Abstract
Objective
This retrospective study aimed to examine the benefits and complications of radiofrequency ablation (RFA) in patients with papillary thyroid microcarcinoma (PTMC) in the isthmus.
Methods
This retrospective study included patients with PTMC in the isthmus and treated at the Chinese People’s Liberation Army hospital from 05/2014 to 05/2018. The patients were divided into the RFA and total thyroidectomy (TT) groups. The outcomes were operation-related complications, rate of recurrence, metastasis rate, and thyroid carcinoma-specific questionnaire of quality of life (THYCA-QOL).
Results
Among 218 patients, 115 patients underwent RFA, and 103 underwent TT. The rates of disappearance of the ablation zone at 1, 3, 6, 12, and 18 months after RFA were 0.8% (1/115), 10.4% (12/115), 51.3% (59/115), 90.4% (104/115), and 100% (115/115), respectively. Surgical time, blood loss, hospital stays, and treatment costs were higher with TT than with RFA (all p < 0.001). The final THYCA-QOL score of the RFA group was significantly higher than in the TT group (p < 0.001). Minor pain at the operation site was seen in all patients in the RFA group. No distant metastasis was detected in all patients, but one patient in the RFA group had a recurrence after 6 months. The final THYCA-QOL score of the RFA group was significantly lower than in the TT group (p < 0.001).
Conclusion
These results suggest that RFA for PTMC in the isthmus had similar outcomes than TT. It will have to be confirmed in future studies.
Introduction
The estimated worldwide incidence of thyroid carcinoma in 2018 was 567,233 in 185 countries [Citation1], and the incidence in the United States of America was 52,890 [Citation2]. Papillary thyroid carcinoma (PTC) is the most common pathological subtype, representing about 80% of all thyroid cancers [Citation3]. In the past two decades, the incidence of thyroid carcinoma and the number of surgeries for this cancer have increased significantly [Citation4–6]. The reasons for this rapidly increasing trend are mainly due to the growing number of small pre-clinical PTCs found because of better imaging systems and/or the use of high-frequency ultrasound examinations during routine health checkups [Citation4–6].
Surgery has been the traditional primary treatment for PTC for decades, and total thyroidectomy (TT) and lobectomy of the thyroid gland (LT) can be performed. Nevertheless, whether TT is better than LT regarding overall survival (OS) and recurrence-free survival (RFS) is an ongoing controversy [Citation7,Citation8]. This debate arises mainly from the fact that the prognosis of thyroid carcinoma is usually quite good. Meta-analyses do not reveal a significant advantage of TT over LT in all patients with PTC [Citation7,Citation8].
The thyroid isthmus is located between the thyroid’s bilateral lobes, connecting both lobes like a bridge. It is generally believed that malignant lesions in the isthmus tend to produce extrathyroidal extension (ETE) due to the small size of the isthmus, but there is no guideline or consensus on the surgical treatment of thyroid carcinoma in the isthmus [Citation9–11]. Some authors suggest that TT should be recommended for patients with PTC in the isthmus because of the high possibility of cervical lymph node metastasis [Citation12]. On the other hand, some authors suggest that isthmectomy might be suitable for selected patients with differentiated thyroid carcinoma [Citation13].
The 2015 ATA guidelines recommend active surveillance as the first-line management for low-risk papillary thyroid microcarcinoma (PTMC) [Citation14,Citation15] due to its asymptomatic nature. This strategy shows a good prognosis and avoids vocal fold paralysis, hypoparathyroidism, and other complications and overtreatments, and unnecessary surgeries. Nevertheless, some patients with low-risk PTMC are worrying about a possible recurrence. Thermal ablation methods such as radiofrequency ablation (RFA), laser ablation (LA), and microwave ablation (MWA) were studied for such patients [Citation16–18]. Thermal ablation can be performed in patients with PTMC and with high operative risk and patients with PTC who are reluctant to undergo an operation [Citation16,Citation19,Citation20]. Good prognosis and relatively low occurrence of complications were found in these studies [Citation16,Citation19,Citation20], but no study focused on using RFA for PTMC in the thyroid isthmus.
