Abstract
Background
Renal denervation (RDN) is a new treatment for hypertension in patients with chronic kidney disease (CKD), but its efficacy is still debated. This meta-analysis aimed to evaluate the efficacy and safety of RDN for hypertension in patients with CKD.
Methods
PubMed, Web of Science, EMBASE, and Ovid databases were searched for relevant studies published. We performed both fixed- and random-effects meta-analyses of the changes in blood pressure, estimated glomerular filtration rate (eGFR), and urinary albumin-to-creatinine ratio (UACR) after RDN.
Results
The meta-analysis included 238 patients from 11 single-center, non-randomized, uncontrolled studies. Office blood pressure and 24-hour ambulatory blood pressure (24 h-ABP) showed a significant reduction 1 month after RDN (p < 0.05). This decrease of 24 h-ABP persisted for 24 months after RDN showed difference systolic blood pressure (p < 0.001) and diastolic blood pressure (p = 0.001). The 24 h-ABP exhibited a similar trend in the subgroup analysis. eGFR measurements obtained at each time point of analysis after RDN were not significantly different from those obtained before (p > 0.05). UACR levels were significantly reduced at 3 months and 6 months after RDN (p < 0.001). After RDN, the heart rate showed no significant changes (p > 0.05), and few major complications were encountered.
Conclusions
The meta-analysis showed that RDN may be effective and safe for treating CKD patients with hypertension. Well-designed randomized controlled trials of RDN are urgently needed to confirm the safety and reproducibility of RDN and to assess its impact on clinical outcomes.
Introduction
Chronic kidney disease (CKD) is a progressive chronic disease that carries a high burden of morbidity and mortality and affects more than 10% of the world’s population [Citation1,Citation2]. CKD often coexists with several chronic conditions, including diabetes, hypertension, and heart failure [Citation3]. Hypertension is also a major factor that causes both CKD mortality and cardiovascular diseases [Citation4].
In patients with CKD, hypertension establishment and maintenance are often critically dependent on numerous factors, including volume overload, activation of the renin-angiotensin system, increased endothelin levels, reduced nitric oxide bioavailability and/or medullary vasodilating factors, and oxidative stress [Citation5]. For a long time, volume overload and renin-angiotensin axis activation have been acknowledged as the most actively involved parameters in the pathogenesis of renal hypertension. However, in clinical practice, treatments for these aspects are not always accompanied by the expected reduction in blood pressure (BP). After years of research, sustained activation of the sympathetic nervous system (SNS) in CKD has been demonstrated to play a crucial role in the pathogenesis and maintenance of hypertension and the progression of CKD [Citation6].
In CKD, stimulation of the renal afferent nerves is caused by factors such as ischemia, and uremic toxins increase systemic sympathetic outflow. Sustained overactivity of SNS increases BP and aggravates further deterioration of the renal function at the same time [Citation7]. Under such circumstances, even with several antihypertensive drugs, drug therapy is usually ineffective in patients with CKD [Citation8].
The kidney is an effector organ for sympathetic nerve outflow and an important regulator of SNS activity; thus, blocking overactivity of the renal sympathetic nerve in CKD may be a reasonable treatment option for lowering BP and delaying the deterioration of kidney function [Citation9].
Renal denervation (RDN), also called catheter-based renal denervation, has been developed in recent years [Citation10]. Compared with surgical denervation, most commonly splanchnicectomy, this more minimally invasive and efficient radiofrequency-induced RDN has been shown to benefit patients with resistant hypertension [Citation11], and convincing results of many experimental hypertension with CKD animal model studies have shown that it effectively reduce BP and protect the kidneys [Citation9]. RDN appears to be a sensible treatment approach for CKD patients with hypertension. Some subgroup analyses from larger clinical trials and smaller research on mechanisms have started to explore this further. Although a comprehensive review on this topic has been published by Schmieder [Citation12], studies have reported inconsistent conclusions regarding the efficacy and safety of RDN, and there is no comprehensive review on RDN for CKD hypertension. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of RDN in the treatment of hypertension in patients with CKD.
