Abstract
Objective
To retrospectively evaluate early clinical outcomes of percutaneous microwave ablation (MWA) for stage T1a renal cell carcinomas (RCCs) in solitary kidney patients.
Materials and methods
15 solitary kidney patients with 16 stage T1a N0M0 biopsy-proved RCCs underwent CT-guided percutaneous microwave ablation between October 2016 and July 2020. The patients were followed up with contrast-enhanced computed tomography or magnetic resonance imaging at 1, 3, and 6 months and every 6 months thereafter. Serum creatinine levels of each patient pre MWA, 1 day after MWA and the most recent record were collected. Technical effectiveness, local recurrence, survival rates and complications were accessed.
Results
Complete ablation was achieved in all 16 tumors (100%) including 13 clear cell carcinomas and 3 papillary carcinomas. Within the follow-up time (median: 24 months) no tumor recurrence or major complication was detected. No significant change in serum creatinine level was noted. The cancer-specific survival rate was 100% (15 of 15), and 1-, 2-, and 3-year overall survival rates were 100%, 93.3%, and 93.3%, respectively.
Conclusion
Percutaneous MWA is an effective and safe treatment option for stage T1a RCCs in solitary kidney patients; it can achieve high complete ablation rate in selected lesions of appropriate size and location.
Introduction
In patients with a history of nephrectomy for renal cell carcinoma (RCC), the contralateral kidney is more likely to develop a new carcinoma during follow-up examinations than in previously healthy patients [Citation1]. In this situation, the standard therapy should be partial nephrectomy or nephron-sparing surgery [Citation2]. However, surgical resection may cause a higher risk of deterioration of kidney function or even renal failure, which may require long-term hemodialysis or kidney transplantation. Furthermore, some patients may refuse surgical treatment or may be poor surgical candidates owning to comorbid conditions. The surgeon and patients should carefully choose the optimal treatment method for renal tumor in this subgroup of patients.
The American Society of Clinical Oncology clinical practice guidelines recommend percutaneous thermal ablation as a treatment option for small renal masses if complete ablation can be reliably achieved [Citation3]. To reduce procedural complications and maintain equivalent oncologic control effectiveness, various thermal ablation techniques have been applied as alternatives for the treatment of RCCs [Citation4], including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation. The local tumor control rate of percutaneous cryoablation was 92% in the management of renal tumors in patients with a solitary kidney, causing minimal loss of renal function [Citation5]. RFA can achieve excellent midterm outcomes that are equivalent to those of open partial nephrectomy [Citation6], and can also serve as an effective and safe alternative treatment for solitary kidney tumors [Citation7,Citation8]. However, publications on MWA in a solitary kidney are limited.
During the past decade, high-power microwave systems have been developed that offer potential advantages over older systems. MWA, such as RFA, is a heat based thermal ablation modality with an identical mechanism of cell death; however, MWA has several theoretical advantages over RFA in producing consistently higher intra-tumoral temperatures, larger ablation volumes, less ablation time, less dependence on the electrical conductivities of tissue, larger ablation zones, and shorter procedural times [Citation9,Citation10]. Percutaneous MWA is an effective and safe treatment option for stage T1a RCCs [Citation11], loco-regional relapse of RCC [Citation12], or metastatic kidney tumors [Citation13]. MWA is also a relatively safe treatment option for patients with benign renal tumor [Citation14].
In this study, we retrospectively reviewed the technical success, midterm oncologic efficacy, and rate of complications in patients with stage T1a RCCs in a solitary kidney treated with MWA under computed tomography (CT) guidance.
Materials and methods
Patients
The retrospective study protocol was approved by local Institutional Review Board. Between October 2016 and July 2020, 15 patients (11 men, four women; mean age: 58.85 years; range: 46–74 years) with 16 biopsy-proved clinical stage T1a (≤ 4 cm) RCCs were treated with percutaneous MWA under CT guidance. Written informed consent for MWA treatment was obtained from each patient. The decision to perform MWA was determined by multidisciplinary tumor board. All patients had a solitary kidney owning to a previous radical nephrectomy for RCC. The characteristics of tumor, such as diameter, were evaluated under contrast-enhanced CT or magnetic resonance imaging (MRI). The inclusion criteria were as follows: RCC lesions <4.0 cm in size; prothrombin coagulation time of <25 s; prothrombin activity higher than 40%; platelet count >40 × 109/L; the absence of renal vein thrombosis and distant metastases beyond the kidney; and presence of an appropriate route for percutaneous puncture. Both exophytic and intraparenchymal tumors were treated.