Therefore, the present retrospective study aimed to examine the benefits and complications of RFA in patients with PTMC in the isthmus. The results could support the use of RFA for this population of patients.
Materials and methods
Patients
This retrospective study included patients with PTMC in the isthmus and diagnosed at the Ultrasound Diagnosis Department of the General Hospital of Chinese PLA from May 2014 to May 2018. The inclusion criteria were 1) diagnosed with PTMC pathologically, 2) a single nodular PTMC (diameter ≤1 cm) limited only to the isthmus by ultrasound (the boundary of isthmus could be defined as two fabricated lines lying just beside the lateral border of the trachea [Citation21]) (), 3) underwent RFA or TT, and 4) noninvasive PTMC confirmed by histopathological examination according to the definition from the World Health Organization [Citation22]. Patients with incomplete follow-up data were excluded.
This study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (S2019-211-01). The requirement for informed consent was waived.
Presurgical staging
All patients underwent laboratory examinations (routine blood tests and thyroid function) and radiographic examination (chest X-ray imaging and thyroid and cervical lymph node ultrasound) before treatment. The diameter, volume (V = πabc/6; V: volume; a: transverse diameter; b: vertical diameter; c: anteroposterior diameter), location, presence of calcifications, and the relation to the capsule membrane of the tumor were carefully evaluated by ultrasound. The blood supply of the tumor was evaluated using contrast-enhanced ultrasound (CEUS). During routine ultrasound examination of the lymph nodes, the following signs suggest a high suspicion of metastasis: absence of the hilum, punctate calcification within the lymph nodes, cystic changes within the lymph nodes, and abundant blood flow around the lymph nodes. LN metastasis was highly suspected if CEUS showed the following signs: perfusion of contrast agent from the periphery to the center, the disappearance of the normal vascular structures in the hilar region of the lymph node, and uneven perfusion of contrast agent in the lymph node. All ultrasound examinations were performed by a specifically qualified sonographer with a professional experience of >10 years.
Operation selection and grouping
The operation was selected by the patients themselves after a comprehensive discussion with the physicians about the indications and pros and cons. Still, if there was a definite diagnosis of cervical lymph node metastasis or extra-glandular invasion by ultrasound, the patient was strongly advised to opt for excision. Ablation was recommended if no metastatic lymph nodes were detected by ultrasound and if there were a significant dislocation between the thyroid and the extraglandular anterior cervical muscle group during swallowing, indicating no extraglandular invasion.
Ultrasound-guided RFA
A specific operation room for all RFA procedures was allocated in the outpatient department. The patients were treated by an interventional sonographer with a professional experience of >10 years. An L3-6 high-frequency linear array probe (3–6 MHz) and the ACUSON Sequoia 512 ultrasound system (Siemens, Erlangen, Germany) were used to perform the ultrasound examinations. The Celon AG RFA System (Olympus, Tokyo, Japan) with a disposable Bipolar RF Electrode (18 G, Olympus, Tokyo, Japan) was used for RFA. The electrode’s non-coated length was 9 mm, and the output power was set to 3–6 W.
The patient was placed in the supine position with the head tilted a bit backward to maintain a good operation field. The area was conventionally disinfected and covered with sterile surgical towels. Local infiltration anesthesia with 1% lidocaine was done between the puncture site and the thyroid’s anterior capsule membrane. Sterile saline was injected into the soft tissue around the isthmus and trachea, which produced a ≥ 10 mm thick zone for protection against heat injury (i.e. the hydrodissection technique). The operation approach was designed according to the tumor’s location, and the electrode was pushed into the center of the tumor under ultrasound guidance. Once the electrode was appropriately positioned, the RFA was initiated with a ‘mobile shooting’ technique developed by Baek et al.: the probe was constantly moved back and forth and in other directions, but the movement was restricted to inside the tumor only [Citation23]. At the very beginning of RFA, a vaporized area appeared around the probe, and a total and transient high-reflection zone over the tumor indicated the termination of RFA. In many patients, the nodules were adjacent to the capsule. Due to the thinness of the isthmus, the capsule was ablated together during the ablation. The efficacy of RFA was evaluated by CEUS. No contrast perfusion into the ablation zone with a safety zone ≥3 mm was defined as complete ablation. If not, supplementing RFA was performed immediately.