Materials and methods
Search strategy
We performed a systematic search in PubMed, Web of Science, Embase, and Ovid databases from their inception to 30 November 2020. The terms used were (‘renal sympathetic denervation’ OR ‘renal nerve ablation’ OR ‘renal denervation’) AND (‘chronic kidney disease’ OR ‘end stage renal disease’) AND (‘blood pressure’ OR ‘hypertension’). We did not impose any filter on text availability or the publication date. This meta-analysis is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and was registered in the International Prospective Register of Systematic Reviews (registration number: CRD42020189455).
Study selection criteria
Participants
This review focused on patients with CKD, including those with CKD stages 1–5 and CKD5D stage, and those older than 18 years of age with hypertension.
Intervention
In this review, we included any transcatheter renal sympathetic denervation procedure performed using contemporary percutaneous catheters and radiofrequency probes.
Design
Prospective or retrospective studies were included.
Comparison
The comparison between the investigated group and the comparator group is not yet possible since there has been no comparator group in the vast majority of included studies.
Outcome measures
The primary outcome measure was BP control (change in office or clinic, 24-h mean ambulatory, ambulatory daytime mean, and night mean systolic and diastolic BPs). Secondary outcomes were kidney function (change in the estimated glomerular filtration rate [eGFR] and urinary albumin-to-creatinine ratio [UACR], and the need for renal replacement therapy), heart rate, use rate of antihypertensive drugs, and adverse effects, including but not limited to bleeding, femoral artery pseudoaneurysm, renal artery dissection, transient dizziness, and flank pain.
Eligibility criteria
The criteria for inclusion of a study in the meta-analysis were as follows. (1) Original research papers written in English. (2) The study participants were humans. (3) The studies were prospective or retrospective. (4) The study compared pre-ablative and post-ablative clinical results, including the primary or secondary outcomes described above. (5) The follow-up period was at least 3 months after ablation. The following publications were excluded: (1) abstracts, case reports, case series, editorials, reviews; (2) studies based on animal experiments; (3) studies based on pediatric populations; and (4) studies lacking relevant outcome data. The included studies were selected independently by two investigators (M.X. and T.L.). A third investigator (Y.H.) was the arbitrator in cases of disagreement.
Data extraction and quality assessment
Two investigators (M.X. and T.L.) independently performed data extraction using a standard electronic data extraction form. The abstracted data included the period, country, and type of study and the baseline characteristics of the included patients, such as age, sex, body mass index, follow-up time, CKD stage, and the aforementioned main outcomes (BP, eGFR, adverse events, and so on).
Study quality was assessed using the Newcastle-Ottawa Scale (NOS), which is an effective tool for evaluating non-randomized studies, including case-control and cohort studies. This ‘star system’ was judged on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively. A study with five or more stars in total was defined as high quality.
Data synthesis and statistical analysis
Before the analysis, data were standardized into equivalent units, and the median and extreme values or quartile data were converted to mean and standard deviation [Citation13]. For continuous variables, weighted mean differences (WMD) with 95% confidence intervals (CIs) were calculated for each study. For categorical variables, each single proportion is expressed as an effect size (ES) and 95% CIs [Citation14]. Heterogeneity was evaluated using the Cochrane Q test and I2 statistic to assess the degree of inter-study variation. I2 values of 0–24.9%, 25–49.9%, 50–74.9%, and 75–100% were considered as having no, mild, moderate, and significant thresholds for statistical heterogeneity, respectively. A fixed-effects model was applied when there was no or low heterogeneity (I2 <50%), whereas a random-effects model was applied when there was moderate or high heterogeneity (I2 >50%) [Citation15,Citation16]. Publication bias was assessed using the Egger’s test [Citation17]. Sensitivity analysis was performed to identify the influence of an individual study on pooled estimates by removing one study at a time [Citation15]. Data analysis was performed using STATA 14.0 (StataCorp, College Station, TX, USA), with p < 0.05 considered statistically significant.
