessed by comparing nomogram-predicted vs. observed Kaplan–Meier estimates of survival probability. The associated scores of each predictor were calculated based on the regression coefficients of the Cox proportional hazards model. The relationship between the overall scores and median OS was calculated using the Kaplan–Meier method. The resulting nomogram was the quantification of the Cox proportional hazards model.
Results
Patient characteristics and clinical outcomes
A total of 234 NSCLC patients (176 in the training cohort and 58 in the validation cohort; ) with 271 tumors received MWA in a mean follow-up of 30.5 ± 11.2 months were included. A total of 41 patients (17.5%) received previous chemotherapy; of these, 22 patients (9.4%) presented with resistance to chemotherapy and received no further post-MWA chemotherapy. The local recurrence rate for NSCLC patients treated with MWA was 24.4% (57/234). There were 195 patients (83.3%) and 39 patients (16.7%) received local anesthesia and intravenous anesthesia, respectively. Detailed demographic characteristics are presented in . Meanwhile, the detailed information of TKIs for NSCLC patients is presented in . Of these, 13 patients (5.6%) received at least two kinds of TKIs before MWA and 27 patients (11.5%) received at least two kinds of TKIs after MWA.
Figure 1. Patient selection flowchart. PLC: primary lung cancer; MWA: microwave ablation; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer.
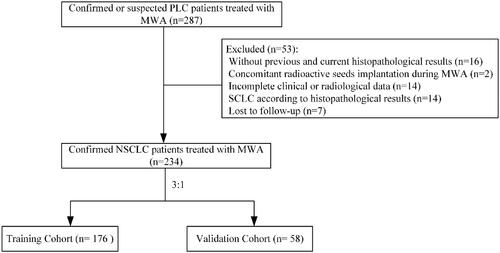
Table 1. Clinical characteristics of NSCLC patients treated with MWA.
Table 2. The detailed kinds of TKIs for NSCLC patients treated with MWA.
The median OS and PFS of the entire cohort were 17.0 ± 12.2 months and 12.5 ± 12.2 months, respectively. Moreover, the incidence rate of complications related to MWA procedures was 23.9% and the details were presented in . Of these, 34 patients (14.5) received intercostal tube placement owing to pneumothorax or/and pleural effusion, including two patients with concomitant pneumothorax and pleural effusion. The tubes were removed when the pneumothorax or/and pleural effusion disappeared and all the patients recovered.
Table 3. Complications of NSCLC patients treated with MWA.
Predictors of OS in the training cohort
The results of univariable and multivariable Cox regression analyses for OS are presented in and , respectively. The predictors of OS in NSCLC patients treated with MWA are tumor diameter (hazard ratio [HR], 2.12; 95% confidence interval [CI], 1.37–3.30; p < 0.001), extrapulmonary metastases (HR, 1.77; 95% CI, 1.06–2.95; p = 0.030), tumor stage (HR, 1.38; 95% CI, 1.07–1.79; p = 0.013), tumor type (HR, 2.00; 95% CI, 1.48–2.72; p < 0.001) and post-MWA TKIs (HR, 0.55; 95% CI, 0.34–0.89; p < 0.001). The Kaplan–Meier analyses of OS are presented in .
Figure 3. Kaplan–Meier analyses of OS. (a) The median OS were 41.0 months for patients with adenocarcinoma, 12.0 months for patients with squamous cell carcinoma, and 8.0 months for patients with other types. (b) The median OS were 39.0 months for patients with Stage I, 24.0 months for patients with Stage II, 21.0 months for patients with Stage III and 15.0 months for patients with Stage IV. (c). The median OS was 35.0 months for patients with <3 cm in diameter compared with 16.0 months for patients with ≥3cm in diameter. (d) The median OS was 36.0 months for patients with post-MWA TKIs compared with 17.0 months than those patients without. (e) The median OS was 13.0 months for patients with extrapulmonary metastases compared with 36.0 months than those patients without.
