Abstract
Objective
To determine whether photothermal polymer nanoparticles (NPs) can interface with bacteria associated with kidney stones, generate heat when stimulated with near infrared (NIR) light, and aid in reducing bacterial burden.
Methods
Two types of kidney stones, artificial, and those removed during percutaneous nephrolithotomy (PCNL), were inoculated with Escherichia coli (E. coli) and then incubated with NPs composed of FITC-labeled Poly[4,4-bis(2-ethylhexyl)-cyclopenta[2,1-b;3,4-b′]-dithiophene-2,6-diyl-alt-2,1,3-benzoselenadiazole-4,7-diyl] (PCPDTBSe). Association of the PCPDTBSe NPs was evaluated using fluorescence microscopy. Infected stones were incubated with NPs and exposed to 800 nm light to generate temperature increases from 25.4 to 68.6 °C on the stones. Following photothermal treatment, the stones were homogenized and the bacteria was enumerated via colony counting assays to evaluate the bactericidal effect. The photothermal effect was also evaluated using scanning electron microscopy of the treated biofilms.
Results
Both kidney stone types sequestered E. coli. Control stones and stones treated with laser only had growth of numerous bacterial colonies, while stones exposed to NPs and laser grew significantly less, or none (p = 0.02).
Conclusions
The polymer NPs interface with E. coli on artificial and patient-derived kidney stones, and they can impart a bactericidal effect, when stimulated with NIR to generate heat. This technique may possibly be extended to treating infected kidney stones in patients.
Introduction
Nephrolithiasis is a common condition affecting up to 10.6% of the population in the USA, with annual healthcare costs exceeding $5 billion [Citation1,Citation2]. The high economic and healthcare burden is further increased by complications of nephrolithiasis including hydronephrosis, decline in kidney function, chronic pain, and infections. Kidney stone-associated infections are a significant challenge to treat, as antibiotic penetration and concentration in the urinary tract is relatively poor compared to other body compartments. Previous research has shown that bacteria may be associated with kidney stones, possibly leading to the development of biofilms on the stone surface [Citation3]. Such microbial biofilms are challenging to eradicate and exhibit a dramatically decreased susceptibility to antimicrobial agents [Citation3–5]. Disruption of biofilms can lead to urosepsis, which is the most frequent complication associated with ureteroscopy (URS) and percutaneous kidney stone surgery, with reported rates of up to 7.6 and 16% of procedures, respectively [Citation6,Citation7]. With the latter, those with infectious complication were more likely to have a positive intraoperative stone culture [Citation6].
Stone surfaces with bacterial biofilms are resistant to penetration by antibiotics and continually shed bacteria within the urinary tract, contributing to the development of urinary tract infections (UTIs) [Citation8–10]. Complete removal of the stone theoretically combats the problem of resistant biofilms and bacterial shedding. However, fragmentation of stones during the removal process (e.g. using laser lithotripsy) has also been linked to the development of infections, via possible release of bacteria from stone surfaces during manipulation [Citation11–13]. When the kidney stones are broken during the process of laser lithotripsy, there is no sufficient thermal energy to kill the bacteria and thus they are dispersed. Moreover, recent studies suggest that bacteria are present in the internal stone structures and are dispersed widely through the urinary tract during endourological procedures [Citation14]. At the present time, no treatment regimens have been developed to address the challenges specific to stone-associated infections.
One strategy that has the potential for disrupting kidney stone-associated biofilms and eradicating bacteria is photothermal ablation [Citation15,Citation16]. Nanoparticles (NPs) that can absorb light and convert it rapidly to heat are advantageous for selective thermal destruction. For example, photothermal NPs have been developed over the past 20 years for selective ablation of tumors in pre-clinical models to evaluate precision heating [Citation17–23]. The same concept has been employed for eliminating biofilms, most frequently using metallic photothermal NPs [Citation15,Citation16,Citation24–27]. Although metallic NPs are proven photothermal agents, there is an exciting new class of heat-generating NPs based on semi-conducting polymers [Citation28–35].
Ablation of biofilms/bacteria associated with kidney stones may be effective for reducing urosepsis; however, the impact of residual NPs that could remain after photothermal treatment needs to be contemplated as potential nucleation sites for crystallization (leading to regrowth of kidney stones). It has previously been demonstrated that polymers can disrupt calcium crystal formation; therefore, we propose that polymer-based NPs might represent an ideal therapeutic for photothermal disruption of bacterial biofilms associated with kidney stones [Citation36,Citation37]. Polymer NPs may not serve as individual polymers to disrupt kidney stone formation, but the use of a softer polymer photothermal NP in lieu of a rigid metallic one might be more suitable for ensuring that the NPs do not serve as nucleation sites for calcium crystal growth.
Our team has previously developed semi-conducting polymer MPs comprised of PCPDTBSe for the generation of heat for ablation of breast and colorectal cancers [Citation38–40]. Although polymer NPs readily enter eukaryotic cells, they do not seem to enter bacteria or penetrate the bacterial cell wall. We have witnessed that they may penetrate the polysaccharide matrix of the biofilm (unpublished results). Since the polymer NPs have a negative surface charge unless they are formulated to be positive, and bacteria are negatively charged there is no reason for the particles to selectively bind to bacteria associated with kidney stones. However, the NPs may passively co-localize with kidney stone-associated bacteria since both the bacteria and NPs can fit into the porous architecture of the stones.