Results
Included studies
We retrieved 444 articles from the databases, and 246 articles remained after eliminating duplicate articles. After reviewing the titles and abstracts, 214 patients were excluded after implementing the eligibility criteria. Of the 35 studies included in the full-text evaluation, seven were excluded because they were case reports, three were excluded because of the study protocol, two were excluded for insufficient data, two were excluded for duplicate patient data, and 10 did not meet the inclusion criteria. Finally, 11 single-center, non-randomized, uncontrolled studies were included in the meta-analysis () [Citation18–28]. These 11 studies included 238 patients and are summarized in . The NOS assessments are presented in . All studies had NOS scores of more than five stars, which were considered to indicate high quality.
Table 1. Base characteristics of included studies.
Table 2. Quality Assessment of the Included Studies
Blood pressure
As shown in and and , the results of the summary analysis of office BP and 24-h ambulatory BP monitoring (24 h ABPM), including office systolic BP (SBP) and office diastolic BP (DBP), showed a decreasing trend between before and after RDN. Among them, two articles [Citation21,Citation28] reported 24 h ABP 1 month after RDN. There was a significant difference in office SBP and office DBP between before and 1 month after RDN (all, p < 0.001), with no heterogeneity (). This significant difference in office SBP continued at 3, 6, and 12 months. By 24 months, there were still two articles [Citation21,Citation24] that recorded 24 h ABP, and there was a significant difference in 24 h ABP between before and 24 months after RDN (SBP: p < 0.001, DBP: p = 0.001), with mild and no evidence of heterogeneity, respectively ().
Figure 2. Forest plots of comparison between (A) office systolic-BP at six months after RDN and that of pre-ablation. (B) Office diastolic-BP at six months after RDN and that of pre-ablation. (C) Office systolic-BP at 12 months after RDN and that of pre-ablation. (D) Office diastolic-BP at 12 months after RDN and that of pre-ablation.
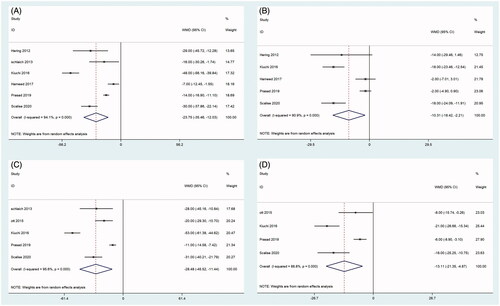
Figure 3. Forest plots of comparison between (A) 24-h ambulatory systolic-BP at six months after RDN and that of pre-ablation. (B) 24-h ambulatory diastolic-BP at six months after RDN and that of pre-ablation. (C) 24-h Ambulatory systolic-BP at 12 months after RDN and that of pre-ablation. (D) 24-h Ambulatory diastolic-BP at 12 months after RDN and that of pre-ablation.
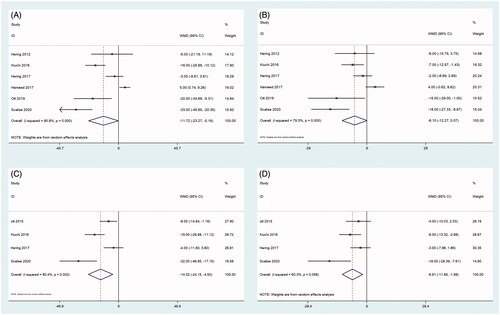
Table 3. Pooled effect on different type blood pressure at different timepoint.
Only two trials [Citation26,Citation28] in which the 24-h ambulatory SBP and DBP could be calculated from the data reported were included in the subgroup analysis of patients with end-stage renal disease (ESRD). The results showed that the treatment may lower 24-h ambulatory SBP and DBP between before and 6 months after RDN for hypertension in patients with ESRD (all p < 0.001), with no significant heterogeneity ().