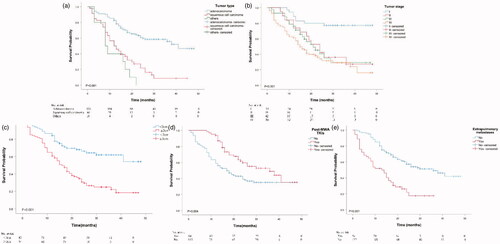
Table 4. Univariate analyses for OS of NSCLC patients treated with MWA in the training cohort.
Development and validation of the nomogram
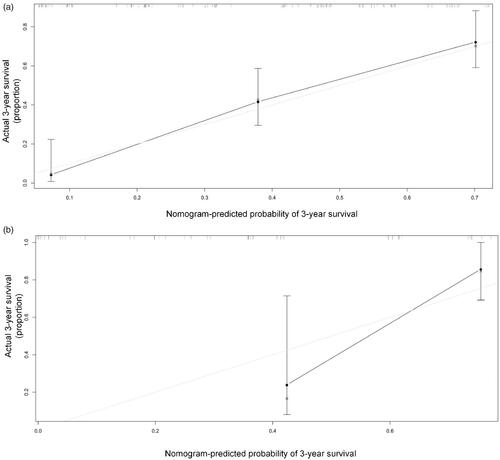
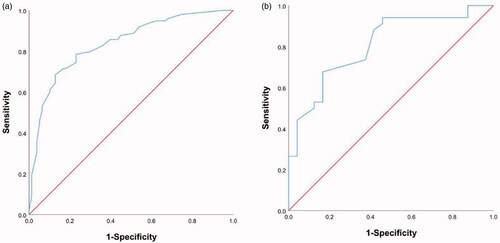
Based on the above five predictors, a nomogram was developed to make prediction of OS after MWA (). With this nomogram, each patient received a total number of points according to the five predictors, to accurately predict the outcome. The median scores predicted by the nomogram was determined as the cutoff for stratifying the patients in the entire cohort into two groups. The group with scores higher than the median score was considered the high-risk group, and the other group was considered the low-risk group. The patients in the low-risk group had better OS than the high-risk group (). According to the internal validation in the training cohort, C-statistic of this nomogram was 0.838 (95% CI, 0.779–0.897; ), while the index of the external validation in the validation cohort was 0.808 (95% CI, 0.695–0.920; ). Both the internal validation and external validation showed good predictive performance. The calibration curve showed a good agreement between the prediction and observation in the probability of 3-year survival in the training and validation cohorts ().
Figure 4. (a). Prognostic nomogram for predicting OS in NSCLC patients treated with MWA. To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Point axis, and a line is drawn downward to the risk axes to determine the likelihood of median OS. The linear predictor represented the product of the independent variable and correlation coefficient. (b) OS of low-risk and high-risk group in the entire cohort.
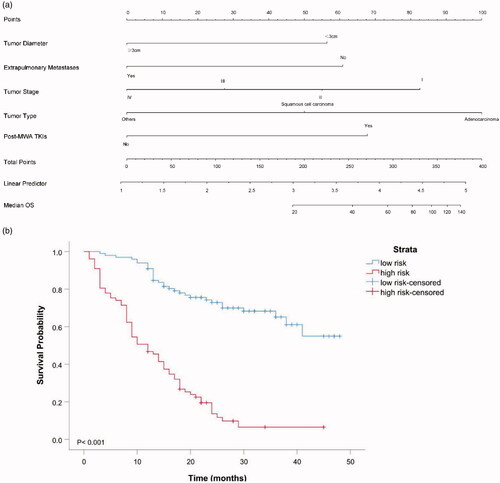
Figure 5. Receiver operating characteristic curve analyses for the nomograms in internal and external validation. The diagonal red line indicates that the index of AUC was 0.5, which was a reference value. C-statistic was calculated as the area between the ROC curve (blue curve) and horizontal axis. (a) The area under the curve (C-statistic) was 0.838 (95% CI, 0.779-0.897) in the training cohort for predicting OS. (b) The area under the curve (C-statistic) was 0.808 (95% CI, 0.695–0.920) in the validation cohort for predicting OS.