Throughout the RFA process, the blood pressure (BP), electrocardiogram, oxygen saturation, heart rate, and respiratory rate of the patient were monitored by a nurse from the ICU. Another registered nurse acted as an assistant to perform the RFA. The patient could not leave the room after RFA until the doctors and nurses were sure that there were no abnormalities in vital signs, hoarseness, and local bleeding after 1–2 h.
Thyroidectomy
TT was performed under general anesthesia by two experienced surgeons (both with >15 years of experience). A 6–8-cm-long transverse incision was made 2 cm above the sternal notch, and the thyroid was reached using an electro-scalpel. The deep cervical fascia was incised in the longitudinal direction, and the anterior fasciculus of the jugular vein was divided to expose the thyroid gland. TT was performed, and the capsule membrane was carefully stripped off. Lymphadenectomy in the central region of the thyroid was performed. Finally, a drainage tube was routinely preserved, and the incision was closed with a subcuticular suture. TSH suppression therapy (TSH was maintained at a relatively middle-lower level, 0.5–2.0 mU/L) was given to all patients to minimize the possibility of recurrence of cancer.
Data collection and definition
Data collected for analysis included demographics (age and sex) and cancer characteristics (largest diameter, volume, and microcalcification). The outcomes included surgical time, blood loss volume during surgery, hospital stays, and treatment costs, operation-related complications (transient hypocalcemia, temporary hoarseness, etc.), the outcomes within 6 months after operation (scar, lymphatic metastasis, rate of recurrence), and thyroid carcinoma-specific questionnaire of quality of life (THYCA-QOL) during follow-up. The complications were reported according to Mauri et al. [Citation24].
The surgical time of RFA started with local anesthetics injection and ended with the patients leaving the operation room. The surgical time of surgery ranged from the incision and ended with incision closure, i.e. not including anesthesia induction or recovery. The costs for RFA mainly included pre-operation examinations, operation process, local anesthesia, and radiofrequency probe, while that for surgery covered pre-operation examinations, surgery process (including vital sign monitoring and consumable medical material), general anesthesia, bed costs, nursing charges, and drugs.
The volume reduction ratio (VRR) was calculated as (volume before RFA-volume after RFA)/volume before RFA × 100%. If there was only a linear scar left in the ablation zone, this reduction was considered as complete as the volume was impossible to be determined.
An effective RFA was defined as no CEUS abnormal enhancement at 6 months. Local tumor progression (LTP) included two situations: 1) newly confirmed PTMC or persistent malignancy by biopsy; and 2) cervical lymph node metastasis confirmed by biopsy. Locoregional recurrence (LRR) was defined as a newly detected malignant lesion in the thyroid bed or the contralateral lobe or metastatic lymph nodes >6 months after the initial surgery and proven to be malignant by cytology and surgical excision [Citation25]. The diagnosis of recurrence was made according to the pathological evidence, and complications were defined with reference to the classification listed in the standard protocol of thyroid ablation [Citation26]. Distant metastases were detected by computed tomography (CT), positron emission tomography (PET), or bone scan, according to symptoms and suspicions.
The specific questionnaire for patients with thyroid carcinoma (THYCA-QOL score) issued by the European Organization for Research and Treatment of Cancer (EORTC) [Citation27] was used to record and analyze seven symptomatic problems (neuro-muscular, voice, attention, sympathetic nerve system, oral cavity/larynx, psychology, and sensation) and six events (scar, coldness, stinging hands/feet, weight gain, headache, and sexual desire).
Follow-up
Routine outpatient follow-ups were scheduled at 1, 3, and 6 months after RFA and then at 6-month intervals. The follow-up data were obtained at 6 months after RFA.
Statistical analysis
SPSS 25.0 (IBM, Armonk, NY, USA) was used for statistical analysis. The Shapiro-Wilk test was used to test whether the distribution of the continuous variables was normal. If the continuous variables were normally distributed, they were presented as means ± standard deviations and analyzed using Student’s t-test; otherwise, they were presented as medians and interquartile range and analyzed using the Mann-Whitney U-test. Categorical data were presented as numbers and percentages and analyzed using the chi-square test or Fisher’s exact test, as appropriate. P-values ≤0.05 were considered statistically significant.