Renal function
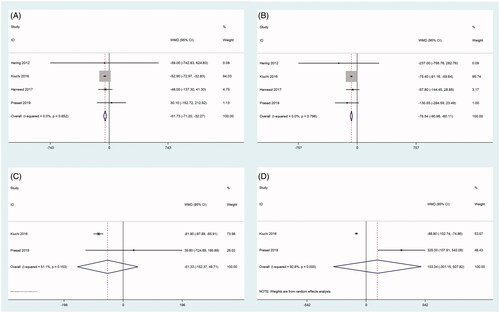
As shown in , two articles reported eGFR values at 1 month [Citation21,Citation23], four articles reported eGFR values at 3 months and 6 months [Citation18,Citation21,Citation23,Citation27], three articles reported eGFR values at 12 months [Citation20,Citation21,Citation27], and two articles reported eGFR values at 24 months after RDN [Citation21,Citation27]. The heterogeneity among the studies was significant; therefore, the random-effects model was used for the analysis (). The eGFR measurements obtained at 1 month, 3 months, 6 months, 12, and 24 months after RDN were not significantly different from those obtained before RDN (all, p > 0.05), as shown in and .
Figure 5. Forest plots of comparison between (A) eGFR at three months after RDN and that of pre-ablation. (B) eGFR at six months after RDN and that of pre-ablation. (C) eGFR at 12 months after RDN and that of pre-ablation. (D) eGFR at 24 months after RDN and that of pre-ablation.
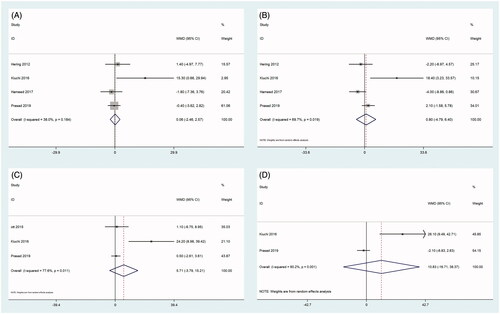
Table 4. Pooled effect on renal function at different timepoint.
Data on UACR levels were reported in four studies [Citation18,Citation21,Citation23,Citation27] (). UACR levels were significantly reduced at 3 months and 6 months after RDN (all, p < 0.001). There was no heterogeneity among the studies; therefore, the fixed-effects model was used for the analysis ( and ). However, UACR levels at 12 and 24 months after RDN were not significantly different from those obtained before RDN (all p > 0.05) (). The heterogeneity among the studies was moderate at 12 months and significant at 24 months after RDN; therefore, the random-effects model was used for the analysis ( and ).
Heart rate
Two articles [Citation18,Citation24] reported changes in the heart rate at 3 months, and three trials [Citation18,Citation24,Citation26] reported data at 6 months after RDN. No difference was found before and after the procedure (all, p > 0.05), with no heterogeneity among the studies (Figure S1).
Antihypertensive drugs
Overall, four trials [Citation19,Citation21,Citation27,Citation28] reported data on antihypertensive medications before and after RDN. The results of the summary analysis showed a significant decrease in antihypertensive medications after RDN (p < 0.001), with moderate heterogeneity among the studies (Figure S2).
Adverse events
Three major complications (hematoma, femoral pseudoaneurysm, and bleeding) were reported after RDN [Citation19,Citation21,Citation23,Citation25]. Only one article reported the data of the bleeding complication [Citation21]. The incidence of pseudoaneurysm was 1.3% (3/238, p = 0.07), with no heterogeneity among the studies, and the incidence of hematoma was 2.9% (7/238, p = 0.206), with significant heterogeneity among the studies (Figure S3).
Two cases of progression to ESRD and one case of dialysis-related complications were recorded in two trials [Citation23,Citation25]; however, these events occurred several months after the procedure, and their occurrence was related to the patients’ kidney disease. One patient had a myocardial infarction that was not ruled out to be related to his own disease [Citation25]. Therefore, they were not considered as procedure-related events.
