Abstract
Objective
To investigate the feasibility, efficacy and safety of ultrasound-guided high-intensity focused ultrasound (HIFU) ablation for treating the broad ligament uterine fibroid (BLUF).
Methods
A total of 236 patients with symptomatic uterine fibroids were enrolled and treated with JC-200 extracorporeal ultrasound-guided HIFU under conscious sedation between January 2017 and December 2018. Of them, data of 12 patients with 13 broad ligament fibroids were retrospectively analyzed. The patients’ mean age was 38.6 ± 6.3 years. The focused ultrasound target was deployed and moved from the deeper layer to the superficial layer of BLUFs. All patients underwent contrast-enhanced MRI (CE-MRI) before, immediate post-operation, and six months after the HIFU ablation procedure. The fibroid size, non-perfusion volume (NPV) ratio, the reduction of fibroid volumes, adverse events, symptom changes, and abnormal MRI findings associated with the HIFU treatment were analyzed.
Results
Ultrasound-guided HIFU ablation in the twelve patients was technically successful with one session treatment. The mean longest diameter of BLUFs was 6.2 ± 2.3 cm. The mean NPV ratio of fibroids was 84.08%± 9.4%. After HIFU ablation, lower abdominal pain occurred in 7 cases, sacrococcygeal pain in 3 cases, and mild skin pain in 6 cases. There were no severe adverse events and complications associated with the treatment. At 6 months post-treatment follow-up, the mean fibroid volume decreased by 56.2%± 9.0% (p < 0.05), and the symptoms related to broad ligament fibroids were improved or disappeared
Conclusions
Ultrasound-guided high-intensity focused ultrasound ablation is feasible, effective, and safe for treating broad ligament fibroids.
Introduction
Uterine fibroid is a common benign tumor with more than 50% morbidities in reproductive-aged women [Citation1]. Subserous fibroids account for about 20–30% of uterine fibroids. Patients with subserous fibroids have urinary frequency, constipation, or back pain due to the effect of pressure [Citation2]. The broad ligament uterine fibroid (BLUF), belonging to the subserous type, is unique and rare. Its incidence among gynecologic tumors is about 1%−3% [Citation3]. Treatment of BLUF includes traditional laparotomy, laparoscopic myomectomy, and total hysterectomy. Due to the presence of complex surrounding structures, all operations can be at risk of injuring the ureter, uterine arteries, iliac vessels, or fallopian tubes, resulting in serious complications such as ureter injury or uncontrollable bleeding for further operation [Citation16]. High-intensity focused ultrasound (HIFU) focuses acoustic energy precisely at a targeted tumor volume, ablating the targeted tissue thermally deep in the body without causing a wound. In recent years, HIFU ablation has been widely used to treat uterine fibroids with minimal morbidity, quick recovery, and uterus preservation [Citation4–5]. To our knowledge, there is no report on HIFU ablation for treating broad ligament fibroids, probably because of the rarity of BLUF as well as technical limitations and safety concerns to the surrounding pelvic organs and vessels. This paper will report a retrospective study of the feasibility, effectiveness, and safety of HIFU ablation for treating broad ligament fibroids.
Materials and methods
This retrospective study was approved by the ethics committee at our institute and the requirement for an informed consent to do the research was waived. (Reference number: IEC-033-02.0-AF02).
Patients
Two hundred and thirty-six patients with symptomatic uterine fibroids were enrolled and treated with ultrasound-guided HIFU under conscious sedation between January 2017 and December 2018. All patients signed the written informed consent before treatment. The inclusion and exclusion criteria were reported in one of our previous studies [Citation5]. MRI scans confirmed the presence of 13 broad ligament uterine fibroids in 12 patients, and their data were collected retrospectively and analyzed. The patients’ mean age was 38.6 years ± 6.3 years (range 24 − 46 years). These patients had mild to moderate pelvic compression symptoms, including frequent urination, low back pain, and constipation. Among them, there were no other symptoms in five patients, and the remaining seven patients simultaneously with submucosal or intramural myoma and BLUF had menorrhagia, dysmenorrhea, anemia or lower abdominal pain. All patients had contrast-enhanced MRI before, immediately post-operation, and at six months after the procedure. The non-perfusion volume ratio (NPV ratio), reduction of fibroid volumes, symptom changes, adverse events, and complications associated with the HIFU ablation were analyzed.
Pretreatment imaging
All patients underwent MRI using a standardized protocol including T1WI, T2WI with and without fat saturation, pre-and post-contrast T1-weighted images at the axial, coronal, and sagittal planes on a 3.0 T MR imager (Verio Tim, Siemens Medical Systems, Germany). They received an intravenous injection of Gd-DTPA-BNA contrast (Omniscan, 0.1mmols/kg of body weight, GE Healthcare). MR images were analyzed to determine the number, location, size, T2-weighted signal intensity, and contrast enhancement patterns of all fibroids [Citation6–7].