When exposed to near-infrared (NIR) light these NPs generate heat, which has been shown to be effective for bacterial ablation in vivo (unpublished data). The goal of the current project was to determine whether these NPs can c-localize with bacteria on artificial and patient-derived kidney stones, and evaluate the effect of heat, generated by NPs stimulated with NIR laser, on inactivating Escherichia coli bacteria. During laser lithotripsy, which is commonly used to break kidney stones and aid in their removal from the patient, intense laser sources of up to 120 W are used [Citation41]. One of the challenges with such a technique is that powerful lasers may be more likely to disperse bacteria rather than apply sufficient heat for long enough to kill them, which may increase the risk of urosepsis. The goal of using NPs is twofold: (1) to develop a precise method of heating the bacteria/biofilm at the stone interface, which harbor pathogens and (2) to deliver the heat rapidly. For future clinical utility, we envision that polymer NPs can be delivered during percutaneous nephrolithotomy (PCNL) for direct interfacing with bacteria that reside in stone crevices. Following their delivery, low powered laser light could be delivered via a fiber optic catheter to stimulate thermal destruction of the bacteria prior to breaking of the kidney stone.
Materials and methods
The polymer NPs used in this study were composed of Poly[4,4-bis(2-ethylhexyl)-cyclopenta[2,1-b;3,4-b]dithiophene-2,6-diyl-alt-2,1,3-benzoselenadiazole-4,7-diyl] (PCPDTBSe) with FITC-labeled polyethyleneglycol (PEG) to offer florescence to the NPs. PCPDTBSe was synthesized in our laboratory according to previously published procedures [Citation42]. To formulate NPs from this polymer, a 2 mg/ml solution of PCPDTBSe was dissolved in tetrahydrofuran and 1 ml was added to 8 mL of a 1 mg/ml solution of FITC-PEG in water under horn sonication for 1 min. Following synthesis, the tetrahydrofuran was evaporated and the NPs were washed in ethanol for both purification and sterilization. Prepared NPs were evaluated by absorption spectroscopy to quantify their optical characteristics and determine their concentration based on previously established calibration curves. They were further characterized by dynamic light scattering (DLS) to determine their size and zeta potentials. The photothermal conversion efficiency (PCE) of PCPDTBSe prepared with Pluronic F127 instead of FTIC-PEG was determined according to the method described by Roper et al. [Citation43].
Artificial kidney stones were manufactured in our lab based on prior experience by mixing 18 grams of gypsum cement (17.1 g of Plaster of Paris and 0.9 g of Portland cement) with 2 g of Velmix (calcium sulfate powder) with 15 ml of deionized (DI) water [Citation44]. The mixture was cast in small cylindrical molds 10 mm in length and 5 mm in diameter and dried for 4 h at 37 °C. Artificial kidney stones were autoclaved to sterilize prior to inoculation. Kidney stones from patients were obtained after our Institutional Review Board (IRB) approved the protocol and patients signed an informed consent prior to surgery to donate a part of their stones to the study. Patient-derived kidney stones were obtained in a sterile manner and handled under sterile conditions throughout the experiment. They were broken into about 5 mm pieces prior to their use in the laboratory. All experimental groups used the same patient-derived kidney stones and thus replicates of the experimental variables were repeated using kidney stones from multiple patients. A component of the standard of care is for patients to be given prophylactic antibiotics prior to kidney stone disruption. Sample fragments of each patient stone were evaluated by the Wake Forest University Health Sciences Clinical Pathology laboratory and Beck Analytical Services to determine the composition of the stone and to identify the presence of bacteria; however, all stones used in this study were negative for patient-derived bacteria. All stones were weighed before their use in culture experiments.
For evaluation of bacterial burden associated with the stones following photothermal treatment the stones were homogenized using a Bullet Blender® Storm, which uses metallic beads in vials to pulverize tissues. Therefore, all of the experiments using infected stones were done in vials with metallic homogenization beads (3 mm in diameter) to minimize disruption of the bacterial biofilms that can occur during transfer if separate growth and treatment containers are used. As the metallic beads used for homogenization can generate heat if exposed to the laser light, the beads were always placed below the stone in the tube, such that the stone blocked the laser light, as shown in . Prior to generating heat on infected stones, we evaluated the rise in temperatures when applying an 800 nm laser (K-Cube from Summus Laser, Inc) at 5 W (1 cm diameter spot size) for 60 or 300 s on uninfected artificial stones. This system has multiple wavelengths, powers and frequencies, although 800 nm, continuous mode, was selected because the PCPDTBSe polymer, from which the NPs are composed, was designed to have a strong absorption near 800 nm. Stones were placed over four metal beads (that are used for homogenizing the stone subsequently) in a Rhino® tube () with 700 µL of sterile water, or 100 µg/ml of PCPDTBSe NPs to simulate similar conditions to the experiments performed with the actual biofilms. The change in temperature of the solutions immediately before and after laser exposure was measured using a Fluke 714 thermometer and 80PK-1 bead probe wire thermocouple. Additionally, temperature increases over time were measured using a Neoptix fiber optic thermocouple connected to a Nomad thermometer.
Figure 1. (A) Rhino® tube set up for the artificial stones above the metal homogenization beads. (B) Laboratory set-up for the treatment of kidney stones placed in tubes and suspended in a water bath to maintain 37 °C.