Sensitivity analyses and publication bias
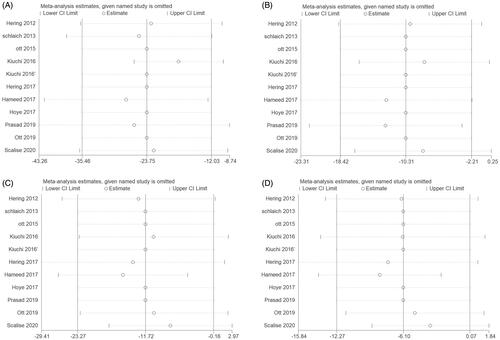
Sensitivity analyses for changes in the BP, eGFR, and UACR level at each month after RDN were used to judge the dependability of the results. One study was deleted at a time, and no such deletion had any significant impact on the results, as shown in .
Figure 7. Results of sensitivity analyses. (A) Office systolic-BP at six months after RDN and that of pre-ablation. (B) Office Diastolic-BP at six months after RDN and that of pre-ablation. (C) 24-h ambulatory Systolic-BP at six months after RDN and that of pre-ablation. (D) 24-h ambulatory Diastolic-BP at six months after RDN and that of pre-ablation.
Publication bias analyses were conducted for primary outcomes. No evidence of publication bias was detected for the BP using the Egger test ().
Table 5. Assessment of publication bias based on different outcome.
Discussion
To our knowledge, the present study is the first meta-analysis to evaluate the efficacy and safety of RDN for hypertension in patients with CKD. The overall outcome showed that individuals treated with RDN experienced significantly greater 24-h BP and diastolic BP reductions, and RDN did not increase the risk of rapid deterioration of renal function and other major adverse events.
In our study, a significant decrease in the average 24-h and office BP was observed 1 month after RDN. Similar to previous studies on the renal sympathetic nerve in patients with resistant hypertension, our review of ABPM and office BP obtained during a follow-up period of 24 months showed a significant decrease in the ambulatory BP, especially the systolic ambulatory BP after RDN. This difference was maintained until 1, 3, 6, 12, and 24 months postoperatively [Citation29,Citation30]. As a more selective method of reducing renal sympathetic secretion, RDN effectively removes sympathetic nerve overactivity and leads to a systemic decrease in sympathetic tone [Citation31]. In particular, sympathetic nerve activity is more obvious in CKD, and the degree of sympathetic nerve overdrive increases with disease progression [Citation32]. Previous research also suggests that the role of sympathetic overactivity in driving sustained hypertension is derived from its effects on renal function; RDN interrupts renal afferent and efferent sympathetic nerves, which modulate central sympathetic outflow and renal physiology to achieve sustained BP reduction in patients with CKD [Citation33,Citation34]. RDN can also reduce increased renin secretion and increased renal tubular sodium reabsorption caused by renal sympathetic nerve activity, thereby inhibiting the renin-angiotensin system and achieving the purpose of lowering BP [Citation35,Citation36]. Furthermore, RDN may affect peripheral vascular resistance by significantly reducing the sympathetic trophic effect on arteriolar walls and arterial stiffness in patients with CKD, which is more conducive to lowering BP [Citation18,Citation37]. However, it is unknown how long BP reduction is maintained as sympathetic nerves recover after the lesion [Citation38]. Data showed that DBP and SBP levels were significantly lower at 24 months after RDN, and neither the recovery of nerve fibers nor the effective counter-regulatory mechanism that elevates BP was found [Citation39]. Even 36 months after RDN, BP was significantly lower than before RDN [Citation40].