CT-guided MWA procedure
All MWA procedures were performed in a CT suite (Philips Brilliance iCT, Philips Healthcare, Cleveland, OH). An ECO-100C MWA system (ECO Microwave Electronic Institute, Nanjing, China) with 2.45 GHz emission frequency was used. The microwave antenna had a single slot, an effective length of 10–18 cm, and an outside diameter of 16 G, with a 15 mm active tip. Inside antenna shaft were dual channels through which distilled water at room temperature was pumped by a peristaltic pump preventing thermal injury along proximal antenna. The ablation power was selected as 45–60 W with 4–8 min duration. One antenna was applied for tumors less than 2.0 cm in diameter, and two antennas were used for those more than 2.0 cm in diameter with an inter-antenna distance of no more than 1.5 cm simultaneously. Local anesthetic was applied at the puncture site of the patient using 1% lidocaine and intravenous anesthesia (propofol 1.5–2 mg/kg) was used when the ablation commenced. The antenna was modified and repositioned according to imaging changes until the tumor was covered completely (plus an ablative margin of at least 5 mm, and ideally 10 mm around the tumor). When the treatment was completed, the track ablation was performed during withdrawal of the electrode to avoid tumor cell seeding along the needle tract and to prevent bleeding by coagulation of the small vessels. Thereafter, a CT scan was performed immediately after the MWA procedure to assess the treatment response and to evaluate immediate complications, such as hemorrhage ().
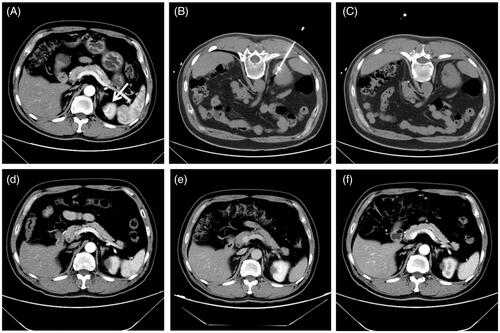
Figure 1. A 1.4 cm tumor lesion in a solitary kidney of a 54-year-old man treated with microwave ablation. (A) Preablation axial contrast-enhanced CT image shows one heterogeneous hyperdensity neoplasm (white arrow) in left kidney. (B) Axial unenhanced CT scan obtained after placement of microwave antenna with CT guidance. (C) Axial unenhanced CT scan after withdrawn of microwave antenna shows the low-density ablation zone. (D, E, F) axial contrast- enhanced CT images show no enhancement in the ablation zone for 1-, 3-, and 12-month after ablation, respectively.
Post-procedural evaluation
According to the standardization of ablation terminology and reporting criteria [Citation15], complete ablation is defined as the absence of enhancement of any areas of the mass on a follow-up enhanced image obtained 1 month after MWA (). If complete ablation was achieved [Citation16], clinical follow-up and imaging with contrast-enhanced CT or contrast-enhanced MRI was performed at 1, 3, and 6 months and every 6 months thereafter (). Two trained abdominal radiologists reviewed the images in consensus for technical success, local tumor progression, metastatic disease, and complications.
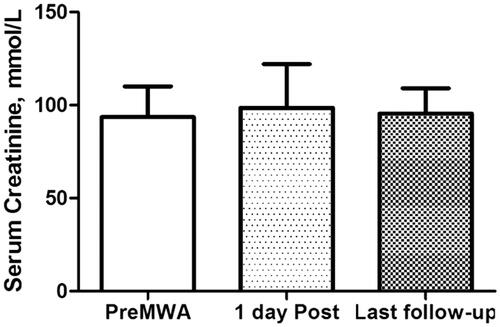
Renal function was evaluated using the serum creatinine levels measured at the following time points: before MWA, 1 day after MWA, and the most recent follow-up at our institution. Complications were defined as major or minor and were followed up at 1 month after ablation.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation and differences were analyzed using Student’s t-test. The time of follow-up is expressed as the median. Actuarially overall survival and cancer-specific survival rate were calculated by the Kaplan–Meier method. All statistical analyses were performed by the Statistical Package for the Social Sciences 16.0 software (SPSS, Chicago, IL), and a p-value of < .05 was considered statistically significant.