Discussion
Thermal ablation is an effective therapeutic strategy for NSCLC patients, which had aimed to induce a zone of thermal coagulative necrosis encompassing the whole tumor and a surrounding safety margin, with 3- and 5-year survival rates of 36–88% and 25–61%, respectively [Citation24,Citation25]. In a randomized controlled trail, Macchi et al. [Citation26] compared RFA with MWA for NSCLC patients, and indicated that MWA can produce less intraprocedural complications but the equal efficacy in survival time.
This study showed that tumor stage is the one of predictors for OS, with the median OS for stage I, II, III and IV are 39.0, 24.0, 21.0 and 15.0 months, respectively. For stage I NSCLC, surgery accompanied with lobectomy and lymph node resection remains the first line treatment [Citation27], with 5-year survival rates for stage I A and I B NSCLC approaching 71–77% and 35–58%, respectively [Citation28]. Radiotherapy and MWA were proven to be effective for unresectable NSCLC patients, especially for those patients with poor cardiopulmonary function and advanced age [Citation29]. In 2014, Yang et al. [Citation30] attempted MWA for 47 unresectable stage I NSCLC patients, and the median OS approached 33.8 months, with the survival rates at 1, 2, 3, and 5 years after MWA were 89, 63, 43, and 16%, respectively. Meanwhile, another study showed the 3-year survival rate exceed 50% for unresectable stage I NSCLC patients treated with thermal ablation [Citation31]. The MWA indicated the similar therapeutic effect with lobectomy, with the 1, 3 and 5-year OS rate of MWA were 100, 92.6, and 50.0% while that OS rate of lobectomy were 100, 90.7, and 46.3%, respectively [Citation15]. Moreover, Ager et al. [Citation32] compared the OS between radiotherapy and ablation by the National Cancer Database of America, and revealed that no OS difference is found in patients with tumor sizes ≤2.0 cm in a median follow-up of 26.2 months despite radiotherapy is associated with the improved OS. However, no significant difference in OS was seen between early-stage NSCLC patients treated with RFA and radiotherapy from another population-based study [Citation33]. For advanced stage NSCLC patients, platinum-based, doublet chemotherapy is the first line treatment option, with the OS ranged from 7.9 to 10.3 months and PFS ranged from 3.6 to 4.8 months [Citation34]. As a supplemental treatment for NSCLC patients treated with chemotherapy, the administration of RFA approached an OS of 14 months [Citation35]. In 2020, a multicenter prospective study showed a longer PFS and OS for advanced NSCLC patients treated with combination therapy of MWA and chemotherapy than chemotherapy alone, with the median OS was not reached in the MWA plus chemotherapy group and 12.6 months in the chemotherapy group [Citation7]. In our study, a total of 136 patients were at advanced stage. Of these, 67 patients received the combination therapy of MWA and chemotherapy; 22 patients with resistance to chemotherapy have received MWA. Even though, whether the MWA is a salvage therapy for those patients needs further investigation.
The patients with tumor <3 cm in diameter were the best candidate for thermal ablation, as recommended by the American College of Chest Physicians [Citation11]. For the early-stage NSCLC, the 2 years survival rate of MWA was improved to 83% in patients with tumor size <2 cm [Citation36]. Vogl et al. [Citation37] found that the patients with tumors larger than 3 cm in diameter have worse outcomes than those smaller than 3 cm. On the contrary, Dupuy et al. [Citation38] indicated that there is no correlation between tumor size and survival when using RFA. In this study, the median OS of NSCLC patients with tumor <3 cm in diameter was significantly longer than that of tumor ≥3 cm. The potential mechanism was the limited size and homogeneity of tumor necrosis achieved by MWA, and the effects of the underlying tumor biology, thereby often resulting in the occurrence of incomplete ablation for the patients with large size in diameter, and increase the risk of remnant or recurrence. Adenocarcinoma is the most common histologic subtype in this study, with the median OS of 41.0 months, which was significant longer than that of squamous cell carcinoma. A potential explain was the more available treatment options for adenocarcinoma than other subtypes, including the combination therapy or monotherapy of chemotherapy, immunotherapy and TKIs.