As prepared, artificial kidney stones are alkaline and rapidly alter the pH of treatment solutions to be highly basic and inhospitable for bacterial culture. Therefore, they were soaked for 48 h in sterile DI H2O in an incubator with CO2 to neutralize the alkaline byproducts prior to bacterial culture. A strain of pathogenic E. coli (ATCC: CFT073) was used to inoculate each set of kidney stones. E. coli were grown in Luria Bertani (LB) broth for 12 h at 37 °C (with orbital shaking) to develop a planktonic suspension that was subsequently diluted to approximately 1.0 × 108 CFU/ml using optical absorption at 600 nm. One milliliter of planktonic suspension was statically incubated at 37 °C with the prepped stones overnight to culture bacteria on the stone surface. The following day the stones were gently rinsed with water to remove bacteria that was not attached to the stone surface. Infected stones were then incubated with NPs at a concentration of 100 µg/ml for 12 h. As described below, some groups were washed twice following incubation with the NPs, and some were not, to remove NPs that were not bound to bacteria on the stone surface. The cultured stones were in homogenization tubes, and were placed in a warm water bath to maintain their temperature at 37 °C ± 1 °C during the laser exposure (see ). The cap of the tube was removed for laser exposure and the entirety of the experiments were performed in a biosafety cabinet. Then 800 nm light was applied at 5 W (1 cm diameter spot size) for 60 or 300 s to generate elevated temperatures on the artificial stones. Immediately following laser exposure, stones were homogenized in a Bullet Blender® and filtered through a 40 micron filter. The bacteria were enumerated via standard serial dilutions up to 10−7 and plated on LB agar incubated for 24 h. The infected stones were divided into eight groups of triplicates: (i) stones that were control and received no NPs and no NIR; (ii) stones incubated with NPs; (iii and iv) stones treated with the NIR, 5 W for 60 or 300 s; (v) stones incubated with NPs and treated with NIR for 60 s; (vi) stones incubated with NPs, washed with sterile water and then treated with NIR for 60 s; (vii) stones incubated with NPs and treated with NIR for 300 s; and (viii) stones incubated with NPs, washed and treated with the laser for 300 s. Stones that were not washed were retained in the NP solution in which they had been incubated. Statistical analyses were performed using the SPSS software version 24.0 for Windows (SPSS). Differences between different treatments were compared by t-test and one-way ANOVA test and were considered significant at values below 0.05.
The FITC-PEG coating of the NPs allowed for fluorescence detection of the NPs co-localized with the kidney stones. The NPs are inherently dark green in color due to the PCPDTBSe. Incubation of the kidney stones with the NPs followed by washing to remove excess NPs changes the color of the stones from white to light green, as observed using an Olympus dissecting microscope with light illumination and 1× magnification. For scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) artificial stones that were 5 mm in diameter and 2 mm high were treated with biofilm and NPs, with and without laser irradiation (300 s), then were fixed in 10% glutaraldehyde in water for 2 h. For fluorescence observation, samples were placed in chambered coverglass and imaged using an Olympus FV 1200 spectral laser scanning confocal microscope at 10×. For SEM imaging additional samples were treated and fixed in the same manner, mounted onto aluminum stubs, sputter coated with silver and imaged using a Zeiss Gemini SEM 300. Additional samples were also prepared for SEM but instead of the photothermal treatment the samples were autoclaved at 121 °C for 30 min prior to fixation.
Results
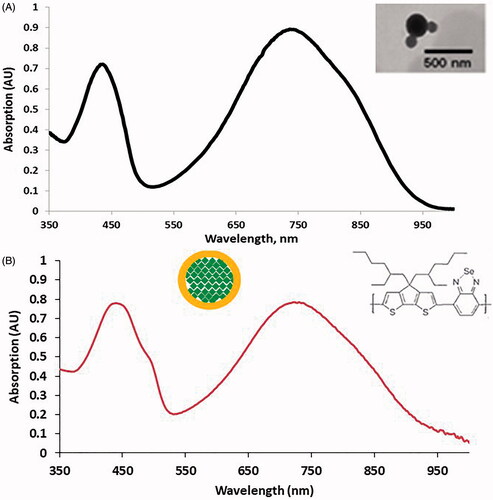
It has been well established that a NIRwindow from 700 to 900 nm exists where water, hemoglobin and deoxyhemoglobin have absorption minima [Citation45]. Therefore, many NPs are designed to absorb wavelengths in this range so that they can selectively absorb this light compared to the surrounding tissues and reduce nonselective thermal damage. As shown in , the optical absorption of the PCPDTBSe NPs shows two clearly defined peaks; one at 420 nm and the second near 750 nm. The inset shows a transmission electron microscopy image of the NPs, indicating that they have a spherical shape. For the use of these NPs as photothermal agents the aim of our research was to stimulate them using an 800 nm source, as this strongly overlaps with a main absorption peak of the NPs. As shown in , the addition of FITC-PEG leads to a slight red-shift shoulder in the 420 nm peak, due to the addition of FITC-PEG, as well as an increase in the peak intensity. DLS indicates that the average size of the NPs was 79 nm, and they had a zeta potential of −32.5 mV. The PCE was calculated to be 53.17%.
Figure 2. (A) Optical absorption of PCPDTBSe NPs, which shows two main absorption peaks near 420 and 750 nm. The inset shows a TEM image of the NPs. (B) Optical absorption of PCPDTBSe NPs prepared with FITC-PEG. Insets show a schematic of where the FITC-PEG (yellow) is in relation to the green PCPDTBSe polymer and the chemical structure of PCPDTBSe.