Subgroup analysis of ESRD patients with hypertension showed similar changes in the BP after RDN treatment. This finding is in line with those of previous reports in patients with ESRD, and shows that BP reduction accompanying renal denervation was greater than that during the whole CKD stage. This may originate from the fact that patients with advanced CKD and ESRD are more likely to have increased sympathetic nerve activity given their impaired capacity for sodium and fluid excretion and increased sympathetic drive [Citation41,Citation42]. Furthermore, in patients with ESRD, the increase in nerve density in the internal area of the periadventitial tissue may have also played a role [Citation42]. This implies that RDN may target more nerves in patients with ESRD than in other hypertensive patients [Citation43]. It is also possible that the increasing improvement and completion of RDN catheters and a higher number of renal artery ablations can be performed [Citation28], thereby more effectively destroying sympathetic fibers within the renal tissue. However, compared to patients with CKD who are not undergoing long-term dialysis, the BP of patients with ESRD is affected by many factors, such as the volume load, electrolytes, and other interference factors [Citation44]. Therefore, further well-designed large clinical trials are needed to determine the potential role of this in ESRD, given that activation of sympathetic nerve activity has been described as a crucial component in the development and progression of CKD [Citation45].
There is growing evidence that therapeutic sympathetic nerve ablation aimed at targeting renal nerves may have potential benefits for CKD [Citation20,Citation24]. The BP-lowering effect of RDN can have a protective effect on the kidney, as the detrimental effect of hypertension on renal function has been well established [Citation46]. Inhibition of renal sympathetic nerve activity may lead to prominent vasodilatation in preglomerular arterioles, decreased renal vascular resistance, and increased renal blood flow, which may be the major determinants of improved renal function [Citation47]. Our data showed that the eGFR was unchanged and urinary albumin excretion, usually expressed as total UACR [Citation48], was significantly reduced at 1, 3, and 6 months after catheter-based RDN, suggesting that RDN and the associated hemodynamic changes have no adverse effects on the kidneys. Although it is unclear whether the unchanged eGFR was related to the progression of individual differences in CKD progression, urinary albumin excretion, as an early marker of CKD progression [Citation49], is encouraging in the early postoperative follow-up.
Regarding complications and adverse effects after RDN, among the 238 patients in the 11 studies included, there was one case of bleeding, three cases of pseudoaneurysm, and seven cases of hematoma among the adverse reactions related to puncture-site complications, which are common with any percutaneous angiographic procedure [Citation23]. The heart rate showed no significant changes after treatment. Moreover, no incidence of electrolyte disturbances, including hyperkalemia, hypokalemia, hypercalcemia, and hypocalcemia, was reported in our included studies after the procedure, supporting that renal denervation may not adversely affect autoregulation of the kidneys [Citation50]. No serious procedure-related complications (including all-cause death, major cardiovascular events, perioperative complications, and hypertension crisis) were reported.
There are several limitations to our analysis. First, significant heterogeneity was found in some pooled results, which may be due to the (1) source and number of populations, (2) different CKD stages, (3) varied ablation types, and (4) the heterogeneity of some measurement methods, such as the office BP measurement. We attempted to explore this relationship between studies by pooling the data using sensitive analysis and subgroup analysis. However, some heterogeneities could not be avoided because of the limited number of included studies. Second, the number of included studies and sample size were small; although this might not have caused obvious publication bias, there is still potential for bias. Third, most of the studies will focus on the efficacy of treatment more than 24 months after surgery in the future, so there is not enough current information to analyze the long-term efficacy of RDN. Finally, after searching the database thoroughly, no relevant randomized controlled trials were found. Thus, well-designed randomized controlled trials of RDN are urgently needed to confirm the safety and reproducibility of this treatment and to assess its impact on clinical outcomes in the future.
Conclusions
In conclusion, our meta-analysis indicates that RDN may be effective and safe for treating CKD patients with hypertension. It helped achieve a highly significant reduction in BP with no adverse reaction to renal function and enabled a substantial reduction in the number of antihypertensive drugs used. Moreover, RDN was associated with reduced albumin excretion during this short follow-up period, and no major complications occurred.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Makhele L, Matlala M, Sibanda M, et al. A cost analysis of haemodialysis and peritoneal dialysis for the management of end-stage renal failure at an academic hospital in Pretoria, South Africa. Pharmacoecon Open. 2019;3(4):631–641.