EGFR-TKIs remain the standard therapy for advanced NSCLC patients with EGFR-sensitive mutations and show a dramatic improvement in PFS and OS [Citation39]. In one study consisted of 15 NSCLC patients with EGFR-mutant, 11 patients achieved complete elimination after MWA. The median PFS and OS were 9.5 months and 23 months despite the sample size was limited, which indicated the continuation of EGFR-TKI beyond focal progression associated to microwave ablation is an efficacious therapeutic strategy to achieve a longer disease control [Citation14]. However, Wei et al. [Citation40] attempted the combination therapy of EGFR-TKIs and MWA for 34 NSCLC patients at advanced stage, which failed to show superior survival in either PFS or OS, compared to 24 advanced NSCLC patients with monotherapy of EGFR-TKIs. As a focal consolidative therapy, MWA leads to better disease control and superior survival than TKIs monotherapy in EGFR-mutant advanced NSCLC patients with extracranial oligometastases [Citation19]. In this study, 27 patients presented with resistance to EGFR or ALK-TKIs have received MWA and present this approach as an effective salvage therapy. Although there were 8 patients with resistance to TKIs continued the regimen after MWA, a total of 98 patients received post-MWA TKIs and the median OS was significant longer than that patients without, which indicated the post-MWA TKIs was a protective predictor of OS for NSCLC patients treated with MWA.
Based on the results of univariable and multivariable analyses, a predictive nomogram was established to predict the OS in NSCLC patients treated with MWA. The data from the training cohort as internal validation and data from the validation cohort as external validation were used to validate the accuracy of this nomogram. The C-statistic values indicated the high accuracy of the nomogram. Moreover, the median scores predicted by the nomogram was determined as the cutoff for stratifying the patients of high-risk and low-risk, which can be applied to identify NSCLC patients who may benefit most from MWA, and be helpful for making decision in clinical practice.
This study has several limitations that should be noted. First, this was a retrospective study; thus, patient selection bias may exist. Second, although a validation cohort was established and showed a high accuracy for predictive performance, external validation from other datasets is warranted. Third, the mean follow-up was 30.5 ± 11.2 months in this study, and more patients with exact termination of mortality in a longer follow-up are warranted. Fourth, the correlation between objective response of MWA and the OS may exist potentially, which should be investigated in a further study.
In conclusion, as a focal therapeutic approach, MWA was effective and safe for NSCLC patients. The predictors of OS in NSCLC patients treated with MWA are tumor diameter, extrapulmonary metastases, tumor stage, tumor type and post-MWA TKIs. The established nomogram was effective in predicting the OS, and could be applied to identify patients who may benefit most from MWA and be helpful for clinical decision making. However, further prospective randomized controlled trials are warranted to validate the results.
Acknowledgments
The authors thank Xin Huang, MS, for his assistance in protocol review and statistical analyses. Funding source had no involvements in the financial support for the conduct of the research and preparation of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71.
- Sun W, Song L, Ai T, et al. Prognostic value of MET, cyclin D1 and MET gene copy number in non-small cell lung cancer. J Biomed Res. 2013;27(3):220–230.
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594.
- Quirk MT, Lee S, Murali N, et al. Alternatives to surgery for early-stage non-small cell lung cancer: thermal ablation. Clin Chest Med. 2020;41(2):197–210.
- Iezzi R, Cioni R, Basile D, et al. Standardizing percutaneous Microwave Ablation in the treatment of Lung Tumors: a prospective multicenter trial (MALT study). Eur Radiol. 2021;31(4):2173–2182.
- Wei Z, Yang X, Ye X, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. Eur Radiol. 2020;30(5):2692–2702.
- Li G, Xue M, Chen W, et al. Efficacy and safety of radiofrequency ablation for lung cancers: a systematic review and meta-analysis. Eur J Radiol. 2018;100:92–98.
- Liu H, Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. 2013;57(4):466–474.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):v1–21.
- Donington J, Thoracic Oncology Network of the American College of Chest Physicians and the Workforce on Evidence-Based Surgery of the Society of Thoracic Surgeons, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142(6):1620–1635.