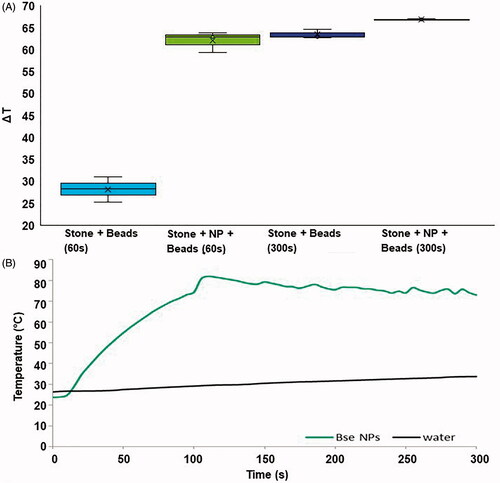
Solution temperatures measured immediately following 60 or 300 s of NIR laser application are shown in . There was a statistically significant difference between the 60 and 60 s with the NPs group (28.2 °C vs. 62 °C, p ≤ 0.001), but there was no significance between the 300 s laser exposure with NPs, compared the without. Exposure of the stones to 60 s of light (over the metal homogenization beads as shown in ) led to a ΔT of 28.2 ± 2.85 °C, and the addition of the NPs (100 µg/ml, 700 µl volume) resulted in a ΔT of 62 ± 2.36 °C. The average temperature increase of a solution of stones and beads with 300 s NIR laser was 63.7 ± 1.1 °C, and the addition of NPs increased this to 66.85 ± 0.15 °C. This result suggests that the NIR exposure of the stones for prolonged times generates as much heat as the NPs when stimulated with 800 nm light for 5 min. One consideration is that the data is a measure of solution temperature, not the temperature of the stone surface. The photothermal technique requires exposure to light and the bottom surface of the stone did not receive laser irradiation in the experimental set-up we used. This could lead to the evaluation of temperature increases of a 700 μl volume of water or NPs without stones exposed to 5 W, 800 nm light over 300 s is provided in . This data shows that water does not increase much in temperature. However, the NPs solution very rapidly accelerates in temperatures, and at 60 s is 59.97 °C. With a beginning room temperature of 22 °C the ▵T = 37.97 °C. Comparatively, water at 60 s is 27.89 °C for a ▵T = 5.89 °C. The temperature plateaus at around 100 s because this was not a thermodynamically closed system and the room temperatures led to a thermal equilibrium. Thus, from about 100 to 300 s an average temperature of 76.59 °C (▵T = 54.59 °C) could be maintained. During our experiments involving bacteria the stones were held at 37 °C during laser exposure, which means that the E. coli were exposed to 91.59 °C for 200 s. Although similar temperatures were reached at the end of the treatment period in the 300 s groups, it was noticeable that the high temperatures were reached much faster when NPs were involved, as bubbles and water vapor were already noticeable within the second minute of NIR exposure in this group.
Figure 3. (A) Temperature increases for artifical kidney stones placed on top of homogenization beads and treated with 60 or 300 s of laser light. Temperatures were measured immediately following laser exposure. The box of each group represents the interquartile range, with the middle black line representing the median and the upper and lower lines representing the maxmium and minimum values (*p < 0.001). (B) Temperature increases of a 700 μl volume of water or 100 μg/ml solution of NPs exposed to 5 W of 800 nm light over time.
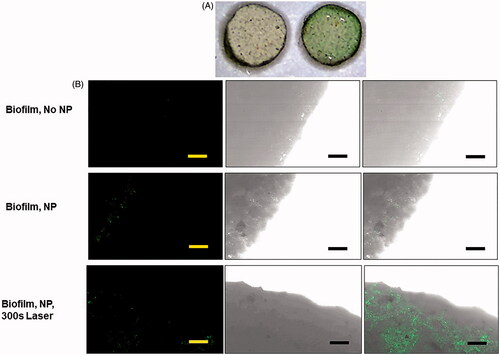
One of the questions we sought to answer in this research was whether non-targeted NPs could attach to bacteria on kidney stones. FITC-PEG was used during NP synthesis to stabilize the polymers and provide a detectable/fluorescent imaging component. The FITC-PEG was used to prepare NPs for visualization with the kidney stones. For hyperthermia experiments, PEG without FITC was used as the stabilizing agent. As shown in , artificial kidney stones coated with E. coli biofilm and incubated for 24 h with PCPDTBSe NPs display a green color, compared to stones without NPs, which appear mostly white. The NPs have a negative zeta potential, as do the calcium components of the kidney stones, and the bacteria have a negative surface charge on their membrane [Citation46]. Thus the mechanism for the NPs to interface with the bacteria or stone surface is most likely not electrostatic interaction. The kidney stones have a porous surface with many grooves and holes for the biofilm to grow upon, and for NPs to settle into. Since the current formulation of NPs has no specific potential for binding, they are most likely passively settling into the grooves of the stone where bacteria also reside. What is quite interesting is that even after washing of the stones, they retained a green tinted color, indicative of the presence of NPs. As shown in , the fluorescent (green) NPs could be seen on stones treated with and without laser stimulation. All artificial and patient-derived kidney stones, obtained from nine different patients, that were inoculated with E. coli grew a biofilm on them as determined visually by examining the stone surface after washing planktonic bacteria away. Of the nine patient stones, four were calcium oxalate based, one calcium phosphate, one uric acid, two carbonate apatite, and one hydroxyapatite.
Figure 4. (A) Microscopy image (1×) of artifical kidney stones with E. coli biofilm, without (left) and with (right) 24 h expsoure to PCPDTBSe/FITC-PEG nanoparticles. (B) Brightfield and fluorescence images of the edge of artifical kidney stones with biofilm and with and without PCPDTBSe/FITC-PEG nanoparticles (green). The scale bar is 100 µm.