Results
Characteristics of the patients
A total of 218 patients pathologically diagnosed with PTMC in the isthmus were included, among whom 115 underwent RFA, and 103 underwent TT (). The baseline characteristics of the two groups, including age, sex, and duration of follow-up, were similar between the two groups (all p > 0.05) (). There were no significant differences in the maximum diameter and the volume of the tumor between the two groups (both p > 0.05) (). Still, the frequency of microcalcification was higher in the TT group than the RFA group (57.3% vs. 32.2%, p < 0.001).
Table 1. Characteristics and clinical features of the patients.
Characteristics of the RFA treatments
None of the patients in the RFA group needed a supplemental ablation. During follow-up, the ablation zone gradually decreased, and the VRRs at 1, 3, 6, 12, and 18 months after RFA were 70.8 ± 19.0%, 90.8 ± 8.7%, 97.6 ± 5.2%, 99.6 ± 1.8%, and 100%, respectively. The rates of disappearance of the ablation zones at 1, 3, 6, 12, and 18 months after RFA were 0.8% (1/115), 10.4% (12/115), 51.3% (59/115), 90.4% (104/115), and 100% (115/115), respectively.
Outcomes and complications in two groups
At 18 months, all the ablation zones were completely absorbed. The surgical time, blood loss, hospital stays, and treatment costs were all higher in the TT group than in the RFA group (all p < 0.001) (). Minor peri-procedural transient pain at the operation site was seen in all patients in the RFA group. Two patients had peri-procedural hoarseness (minor complication) that resolved by itself by 1 and 3 months, respectively. No patient in the RFA group needed a second surgery. In the TT group, transient minor hypocalcemia was found in one patient after surgery, and this patient gradually recovered when calcium was administered. A cervical hematoma or recurrent laryngeal nerve injury was not found in any patients in either group.
Table 2. Outcomes of the thyroid carcinoma.
Two patients in the TT group were confirmed with cervical lymph node metastasis in the central area by needle puncture biopsy (). No patient in the RFA group was found with signs of metastasis at ultrasound. No distant metastasis was detected in all patients, but one patient in the RFA group had a recurrence after 6 months, showing as multiple dots with strong echo in the gland tissue at ultrasound. The biopsy of the dots was pathologically proven to be PTC.
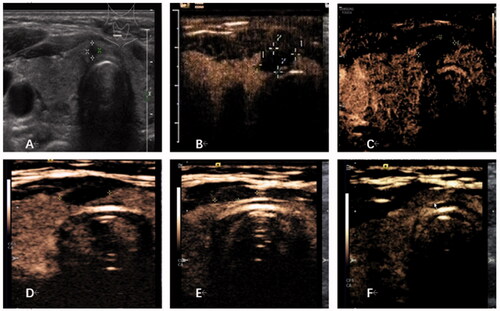
showed the imaging evaluation of one patient during treatment.
Figure 2. Imaging evaluation of one patient. (A) B-mode ultrasound revealed a hypoechoic nodule on the thyroid isthmus, 3 × 3 × 4 mm in size. (B) Contrast-enhanced ultrasound (CEUS) examination was performed after RFA, and the ablation area was 14 × 6 × 15 mm. (C) One month after ablation, CEUS showed that the ablation area was 10 × 4 × 10 mm, with a volume reduction rate of about 68%. (D) At 3 months after ablation, CEUS showed that the ablation area was 8 × 4 × 7 mm, with a volume reduction rate of about 82%. (E) At 6 months after ablation, CEUS showed that the ablation area was 8 × 3 × 6 mm, with a volume reduction rate of about 89%. (F) At 12 months after ablation, the ablation area was completely absorbed, and the isthmus was uniformly enhanced by contrast-enhanced ultrasound.
Quality of life
The final THYCA-QOL score of the RFA group was significantly higher than that of the TT group (p < 0.001) ().