- Cheng M, Fay M, Steinke K. Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: a retrospective study. Int J Hyperthermia. 2016;32(3):316–323.
- Ni Y, Bi J, Ye X, et al. Local microwave ablation with continued EGFR tyrosine kinase inhibitor as a treatment strategy in advanced non-small cell lung cancers that developed extra-central nervous system oligoprogressive disease during EGFR tyrosine kinase inhibitor treatment: a pilot study. Medicine. 2016;95(25):e3998.
- Li X, Qi H, Qing G, et al. Microwave ablation with continued EGFR tyrosine kinase inhibitor therapy prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Thorac Cancer. 2018;9(8):1012–1017.
- Yao W, Lu M, Fan W, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia. 2018;34(8):1329–1336.
- Ni X, Han JQ, Ye X, et al. Percutaneous CT-guided microwave ablation as maintenance after first-line treatment for patients with advanced NSCLC. Onco Targets Ther. 2015;8:3227–3235.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32(2):464.
- Wei Z, Ye X, Yang X, et al. Advanced non small cell lung cancer: response to microwave ablation and EGFR Status. Eur Radiol. 2017;27(4):1685–1694.
- Ni Y, Ye X, Yang X, et al. Microwave ablation as local consolidative therapy for patients with extracranial oligometastatic EGFR-mutant non-small cell lung cancer without progression after first-line EGFR-TKIs treatment. J Cancer Res Clin Oncol. 2020;146(1):197–203.
- Lim W, Ridge CA, Nicholson AG, et al. The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg. 2018;8(7):709–718.
- Venturini M, Cariati M, Marra P, et al. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc Intervent Radiol. 2020;43(5):667–683.
- Wang D, Li B, Bie Z, et al. Synchronous core-needle biopsy and microwave ablation for highly suspicious malignant pulmonary nodule via a coaxial cannula. J Cancer Res Ther. 2019;15(7):1484–1489.
- Xu S, Shi BQ, Chao LM, et al. Prognostic nomogram for the combination therapy of percutaneous catheter drainage and antibiotics in pyogenic liver abscess patients. Abdom Radiol. 2020;45(2):393–402.
- Hiraki T, Gobara H, Iguchi T, et al. Radiofrequency ablation for early-stage non-small cell lung cancer. Biomed Res Int. 2014;2014:152087.
- Palussiere J, Lagarde P, Auperin A, et al. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years' experience in 87 patients from two centers. Cardiovasc Intervent Radiol. 2015;38(1):160–166.
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34(5):96.
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2(7):593–602.
- Goldstraw P, Participating Institutions, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714.
- Videtic GM, Paulus R, Singh AK, et al. Long-term follow-up on NRG oncology RTOG 0915 (NCCTG N0927): a randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103(5):1077–1084.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110(6):758–763.
- Narsule CK, Sridhar P, Nair D, et al. Percutaneous thermal ablation for stage IA non-small cell lung cancer: long-term follow-up. J Thorac Dis. 2017;9(10):4039–4045.
- Ager BJ, Wells SM, Gruhl JD, et al. Stereotactic body radiotherapy versus percutaneous local tumor ablation for early-stage non-small cell lung cancer. Lung Cancer. 2019;138:6–12.
- Lam A, Yoshida EJ, Bui K, et al. A National Cancer Database Analysis of radiofrequency ablation versus stereotactic body radiotherapy in early-stage non-small cell lung cancer. J Vasc Interv Radiol. 2018;29(9):1211–1217.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551.
- Li X, Zhao M, Wang J, et al. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancers. AJR Am J Roentgenol. 2013;201(6):1362–1367.
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer-Am Cancer Soc. 2015;121(19):3491–3498.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261(2):643–651.
- Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue-is microwave not just for popcorn anymore? Radiology. 2009;251(3):617–618.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128.
- Wei Z, Ye X, Yang X, et al. Microwave ablation combined with EGFR-TKIs versus only EGFR-TKIs in advanced NSCLC patients with EGFR-sensitive mutations. Oncotarget. 2017;8(34):56714–56725.