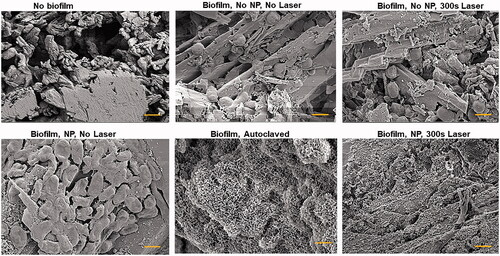
SEM images of artificial kidney stones subjected to various treatments are shown in . As shown in the image of stones with no biofilm, the stone surface is very jagged, with a crystalline appearance and many pores. Biofilm without NPs or laser show the intact morphology of E. coli peppered along crystalline edges. Biofilm treated with laser only, or NPs only indicate that the bacterial morphology is retained. However, biofilms that were autoclaved (no NP or laser exposure) as a control for visualizing thermal damage of the biofilm have a disrupted morphology. There does not appear to be intact bacteria and there is small debris covering the surface of the stone. Artificial kidney stones were autoclaved prior to the formation of biofilm, and hence the morphological changes observed in the biofilm coated, and then autoclaved sample, are due to thermal disruption of the biofilm specifically. Similar changes were observed in the E. coli biofilm treated with NPs overnight, with no washing to remove excess NPs, and then exposed to 300 s of 800 nm of 5 W of laser light.
Figure 5. Scanning electron microscopy images of artificial kidney stones without biofilm or with biofilm and various nanoparticle treatments. Also included is a biofilm-coated stone that was autoclaved. All images were taken at 100,000× and the scale bar is 1 µm.
In the control groups of artificial kidney stones without NPs, and the groups of stones that were just treated with the NIR laser without NPs for 60 and 300 s there was no statistically significant reduction in CFUs/g, (), although 300 s NIR laser treatment exhibited a 30.52% in reduction. The control groups of stones without NPs and treated with the NIR laser for 60 and 300 s had growths ranging from 768 to 1594 CFU/g per stone, and there was no difference when comparing CFU/g between the groups (). When comparing CFU/g between the artificial kidney stones without NPs or laser and the treatment groups that used the 5 W laser for 300 s there was an average reduction in growth from 1284.6 to 237.4 CFU/g (an 82% reduction) when NPs were washed and to 438.3 CFU/g (a 66% reduction) when NPs were not washed away before treatment (p = 0.02, p = 0.03, respectively (). There was a statistically significant reduction (51%) in the NP-treated group with no washing compared to the group that received 300 s of laser light and no NPs. The group incubated with NPs and washed had a 73% decrease compared to the group that did not receive NPs but did receive 300 s of laser light. The groups incubated with NPs, then washed and exposed to 60 s of laser light did not have a significant reduction in CFU/g of stone material. However, the group incubated with NPs, but not washed prior to exposure to 60 s of laser light had a statistically significant reduction (95%) compared to the group of stones treated with only 60 s of light. Compared to either the no NPs, no laser group (1284.66 CFU/g) or the no NPs, 60 s NIR group (1181.13), the result of the artificial kidney stones treated with NPs and not washed before exposure to 60 s of NIR (54.98 CFU/g) led to a ∼95% reduction compared to either control group. However, there was no significant difference in the 60 s group when the NPs were washed prior to NIR exposure (1110.27 CFU/g).
Figure 6. (A) CFU/g of artificial kidney stones treated with no NPs and no NIR (gray); no NPs, 60 s NIR (blue); no NPs, 300 s NIR (orange); NPs, no wash, 60 s NIR (blue with green horizontal lines); NPs, washed, 60 s NIR (blue with diagonal green lines); NPs, no wash, 300 s NIR (orange with green horizontal lines); NPs, washed, 300 s NIR (blue with diagonal green lines) (* indicates statistical significance between groups p < 0.05). (B) CFU/g of patient-derived kidney stones treated with no NPs, no NIR (gray); NPs only, no NIR (green); NPs, no wash, 60 s NIR (blue with green horizontal lines); NPs, washed, 60 s NIR (blue with diagonal green lines); NPs, no wash, 300 s NIR (orange with green horizontal lines); NPs, washed, 300 s NIR (orange with diagonal green lines) (* indicates statistical significance between groups p < 0.05).
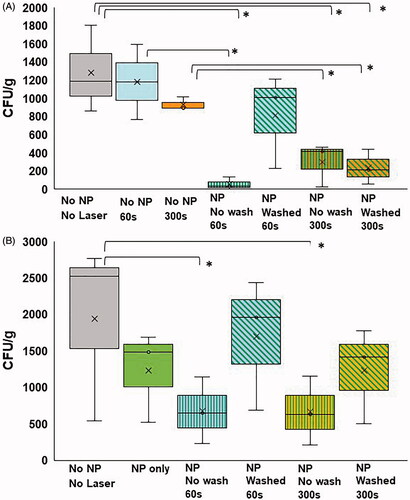
Patient-derived kidney stones treated with NPs had a slight, but non-significant reduction in CFUs (2521 ± 703.67 to 1483 ± 359.17 CFU/g). But when comparing CFU/g between the stones without NPs and the unwashed treatment groups that used the 5 W NIR laser for 60 s and 300 s there was a statistically significant reduction in growth from 2521 ± 703.67 CFU/g to 650 ± 263.83 CFU/g and 633 ± 273.02 CFU/g (p = 0.02. ). There was no such difference in the washed groups (Washed, 60 s was 1956 ± 521.64 and washed, 300 s was 1414 ± 379.75 CFU/g).