Results
Sixteen tumors in 15 patients with a mean diameter 2.3 cm (range: 1.2–3.6 cm) were managed using MWA treatment under CT guidance. All the 16 tumors were treated once; there were no repeated treatments. One patient had two tumors that were ablated in a single session. RCC was confirmed in 16 tumors by biopsy prior to MWA procedure. Pathological results indicated clear cell carcinoma in 13 patients and papillary carcinoma in three patients. Patient and tumor characteristics are summarized in .
Table 1. Patient and tumor characteristics.
Complete ablation was achieved in all 16 tumors (100%). The median follow-up time was 24 months (range: 1–41 months). During the follow-up, no patient showed local recurrence in all completely ablated tumor lesions. There were no metastatic lesions reported. One patient died of myocardial infarction at 21 months. The cancer-specific survival rate was 100%, and 1-, 2-, and 3-year overall survival rates were 100%, 93.3%, and 93.3%, respectively.
No major complications were encountered in our study. However, two minor complications (12.5%) were observed. One patient exhibited a slight bleeding into the surrounding tissue after MWA, without any clinical consequences. The resulting hematoma was reabsorbed spontaneously. One patient complained of mild pain at the ablation site, which regressed with analgesics. No hematuria or post-ablation syndrome [Citation17] was reported.
Renal function parameters prior to and after the therapy were obtained routinely. No significant changes were observed in serum creatinine levels before and 1 day after MWA treatment (93.5 ± 9.3 mmol/L versus 98.6 ± 12.1 mmol/L, p = .21) (). The mean serum creatinine after treatment was 95.4 ± 6.8 mmol/L, which was not significantly changed when compared with pre-ablation levels (p = .53).
Discussion
The results of our study demonstrated that percutaneous MWA was a safe and effective treatment for stage T1a RCCs in patients with a solitary kidney. In all 16 tumors that were treated by MWA, complete ablation was achieved with minimal impact on the renal function and a few ablation-related complications.
Choosing the optimal treatment for patients with solitary kidney tumors is crucial. Complete removal of the tumor and maintenance of renal function should be carefully considered while deciding the treatment strategy. Traditionally, partial nephrectomy or nephron-sparing surgery is considered as standard treatment for these renal tumors. Although the local recurrence rate was lower for partial nephrectomy than that for ablation therapy [Citation18], surgical resection may cause potential injury to the remaining kidney [Citation19], causing renal dysfunction or even renal failure. Minimally invasive ablation therapy is successfully applied for RCCs treatment and has gained preliminary satisfactory results [Citation20–22]. Talenfeld et al. [Citation23] retrospectively evaluated T1a lesions treated either with RFA, cryoablation, or MWA, and reported similar oncologic outcomes of total or partial nephrectomy (5-year cancer-specific survival rate: 95–98%); however, less periprocedural complications, reduced 90 days mortality, and a lower risk of long-term renal insufficiency were observed. Katsanos et al. [Citation24] conducted a systematic review comparing thermal ablation with surgical nephrectomy for small renal tumors. They found that local recurrence (3.6% versus 3.6%;) and disease-free survival (hazard ratio: 1.04) were almost similar between the two groups. However, postoperative decline of the estimated glomerular filtration rate (mean difference: −14.6 mL/min/1.73 m2) and complication rate (7.4% versus 11%) were significantly lower in the ablation group that in the surgical nephrectomy group.
A previous study has reported the use of MWA for treating RCCs under ultrasonic (US) guidance in patients with a solitary kidney [Citation25]. In this study, complete ablation was achieved in 15 of 16 (93.8%) lesions without deterioration of preablation renal function or occurrence of major complications. The tumor size was relatively large (average: 3.2 cm, range: 1–8.4 cm) in this study, and only one tumor (size: 5.5 cm) was not completely ablated. Another difference between the two studies was image-guidance. CT guidance was used in our study. CT can provide a large field of view and is less limited by patient structure or gas formation; hence, it can provide proper visualization of the ablation area, making post-procedural assessment more accurate than US guidance [Citation4].