Proportion of patients on L-thyroxine therapy
All patients in the TT group experienced hypothyroidism after surgery, and replacement therapy with L-thyroxine was needed life-long. For the patients in the RFA group, 12–33 months of L-thyroxine therapy was given to suppress the TSH to a middle-lower level. Many patients could not be weaned immediately from TSH suppression therapy, and the dose had to be tapered ¼ of tablet once every 3 months. The therapy was stopped once the coagulative necrotic area was completely absorbed, except for four patients with abnormalities of the thyroid function (one with hyperthyroidism and three with elevated blood levels of thyroglobulin antibody).
Other unfavorable events
The patients in the RFA group had no scars on the neck, but all patients in the TT group had scars after surgery.
Discussion
RFA can be used to treat PTMC in selected patients [Citation16,Citation19,Citation20,Citation28–31], but no study specifically examined the RFA treatment of PTMC in the thyroid isthmus. This retrospective study aimed to examine the benefits and complications of RFA in patients with PTMC in the isthmus. The results suggest that RFA for PTMC in the isthmus had similar outcomes to TT. It will have to be confirmed in future studies.
The incidence of malignancy in the thyroid isthmus is 2.2–9.2% [Citation21,Citation32]. For benign lesions, RFA has been reported to have excellent outcomes and few complications [Citation33–36], with similar efficacy to surgery [Citation37,Citation38]. For recurrent thyroid malignant lesions, RFA has also been reported to have excellent outcomes [Citation39–41] and no metastatic spread [Citation26], but recurrences can be observed [Citation42]. Malignant lesions in the isthmus are more likely to produce ETE and lymph node metastasis in the central area than malignant lesions elsewhere in the thyroid. The reasons for this might be because the isthmus is relatively thin, and lymph vessels are less abundant than in the lobes, and the lymphatic fluid from the isthmus usually flows into the prelaryngeal lymph nodes and pretracheal lymph nodes [Citation43]. Therefore, carcinoma in the isthmus is routinely treated by surgery, but due to its good prognosis features, RFA could be a possible treatment [Citation16,Citation19,Citation20].
In 2009, the ATA recommended TT for PTC patients with tumors >1.0 cm in diameter [Citation10], while the updated guidelines in 2015 recommended either TT or LT for patients without ETE and lymph node metastasis on the condition that the diameter of the tumor was between 1.0 and 4.0 cm [Citation11]. Nevertheless, it is still unclear that TT is superior to LT in OS and RFS [Citation7,Citation8]. Several authors systematically reviewed the effects of the operating range of the PTMC on the OS and RFS and found that the operating range did not lead to a significant variation in survival in patients with low-risk or middle-risk PTMC (low-risk patients usually got single or multiple lesions in the thyroid gland while middle-risk patients tended to have multiple tumors) [Citation8,Citation25].
Because of the asymptomatic and indolent characteristics of PTMC, the 2015 ATA guidelines recommended active surveillance as the first-line management for low-risk PTMC [Citation11,Citation14,Citation15]. The persistent active surveillance did reveal that low-risk PTMC develops quite slowly, and the cumulative percent of lymph node metastasis after 10 years was only 3.8%, without distant metastasis or death [Citation44]. Compared with immediate surgery, this strategy had a prognosis and significantly avoided not only vocal fold paralysis, hypoparathyroidism, life-long levothyroxine administration, and other complications but also overtreatment and scars [Citation45]. Nevertheless, active surveillance is not accepted by all patients with PTMC.
Therefore, thermal therapy for low-risk PTMC might be an option [Citation16–20]. According to the present study, the therapeutic effects of ultrasound-guided RFA on PTMC in the isthmus might not be inferior to TT. Of course, it cannot be denied that the indolent nature of PTMC might be the cause for the low rate of recurrence and metastasis and that the treatment strategies did not affect the outcomes. Nevertheless, patients who chose RFA instead of surveillance can still undergo TT if needed.