Discussion
The current treatment options that are available for actively removing kidney and ureteral stones are PCNL and URS. One of the more dreaded complications following these procedures is postoperative infection, ranging from fever to severe sepsis and risk of death [Citation7,Citation12,Citation44,Citation47]. Risk factors for developing infection complications after percutaneous and endoscopic procedures include complex stone burden, hydronephrosis, recurrent urinary infections, external drain tubes, internal stents, diabetes mellitus, immunosuppression and intraoperative time and bleeding [Citation6,Citation11,Citation47]. Koras et al. investigated risk factors leading to infection-related complications following PCNL [Citation7]. Stone burden, recurrent UTI, and infected stones were all independently associated with the development of urosepsis. Rivera et al. [Citation6] looked at 227 patients who underwent primary PCNL with infectious complications occurring in 16%. Additionally, there was a threefold increased risk of postoperative infection-related complications when multiple stones were removed. Large renal stones can serve as a foreign body that harbor colonized bacteria and making urine sterilization difficult even with antibiotic therapy [Citation6,Citation11]. The goal of using photothermal NPs is to thermally inactivate the bacteria on the stone surface immediately prior to breakage of the stone. The data in demonstrate that the bacteria are inactivated. The SEM images in indicate changes in the morphology of the biofilm to become more compacted onto the stone surface, which may be beneficial by trapping bacteria fully or partially inactivated bacteria onto the stone surface prior to breakage.
Prior studies have shown that NPs are capable of localizing to target tissues and generating heat for tissue destruction [Citation38]. One of the challenges of targeting any type of NPs to bacteria associated with kidney stones is that the species of bacteria is often unknown at the time of treatment. It would be advantageous to have a soft NP that can fit into the crevices of kidney stones and thermally ablate bacteria prior to the intense destruction of the stone. It may not be possible to target the NPs to bacteria as is the case for other types of photothermal ablation techniques where the cell type is known or the bacteria can be cultured prior to therapeutic intervention. Although the Ho:YAG laser is used frequently in urological endoscopic procedures, it emits at 2120 nm and the NPs used in this work are designed to absorb at 800 nm. Photothermal NPs are often designed to absorb infrared light in the tissue transparent window between 700 and 900 nm in order to by-pass the inherent tissue absorption. Even though the kidney stones would be able to be directly visualized in the operating room, photothermal polymers that absorb at 2010 nm have not yet been developed. Therefore, to first prove the concept that NPs can interface with E. coli biofilms associated with kidney stones, we used a polymer NP already being researched in other photothermal applications. It may be theorized that since polymer NPs are softer than their metallic photothermal counterparts, they may not serve as rigid nucleation sites for calcium crystal growth, although this aspect needs to be evaluated. Other NPs that can interface with bacteria and biofilms contained within kidney stones could be evaluated to further elucidate the mechanisms for thermally disrupting the biofilms. NIR can be delivered through the optical fiber of an endoscope, which is becoming a common technique for neurological procedures [Citation48]. Our vision is that these photothermal polymer NPs can be injected during PCNL/URS via ureteral catheter and syringe. We have previously demonstrated that the NPs are stable at either high or low pH, or subjected to multiple heating and cooling cycles without loss of their heat generating capacity [Citation38]. NPs that have not localized to biofilms or bacteria should be able to be washed away from the stone surface using a saline rinse and suction to remove excess material. Although we used 24 h of incubation to co-localize the NPs with the kidney stones, it is possible that a much shorter time, only a few minutes of dwell time, may be needed to localize the NPs with the bacteria. It is envisioned that this could be done intra-operatively, once the exact dwell time and concentration of NPs that remain co-localized to bacteria on the stone surface are determined. This was the rationale for evaluating both washed and unwashed stones, and the data in indicates that there is sufficient NP concentration interfacing with the biofilm and kidney stone surface to induce photothermal disruption of the biofilms. Then, NIR can be delivered through an optical fiber to impart a bactericidal effect prior to the stone fragmentation. We believe that this effect can be performed locally with minimal collateral tissue injury because the high temperatures will be localized to the stone surface and treatment will be rapid. In addition, our results have demonstrated that NPs may co-localize with kidney stones and washing to remove non-bound NPs still leads to sufficient bacterial elimination, indicating that the PCPDTBSe NPs may be well interfaced with the stone or bacteria. The same premise can be achieved clinically by washing excess NPs away from a kidney stone prior to NIR stimulation. E. coli was effectively reduced from both the artificial and patient-derived kidney stones. Previous literature has alluded to the use of a photothermal agents, including NPs, for ablation and breaking of kidney stones. The current work shows that low energy photothermal ablation can disrupt bacteria that may be associated with kidney stones and does not break the stone, as neither the artificial or patient-derived stones were broken using our techniques. This could be beneficial for inhibiting the dissemination of bacteria that occurs with ablation of kidney stones, and could therefore help limit the potential for urosepsis. The current work demonstrates that the PCPDTBSe NPs seem to co-localize with the E. coli cultured on the kidney stones, most likely via passively localizing within the grooves of the stones where bacteria thrive. Even washing to remove excess NP material seems to leave sufficient numbers of NPs behind to impart enough heat for disruption of pathogenic biofilms that cause significant clinical complications. This is the first time that this benefit has been demonstrated, and further refinements could easily be made by specifically targeting the NPs to negatively charged bacteria by coating the NP with positively charged materials.