- Sato Y, Yanagita M. Functional heterogeneity of resident fibroblasts in the kidney. Proc Jpn Acad Ser B Phys Biol Sci, 2019.95(8):468–478.
- Blankenburg M, Fett A-K, Eisenring S, et al. Patient characteristics and initiation of mineralocorticoid receptor antagonists in patients with chronic kidney disease in routine clinical practice in the US: a retrospective cohort study. BMC Nephrol, 2019;20(1):171.
- Lin Y-C, Lin J-W, Wu M-S, et al. Effects of calcium channel blockers comparing to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with hypertension and chronic kidney disease stage 3 to 5 and dialysis: a systematic review and meta-analysis. PLOS One, 2017;12(12):e0188975.
- Schalekamp MA, Man in't Veld AJ, Wenting GJ. The second Sir George Pickering memorial lecture. What regulates whole body autoregulation? Clinical observations. J Hypertens. 1985;3(2):97–108.
- Converse RL, Jacobsen TN, Toto RD, Jr., et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327(27):1912–1918.
- Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension, 1995;25(4):878–882.
- Verdalles U, de Vinuesa SG, Goicoechea M, et al. Utility of bioimpedance spectroscopy (BIS) in the management of refractory hypertension in patients with chronic kidney disease (CKD). Nephrol Dial Transplant. 2012;27(Suppl 4):iv31–iv35.
- Sata Y, Schlaich MP. The potential role of catheter-based renal sympathetic denervation in chronic and end-stage kidney disease. J Cardiovasc Pharmacol Ther, 2016;21(4):344–352.
- Mancia G. Renal nerve ablation. Eur Heart J. 2018;39(46):4060–4061.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens. 2011;20(6):647–653.
- Schmieder RE. Renal denervation: where do we stand and what is the relevance to the nephrologist? Nephrol Dial Transplant, 2020:gfaa237.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol, 2014;14(1):135.
- Miller JJ. The inverse of the Freeman – Tukey double arcsine transformation. Am Statist. 1978;32(4):138.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ, 2003;327(7414):557–560.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21(11):1539–1558.
- Egger M, Davey Smith G, Schneider M, et al., Bias in meta-analysis detected by a simple, graphical test. BMJ, 1997;315(7109):629–634.
- Hering D, Mahfoud F, Walton AS, et al., Renal denervation in moderate to severe CKD. JASN. 2012;23(7):1250–1257.
- Schlaich MP, Bart B, Hering D, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol, 2013;168(3):2214–2220.
- Ott C, Mahfoud F, Schmid A, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33(6): p. 1261–1266.
- Kiuchi MG, Chen S. Effectiveness of renal sympathetic denervation in renal function and blood pressure in CKD and non-CKD patients with controlled vs. uncontrolled hypertension. Int J Cardiol Heart Vasc. 2016;13:3–5.
- Kiuchi MG, Graciano ML, Carreira MAMQ, et al. Long-term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens. 2016;18(3):190–196.
- Hameed MA, Freedman JS, Watkin R, et al. Renal denervation using carbon dioxide renal angiography in patients with uncontrolled hypertension and moderate to severe chronic kidney disease. Clin Kidney J. 2017;10(6):778–782.
- Hering D, Marusic P, Duval J, et al., Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol. 2017;232:93–97.
- Hoye NA, Wilson LC, Wilkins GT, et al. Endovascular renal denervation in end-stage kidney disease patients: cardiovascular protection-a proof-of-concept study. Kidney Int Rep. 2017;2(5):856–865.
- Ott C, Schmid A, Ditting T, et al. Effects of renal denervation on blood pressure in hypertensive patients with end-stage renal disease: a single centre experience. Clin Exp Nephrol. 2019;23(6):749–755.