Since there is no recommendation from the ATA or BTA to treat lymph node-negative malignancy in the isthmus [Citation11], several authors tried to perform isthmectomy alone or with limited cervical lymphadenectomy [Citation13,Citation46], and the present study was based on the same hypothesis. The patients with ETE, according to the US risk evaluation protocol [Citation11], were excluded, although minimal ETE could still not be ruled out. ETE indicates a poor prognosis for differentiated thyroid carcinoma [Citation47], and the incidence of ETE in isthmic PTCs is significantly higher than that in lobar PTCs [Citation21]. Nevertheless, ETE in lobar PTCs, but not in isthmic PTCs, is a risk factor for central and lateral cervical lymph node metastasis [Citation48]. Whether ETE in isthmic PTCs plays an equally important role as ETE in lobar PTCs is still unknown. Furthermore, as the capsule membrane of the gland is not intact at the site of the isthmus and contains a various proportion of connective and fat tissues [Citation49], the role of ETE in isthmic PTCs needs further evidence and researches. In the present study, no significant ETE was detected after RFA. It suggests that the pre-ablation evaluation of ETE by ultrasound was relatively reliable.
The treatment costs cannot be ignored, especially considering the high incidence of PTMC [Citation4–6]. In the present study, RFA was carried out in the interventional room of the outpatient department, and the patients could go back home 1–2 h after the operation if there were no discomfort. On the other hand, in China, TT surgery usually requires 3–4 days of hospitalization due to the preoperative and postoperative regulations. Another issue that should be noted is that the patients in the TT group had to take life-long thyroid hormone replacement therapy to prevent hypothyroidism and minimize the stimulating effect of TSH on tumor growth, while TSH suppression lasted 2–3 years for the patients in the RFA group [Citation22]. This difference might somehow affect the THYCA-QoL questionnaire scores and even determine the development or recurrence of cancer. Nevertheless, this will have to be examined in future studies.
Occult PTMC and minor central cervical lymph-node metastasis were not considered in the present study. An early autopsy study found that in patients who did not die of thyroid diseases, 5.2% were confirmed with thyroid carcinoma of >3 mm in diameter [Citation50], indicating that the role of occult PTMC is unclear.
There are limitations to this study. Dr. Luo, who oversees the thermal ablations at our center, has a high experience, and many patients from all over China went to Beijing to seek her help, which might cause a selection bias. In addition, the patients themselves selected their operation after a discussion with the physician, possibly introducing some bias. The possibilities of complications, recurrence, and metastasis were greatly reduced because of her skillful operations. Second, some studies showed that for patients whose isthmic PTMC was close to the lateral lobe, the incidence of metastasis to the prelaryngeal lymph node or neighboring tissue was significantly higher than that in patients whose tumor was in the center of isthmus [Citation48], but subgroups of the location of the tumor were not designed in our study due to the small number of patients. Third, a detailed analysis of the therapeutic difference between thermal coagulation zones of ≤5 and >5 mm was not carried out. Finally, this retrospective study could not guarantee the homogeneity of the TT procedures among the patients. Moreover, the data were extracted from the 2013–2018 period, meaning that some patients received treatments before the 2015 ATA guideline release. This might why TT was more likely to be chosen for some patients in the early study period.
In conclusion, the results suggest that RFA for PTMC in the isthmus had similar outcomes than TT in terms of recurrence, metastasis, and complications, but RFA led to a better quality of life and lower treatment costs. It will have to be confirmed in future studies.
Author contributions
Qing Song, Hanjing Gao, and Ling Ren carried out the studies, collected data, and drafted the manuscript. Xiaoqi Tian, Yu Lan, and Lin Yan performed the statistical analysis and critically for important intellectual content. Yukun Luo participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The China Postdoctoral Science Foundation-funded project (No. 2018M643876) and the Innovation Cultivation Fund Project of PLA Army General Hospital are gratefully acknowledged. Ling Ren, Lin Yan, and Yu Lan are gratefully acknowledged.
Disclosure statement
The authors of this manuscript declare no relationship with any company whose products or services may be related to the subject matter of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. A Cancer J Clinic. 2018;68(6):394–424.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. A Cancer J Clinic. 2020;70(1):7–30.
- Buj R, Mallona I, Diez-Villanueva A, et al. Kallikreins stepwise scoring reveals three subtypes of papillary thyroid cancer with prognostic implications. Thyroid. 2018;28(5):601–612.
- Vaccarella S, Franceschi S, Bray F, et al. L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617.
- Colonna M, Uhry Z, Guizard AV, et al. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015;39(4):511–518.