Of significant note is that the temperatures measured in these experiments are indicative of bulk heating of a solution and it is not known how hot the surface of the stone with NPs actually is. There are a number of conclusions that can be drawn based on the energies used and temperatures obtained in this work. Application of 5 W of laser power over a 1 cm spot size for 60 and 300 s yields drastically different energy inputs into the kidney stone/NPs system. Sixty seconds of 800 nm light is 382.17 J/cm2, compared to 1910.83 J/cm2 for 300 s. However, the average temperature over 60 s is 41.99 °C and for 300 s it is 49.5 °C, thus beginning at a basal temperature of 37 °C, the average temperatures are 79 °C for 60 s and 86.5 °C for 300 s exposures. For bacteria that are killed by temperatures above 65 °C, these average temperatures most likely yield the reduction in bacteria observed at 300 s because they are held above 65 °C for a prolonged time. However, for both the artificial and patient-derived kidney stones treated with NPs, not washed and exposed to 60 s of NIR, there were statistically significant reductions in CFUs, on par with the results of the 300 s group. These results indicate that the rapid temperature spike using 60 s of NIR stimulation is sufficient for eliminating E. coli from the stones, and supports the notion that prolonged hyperthermia may not be needed for bacterial disruption, which would be valuable for clinical translation. By comparing the groups shown in , artificial kidney stones treated with NPs and not washed and then exposed to 300 s had a 73.4% decrease in CFU/g compared to the stones treated with 300 s NIR alone. Stones treated with NPs, washed and exposed to 300 s had a 50.89% decrease in CFU/g compared to the stones treated with 300 s NIR alone. Compared to stones treated with no NPs and no NIR, stones treated with 300 s of NIR (no NPs) had a 30.52% reduction, whereas stones with 300 s of NIR, NPs and washed had an 81.52% reduction, while stones with 300 s of NIR, NPs and not washed had a 65.88% reduction.
The data in shows that 5 W, 60 s of NIR without NPs generates heat (▵T of 25–28 °C), whereas 300 s without NPs leads to a ▵T of 63–68 °C. This indicates that the stones generate significant heat when stimulated with 800 nm. Ideally, is it desired that only the NPs stimulate heat, as can be observed in . Although there are some differences between not washing to remove excess NPs, compared to washing and with 300 s NIR, the differences between the groups are not statistically significant. Stones treated with NPs and washed to remove excess NPs had a 6% reduction in CFU/g compared to stones treated with 60 s of NIR alone, and a 13.57% reduction compared to stones that received neither NPs, nor NIR. By not washing the stones to remove excess NPs prior to 60 s NIR the elevated temperatures that occur in the bulk solution and at the stone interface led to a dramatic reduction (∼95%) compared to stones not treated with either NPs or NIR. Although this result seems better than the ∼73-81% reduction in the 300 s groups there is no statistical difference between NIR applied for either 60 or 300 s. Since water alone stimulated for 300 s does not generate much heat, the stones alone must be absorbing the NIR and converting it to heat. What is most notable is that the NPs enhance this effect. As shown in , 300 s without NPs, reduces the bacterial burden, but not significantly, even though a lot of heat was generated in the bulk solution when temperatures were measured. The fact that NPs (either washed away from the stone or not) can significantly decrease the bacteria indicate that the NPs must be doing the work, and must be locally interfaced with the E. coli to lead to such a reduction.
Our findings concur that NPs appear to co-localize with bacteria associated with kidney stones, and applying NIR laser can create localized heating that has a bactericidal effect. We found this effect to be significant from the control group when the laser was applied for either 60 or 300 s. While the work presented here does not show that NPs can be selectively targeted to E. coli (although this could be enhanced by functionalizing their surface), it does show that NPs can be localized to kidney stones, which harbor pathogenic bacteria, and that 60 s of NIR exposure is sufficient for reducing the bacterial burden through the generation of heat from the NPs. This work represents opportunities for further exploration into evaluating the role of kidney stone dimensions, NP targeting and concentration with optimal laser parameters for disrupting biofilms associated with kidney stones.
Conclusions
NPs have proven that they can interface with E. coli associated with artificial and patient-derived kidney stones to impart a bactericidal effect when stimulated with NIR light to generate elevated local temperatures. This is a promising property that has not been demonstrated previously. In the future, this tool can be utilized as a means of decreasing the chance of urosepsis that occurs following some endo-urological procedures. As there is currently no treatment regimen developed to address the challenges specific to stone-associated UTIs, this study investigates the possibility to precisely ablate bacteria adherent to kidney stones.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Scales CD, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165.
- Saigal CS, Joyce G., Timilsina AR. The urologic diseases in America project, direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68:1808–1814.
- Marcus RJ, Post JC, Stoodley P, et al. Biofilms in nephrology. Expert Opin Biol Ther. 2008;8:1159–1166.
- Tolordava E, Tiganova I, Alekseeva N, et al. Renal calculus microflora in urolithiasis and search for agents of control of biofilms formed by uropathogenic bacteria. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii. 2012;4:56–62.
- Li X, Lu N, Brady HR, et al. Ureolytic biomineralization reduces Proteus mirabilis biofilm susceptibility to ciprofloxacin. Antimicrob Agents Chemother. 2016;60:2993–3000.
- Rivera M, Viers B, Cockerill P, et al. Pre-and postoperative predictors of infection-related complications in patients undergoing percutaneous nephrolithotomy. J Endourol. 2016;30:982–986.
- Koras O, Bozkurt IH, Yonguc T, et al. Risk factors for postoperative infectious complications following percutaneous nephrolithotomy: a prospective clinical study. Urolithiasis. 2015;43:55–60.
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108.
- Niveditha S, Pramodhini S, Umadevi S, et al. The isolation and the biofilm formation of uropathogens in the patients with catheter associated urinary tract infections (UTIs). J Clin Diagn Res. 2012;6:1478–1482.
- Schaffer JN, Pearson MM. Proteus mirabilis and urinary tract infections. Microbiol Spectr. 2015;3(5). DOI: https://doi.org/10.1128/microbiolspec.UTI-0017-2013.
- Singh I, Shah S, Gupta S, et al. Efficacy of intraoperative renal stone culture in predicting postpercutaneous nephrolithotomy urosepsis/systemic inflammatory response syndrome: a prospective analytical study with review of literature. J Endourol. 2019;33:84–92.
- Fan S, Gong B, Hao Z, et al. Risk factors of infectious complications following flexible ureteroscope with a holmium laser: a retrospective study. Int J Clin Exp Med. 2015;8:11252.