- Prasad B, Berry W, Goyal K, et al. Central blood pressure and pulse wave velocity changes post renal denervation in patients with stages 3 and 4 chronic kidney disease: the Regina RDN Study. Can J Kidney Health Dis. 2019;6:205435811982838.
- Scalise F, Sole A, Singh G, et al. Renal denervation in patients with end-stage renal disease and resistant hypertension on long-term haemodialysis.J Hypertens. 2020;38(5):936–942.
- Daemen J, Mahfoud F, Kuck K-H, et al. Safety and efficacy of endovascular ultrasound renal denervation in resistant hypertension: 12-month results from the ACHIEVE study. J Hypertens. 2019;37(9):1906–1912.
- Kario K, Yamamoto E, Tomita H, et al., on behalf of the SYMPLICITY HTN-Japan Investigators. Sufficient and persistent blood pressure reduction in the final long-term results from SYMPLICITY HTN-Japan-safety and efficacy of renal denervation at 3 years. Circ J. 2019;83(3):622–629.
- Jaén-Águila F, Vargas-Hitos JA, Mediavilla-García JD. Implications of renal denervation therapy in patients with sleep apnea. Int J Hypertens. 2015;2015:408574.
- Baek SH, Cha R-H, Kang SW, et al. Circulating renalase predicts all-cause mortality and renal outcomes in patients with advanced chronic kidney disease. Korean J Intern Med. 2019;34(4):858–866.
- Wang T-D, Lee Y-H, Chang S-S, et al., 2019 Consensus statement of the Taiwan Hypertension Society and the Taiwan Society of Cardiology on renal denervation for the management of arterial hypertension. Acta Cardiol Sin. 2019;35(3):199–230.
- Weber MA, Mahfoud F, Schmieder RE, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Interv, 2019;12(12):1095–1105.
- Kannan A, Medina RI, Nagajothi N, et al., Renal sympathetic nervous system and the effects of denervation on renal arteries.World J Cardiol. 2014;6(8):814–823.
- Sata Y, Head GA, Denton K, et al. Role of the sympathetic nervous system and its modulation in renal hypertension. Front Med. 2018;5:82.
- Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116(6):1007–1021.
- Norvell JE, Weitsen HA, Dwyer JJ. Degeneration and regeneration of adrenergic nerves in the autotransplanted kidney. Transplantation, 1969;7(3):218–220.
- Kiuchi MG, Maia GLM, de Queiroz Carreira MAM, et al. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J. 2013;34(28):2114–2121.
- Naduvathumuriyil T. et al. Clinical benefits and safety of renal denervation in severe arterial hypertension: a long-term follow-up study. J Clin Hypertens, 2020;22(10):1854–1864.
- Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61(2):457–464.
- Mauriello A, Rovella V, Anemona L, et al. Increased sympathetic renal innervation in hemodialysis patients is the anatomical substrate of sympathetic hyperactivity in end-stage renal disease. J Am Heart Assoc. 2015;4(12):e002426.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64(7):635–643.
- Miskulin DC, Weiner DE. Blood pressure management in hemodialysis patients: what we know and what questions remain. Semin Dial. 2017;30(3):203–212.
- Noh MR, Jang H-S, Kim J, et al. Renal sympathetic nerve-derived signaling in acute and chronic kidney diseases. Int J Mol Sci. 2020;21(5):1647.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab. 2014;5(5):124–136.
- Lohmeier TE, Iliescu R, Liu B, et al., Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59(2):331–338.
- Chen H-Y, Zhong X, Huang XR, et al. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther. 2014;22(4):842–853.
- Meneses GC, Libório AB, de Daher EF, et al. Urinary monocyte chemotactic protein-1 (MCP-1) in leprosy patients: increased risk for kidney damage. BMC Infect Dis. 2014;14(1):451.
- DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension. 2004;43(2):147–150.