- Topstad D, Dickinson JA. Thyroid cancer incidence in Canada: a national cancer registry analysis. CMAJ Open. 2017;5(3):E612-E6.
- Vargas-Pinto S, Romero Arenas MA. Lobectomy compared to total thyroidectomy for low-risk papillary thyroid cancer: a systematic review. J Surg Res. 2019;242:244–251.
- Zhang C, Li Y, Li J, et al. Total thyroidectomy versus lobectomy for papillary thyroid cancer: a systematic review and meta-analysis. Medicine. 2020;99(6):e19073.
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(12):1856–1883.
- Cooper DS, Doherty GM, Haugen BR, et al.; American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Vasileiadis I, Boutzios G, Karalaki M, et al. Papillary thyroid carcinoma of the isthmus: total thyroidectomy or isthmusectomy? Am J Surg. 2018;216(1):135–139.
- Nixon IJ, Palmer FL, Whitcher MM, et al. Thyroid isthmusectomy for well-differentiated thyroid cancer. Ann Surg Oncol. 2011;18(3):767–770.
- Tufano RP, Clayman G, Heller KS, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015;25(1):15–27.
- Sakai T, Sugitani I, Ebina A, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019;29(1):59–63.
- Zhang M, Luo Y, Zhang Y, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Jeong SY, Baek JH, Choi YJ, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2018;34(5):611–616.
- Yue W, Wang S, Yu S, et al. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2014;30(2):150–157.
- Hahn SY, Han BK, Ko EY, et al. Ultrasound findings of papillary thyroid carcinoma originating in the isthmus: comparison with lobe-originating papillary thyroid carcinoma. AJR Am J Roentgenol. 2014;203(3):637–642.
- Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years’ follow-up. Thyroid. 2020;30(3):408–417.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194(4):1137–1142.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Jeon YW, Gwak HG, Lim ST, et al. Long-term prognosis of unilateral and multifocal papillary thyroid microcarcinoma after unilateral lobectomy versus total thyroidectomy. Ann Surg Oncol. 2019;26(9):2952–2958.
- Kim JH, Baek JH, Sung JY, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2017;33(2):212–219.
- Husson O, Haak HR, Mols F, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncologica. 2013;52(2):447–454.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2019;36(2):13–20.
- Kim JH, Baek JH, Lim HK, et al. 2017 thyroid radiofrequency ablation guideline: korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Mauri G, Nicosia L, Della VP, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Hahn SY, Shin JH, Na DG, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean society of thyroid radiology. Korean J Radiol. 2019;20(4):609–620.
- Karatzas T, Charitoudis G, Vasileiadis D, et al. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: a case control study. Inter J Surg. 2015;18:64–68.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Euro Radiol. 2008;18(6):1244–1250.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Euro Radiol. 2013;23(4):1044–1049.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2018;34(5):617–623.
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321–1325.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26(3):420–428.
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015;276(3):909–918.
- Choi Y, Jung SL, Bae JS, Lee SH, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North Am Hyperthermia Group. 2019;36(1):359–367.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Euro Radiol. 2019;29(9):4897–4903.
- Lee YS, Jeong JJ, Nam KH, et al. Papillary carcinoma located in the thyroid isthmus. World J Surg. 2010;34(1):36–39.
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34.
- Oda H, Miyauchi A, Ito Y, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26(1):150–155.
- Lim ST, Jeon YW, Suh YJ. Correlation between surgical extent and prognosis in node-negative, early-stage papillary thyroid carcinoma originating in the Isthmus. World J Surg. 2016;40(2):344–349.
- Doescher J, Veit JA, Hoffmann TK. [The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery]. Hno. 2017;65(12):956–961.
- Seok J, Choi JY, Yu HW, Jung YH, et al. Papillary thyroid cancers of the thyroid isthmus: the pattern of nodal metastasis and the significance of extrathyroidal extension. Ann Surg Oncol. 2020;27(6):1937–1944.
- Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol. 2010;17(2):386–391.
- Fukunaga FH, Yatani R. Geographic pathology of occult thyroid carcinomas. Cancer. 1975;36(3):1095–1099.