- Zanetti G, Paparella S, Trinchieri A, et al. Infections and urolithiasis: current clinical evidence in prophylaxis and antibiotic therapy. Archivo Italiano Di Urologia Andrologia. 2008;80:5.
- Eswara JR, Sharif-Tabrizi A, Sacco D. Positive stone culture is associated with a higher rate of sepsis after endourological procedures. Urolithiasis. 2013;41:411–414.
- Castillo-Martínez JC, Martínez-Castañón GA, Martínez-Gutierrez F, et al. Antibacterial and antibiofilm activities of the photothermal therapy using gold nanorods against seven different bacterial strains. J Nanomater. 2015:783671. DOI:https://doi.org/10.1155/2015/783671
- Meeker DG, Jenkins SV, Miller EK, et al. Synergistic photothermal and antibiotic killing of biofilm-associated staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis. 2016;2:241–250.
- Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem C. 2016;120:4691–4716.
- Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34–55.
- Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28.
- Iancu C, Mocan L. Advances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermia. Int J Nanomed. 2011;6:1675–1684.
- Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1449.
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41:1842–1851.
- O’Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176.
- Pihl M, Bruzell E, Andersson M. Bacterial biofilm elimination using gold nanorod localised surface plasmon resonance generated heat. Mater Sci Eng C Mater Biol Appl. 2017;80:54–58.
- Ibelli T, Templeton S, Levi-Polyachenko N. Progress on utilizing hyperthermia for mitigating bacterial infections. Int J Hyperthermia. 2018;34:144–156.
- Kirui DK, Weber G, Talackine J, et al. Targeted laser therapy synergistically enhances efficacy of antibiotics against multi-drug resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Nanomedicine. 2019;20:102018.
- Zharov VP, Mercer KE, Galitovskaya EN, et al. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J. 2006;90:619–627.
- Li D-D, Wang J-X, Ma Y, et al. A donor–acceptor conjugated polymer with alternating isoindigo derivative and bithiophene units for near-infrared modulated cancer thermo-chemotherapy. ACS Appl Mater Interfaces. 2016;8:19312–19320.
- Korupalli C, Huang C-C, Lin W-C, et al. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials. 2017;116:1–9.
- Geng J, Sun C, Liu J, et al. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. Small. 2015;11:1603–1610.
- Zhang J, Yang C, Zhang R, et al. Biocompatible D–a semiconducting polymer nanoparticle with light-harvesting unit for highly effective photoacoustic imaging guided photothermal therapy. Adv Funct Mater. 2017;27:1605094.
- Sun H, Lv F, Liu L, et al. Conjugated polymer materials for photothermal therapy. Adv Therap. 2018;1:1800057.
- Sarkar S, Levi-Polyachenko N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv Drug Deliv Rev. 2020;163–164:40–64.
- Feng G, Fang Y, Liu J, et al. Multifunctional conjugated polymer nanoparticles for image‐guided photodynamic and photothermal therapy. Small. 2017;:1602807.
- Wang Y, Li S, Liu L, et al. Photothermal-responsive conjugated polymer nanoparticles for the rapid and effective killing of bacteria. ACS Appl Bio Mater. 2018;1:27–32.
- Akyol E, Öner M. Inhibition of calcium oxalate monohydrate crystal growth using polyelectrolytes. J Cryst Growth. 2007;307:137–144.
- Jung T, Sheng X, Choi CK, et al. Probing crystallization of calcium oxalate monohydrate and the role of macromolecule additives with in situ atomic force microscopy. Langmuir. 2004;20:8587–8596.
- Graham-Gurysh E, Kelkar S, McCabe-Lankford E, et al. Hybrid donor–acceptor polymer particles with amplified energy transfer for detection and on-demand treatment of breast cancer. ACS Appl Mater Interfaces. 2018;10:7697–7703.
- McCabe‐Lankford EE, Brown TL, Levi, NH, et al. Assessing fluorescence detection and effective photothermal therapy of near-infrared polymer nanoparticles using alginate tissue phantoms. Lasers Surg Med. 2018;50:1040–1049.
- Kelkar SS, McCabe ‐Lankford E, Albright, R, et al. Dual wavelength stimulation of polymeric nanoparticles for photothermal therapy. Lasers Surg Med. 2016;48:893–902.
- Hardy LA, Vinnichenko V, Fried NM. High power holmium:YAG versus thulium fiber laser treatment of kidney stones in dusting mode: ablation rate and fragment size studies. Lasers Surg Med. 2019;51:522–530.
- MacNeill CM, Coffin RC, Carroll DL, et al. Low band gap donor-acceptor conjugated polymer nanoparticles and their NIR-mediated thermal ablation of cancer cells. Macromol Biosci. 2013;13:28–34.
- Roper DK, Ahn W, Hoepfner M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J Phys Chem C. 2007;111:3636–3641.
- Quintero MDS, Álvarez UM, Wacher C, et al. Interaction of shockwaves with infected kidney stones: is there a bactericidal effect? J Endourol. 2008;22:1629–1638.,
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317.
- Robinson TE, Hughes EAB, Wiseman OJ, et al. Hexametaphosphate as a potential therapy for the dissolution and prevention of kidney stones. J Mater Chem B. 2020;8:5215–5224.
- Gutierrez J, Smith A, Geavlete P, et al. On behalf of the CROES PCNL Study Group, Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol. 2013;31:1135–1140.
- Rennert RC, Khan U, Bartek J, et al. Laser ablation of abnormal neurological tissue using robotic neuroblate system (LAANTERN): procedural safety and hospitalization. Neurosurgery. 2020;86:538–547.