Several studies have reported on the feasibility and efficacy of RFA for managing tumors in solitary kidneys. RFA showed similar oncological and functional outcomes compared to partial nephrectomy [Citation26]. Hoffmann et al. [Citation7] and Grasso et al. [Citation27] reported a 100% complete ablation rate for RCCs treatment in patients with a solitary kidney without major complications or renal functional changes. In their studies, the maximum tumor diameter was approximately 4.0 cm and none of tumors was close to the renal pelvis. Another RFA study [Citation28] showed that complete ablation was achieved in 17 tumors (89.4%) after one or two RFA sessions. Furthermore, 100% of exophytic and parenchymal tumors, and 3 cm or smaller sized tumors, were completely ablated. Therefore, tumor size and location were essential factors impacting ablation success rate. Cryoablation is also a promising choice for treating solitary kidney tumors. Renal cryoablation provided better perioperative outcomes in terms of median operative time, estimated blood loss, transfusion, hospital stay, and complications [Citation29].
In our study, the target tumors did not exceed 4.0 cm, which is described as the upper threshold of T1a tumor. Small sized tumors (<4.0 cm) are ideal candidates for ablation treatment [Citation4,Citation30]. Complete ablation is easily attainable and local recurrence rate is low. In terms of location, complete ablation is more difficult in a centrally located tumor and the blood flow causes a heat-sink effect that limits the ablation temperature. Furthermore, complete ablation of central tumors may cause damage to the collecting system or kidney vessels, causing renal function abnormality and hematuria. Ablation should be cautiously opted for treating tumors close to the renal pelvis. Therefore, only exophytic and parenchymal tumors were selected in our study.
The encouraging results of our study add to the literature indicating that stage T1a RCCs can be successfully treated with different percutaneous thermal ablation modalities. MWA procedure can achieve larger ablation zones and is less influenced by heat-sink effect in contrast to RFA. MWA has a similar or slightly better technical efficacy rate and local tumor recurrence rate than RFA or cryoablation with comparable complications rates and lower incidence of major complications [Citation31]. However, comparison between modalities is difficult. Each technique has its own advantages and disadvantages, which should be considered by interventional radiologist for each patient [Citation4].
The complication rate was low, which is consistent with the reported data [Citation31,Citation32]. Only minor complications were observed, which did not need clinical intervention. We did not observe hematuria, massive hemorrhage, neoplastic seeding, or damage to the collecting system or adjacent organs [Citation33]. Moreover, no apparent impairment to renal function was observed because of MWA. In our study, no significant difference in the serum creatinine levels was observed after MWA treatment.
This study has several limitations. First, the study is a retrospective and single-center study, which may be biased by institutional expertise. Second, the sample size was relatively small, a large cohort is needed. Third, there was a patient selection bias regarding tumor size and location. Further studies are needed to include more large size tumors and evaluate the treating effectiveness of tumors adjacent to important structures. Finally, the follow-up period was not sufficiently long to estimate long-term oncological results.
In conclusion, percutaneous MWA of stage T1a RCCs is an effective and safe treatment option for patients with a solitary kidney. Tumor size and location should be considered while opting for renal tumor ablation
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hoffmann RT, Jakobs TF, Trumm C, et al. RFA of renal cell carcinoma in a solitary kidney. Abdom Imaging. 2008;33(2):230–236.
- Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166(1):6–18.
- Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(6):668–680.
- Filippiadis D, Mauri G, Marra P, et al. Percutaneous ablation techniques for renal cell carcinoma: current status and future trends. Int J Hyperthermia. 2019;36(2):21–30.
- Weisbrod AJ, Atwell TD, Frank I, et al. Percutaneous cryoablation of masses in a solitary kidney. Am J Roentgenol. 2010;194(6):1620–1625.
- Sung HH, Park BK, Kim CK, et al. Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hyperthermia. 2012;28(3):227–234.
- Hoffmann RT, Jakobs TF, Kubisch CH, et al. Renal cell carcinoma in patients with a solitary kidney after nephrectomy treated with radiofrequency ablation: mid term results. Eur J Radiol. 2010;73(3):652–656.
- Olweny EO, Park SK, Tan YK, et al. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–1161.
- Yu J, Liang P, Yu X, et al. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79(1):124–130.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–143.
- Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology. 2017;284(1):272–280.
- Ierardi AM, Carnevale A, Rossi UG, et al. Percutaneous microwave ablation therapy of renal cancer local relapse after radical nephrectomy: a feasibility and efficacy study. Med Oncol. 2020;37(4):27.
- Carrafiello G, Dionigi G, Boni L, et al. Current role of interventions in metastatic kidney tumors: single center experience. Updates Surg. 2011;63(4):259–269.
- Ierardi AM, Petrillo M, Coppola A, et al. Percutaneous microwave ablation of renal angiomyolipomas in tuberous sclerosis complex to improve the quality of life: preliminary experience in an Italian center. Radiol Med. 2019;124(3):176–183.
- Ahmed M, Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update: supplement to the consensus document . J Vasc Interv Radiol. 2014;25(11):1706–1708.
- Russo U, Maestroni U, Papapietro RV, et al. Imaging after radiofrequency ablation of renal tumors. Future Oncol. 2018;14(28):2915–2922.
- Carrafiello G, Lagana D, Ianniello A, et al. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas Radiol. 2007;51(6):550–554.
- Uhlig J, Strauss A, Rucker G, et al. Partial nephrectomy versus ablative techniques for small renal masses: a systematic review and network meta-analysis. Eur Radiol. 2019;29(3):1293–1307.
- Zabell J, Isharwal S, Dong W, et al. Acute kidney injury after partial nephrectomy of solitary kidneys: impact on long-term stability of renal function. J Urol. 2018;200(6):1295–1301.
- Floridi C, De Bernardi I, Fontana F, et al. Microwave ablation of renal tumors: state of the art and development trends. Radiol Med. 2014;119(7):533–540.
- Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renal lesions Bosniak III or IV after 24 months follow up. Int J Surg. 2013;11(Suppl 1):S30–S35.
- Carrafiello G, Lagana D, Ianniello A, et al. Percutaneous radiofrequency thermal ablation of renal cell carcinoma: is it possible a day-hospital treatment? Int J Surg. 2008;6(Suppl 1):S31–S35.
- Talenfeld AD, Gennarelli RL, Elkin EB, et al. Percutaneous ablation versus partial and radical nephrectomy for T1a renal cancer: a population-based analysis. Ann Intern Med. 2018;169(2):69–77.
- Katsanos K, Mailli L, Krokidis M, et al. Systematic review and meta-analysis of thermal ablation versus surgical nephrectomy for small renal tumours. Cardiovasc Intervent Radiol. 2014;37(2):427–437.
- Lin Y, Liang P, Yu XL, et al. Percutaneous microwave ablation of renal cell carcinoma is safe in patients with a solitary kidney. Urology. 2014;83(2):357–363.
- Xiaobing W, Wentao G, Guangxiang L, et al. Comparison of radiofrequency ablation and partial nephrectomy for tumor in a solitary kidney. BMC Urol. 2017;17(1):79.
- Grasso RF, Luppi G, Faiella E, et al. Radiofrequency ablation of renal cell carcinoma in patients with a solitary kidney: a retrospective analysis of our experience. Radiol Med. 2012;117(4):606–615.
- Jimenez E, Zurera L, Espejo JJ, et al. Percutaneous radiofrequency ablation of renal tumors in solitary kidney patients. Arch Espanoles Urol. 2011;64(1):51–58.
- Panumatrassamee K, Kaouk JH, Autorino R, et al. Cryoablation versus minimally invasive partial nephrectomy for small renal masses in the solitary kidney: impact of approach on functional outcomes. J Urol. 2013;189(3):818–822.
- Carrafiello G, Mangini M, Fontana F, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(2):367–374.
- Choi SH, Kim JW, Kim JH, et al. Efficacy and safety of microwave ablation for malignant renal tumors: an updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19(5):938–949.
- Papa M, Suardi N, Losa A, et al. ABLATE: a score to predict complications and recurrence rate in percutaneous treatments of renal lesions. Med Oncol. 2020;37(4):26.
- Ierardi AM, Puliti A, Angileri SA, et al. Microwave ablation of malignant renal tumours: intermediate-term results and usefulness of RENAL and mRENAL scores for predicting outcomes and complications. Med Oncol. 2017;34(5):97.