Ultrasound-guided HIFU equipment and procedure
The Ultrasound-guided HIFU procedure was performed using a JC-200 extracorporeal ultrasound-guided HIFU system (Chongqing Haifu Medical Technology Co, Ltd., Chongqing, China), which was equipped with a diagnostic ultrasonic probe (Esaote MyLab70, Italy). A single 20 cm diameter focused piezoelectric ceramic ultrasound transducer with a focal length of 14 cm and operating at a frequency of 1.0 MHz generated the ultrasound energy. The transducer can be moved smoothly in 6 directions (left and right, cranial and caudal, and up and down) using computer control. The corresponding mobile ranges are 120 mm, 120 mm, and 180 mm, respectively.
Patients underwent bowel and skin preparation before the treatment. During the HIFU procedure, they took up a prone position on the treatment table. Intravenous sedation and analgesic medication was administered to them to maintain conscious sedation. A degassed water balloon was used if necessary to push the bowels away from the acoustic pathway to avoid intestinal damage. All patients received intravenous fentanyl, 50–400 μg, and midazolam hydrochloride, 1–4 mg, for conscious sedation. Their vital signs were monitored during the therapeutic procedure. Patients were requested to inform the operator if there was any pain or discomfort.
The focused ultrasound target was deployed on a deeper layer of the broad ligament fibroid initially. Then, the focal ablation gradually moved from its subdeep layer toward more superficial layers. The therapeutic energy was determined based on the patients’ feedback or/and the grayscale changes on the sonographic images. When the grayscale covered most of the fibroid, the treatment was considered completed. Treatment would be stopped or the focal spot changed if the patient felt severe or intolerant pain or complained of nerve numbness or pain radiating to the lower leg. One of the authors (YX), who has had more than ten years’ HIFU ablation experience, performed these 12 patients’ procedures. All patients had just one treatment session.
Post-treatment imaging and follow-up
Following HIFU ablation, axial, sagittal, and coronal contrast-enhanced MR imaging were taken within the next day, and the images were analyzed to assess the non-perfused volume (NPV). The NPV and fibroid volumes were determined from the T1-weighted images by manually contouring the total non-perfused area and tumor area on each imaging slice. The NPV ratio, defined as the NPV divided by the fibroid volume, was then calculated. After treatment, the volume reduction was calculated by the reduced tumor volume at six months follow-up. The MRI scanning protocol and parameters were the same as used for the pretreatment MRI. Where applicable, the author team will study in detail any treatment-related abnormal MRI findings before and after the HIFU ablation treatment.
Data analysis and statistics
SPSS software (SPSS 18.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All data were presented as mean ± SD. The student’s t-test and Chi-squared test were performed for statistical analyses. A p-value of < 0.05 was used to define the statistical significance.
Results
The mean longest diameter of the 13 BLUFs was 6.2 cm ± 2.3 cm (range: 4.2−9.1 cm), and their mean volume was 79.0 cm3 ± 37.0 cm3 (range: 25.7 cm3−152.1 cm3). Three fibroids showed MRI T2-weighed isointense signals; 10 showed low intense signals. The vascularity of three fibroids was moderately vascular and 10 hypovascular ().
Table 1. The data of patients with broad ligament fibroids and ultrasound-guided HIFU treatment results.
Ultrasound-guided HIFU ablation for these 12 patients was technically successful, and the average NPV ratio of the BLUFs was 84.1% ± 9.4% (range: 63.6–97.8%) (). After treatment, seven patients had lower abdominal pain (7/12), three had sacrococcygeal pain (3/12), six had mild skin-burning pain (6/12), and one patient complained of discharging vaginal fluid for a week. All these symptoms were mild and had dissipated within one week. No severe adverse events or complications occurred.
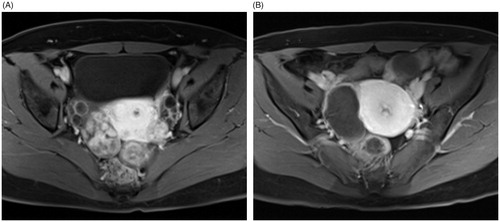
Figure 1. Contrast enhanced-MRI images of a 36-year-old woman with a right broad ligament fibroid. These showed a moderately vascular fibroid before treatment (A) and more than 90% NPV ratio immediately after HIFU ablation (B).
At six months follow-up, the mean volume and reduction rate of the broad ligament fibroids was 34.7 cm3 ± 17.3 cm3 (range:12.2 cm3−65.1 cm3) and 56.2% ± 9.0% (range: 41.7–68.7%) respectively (p < 0.05) () . The pelvic pressure and other related symptoms were improved or disappeared in all 12 cases, except one patient still had heavy periods because of a submucosal fibroid (). This patient had localized edema and swelling of abdominal muscles immediately after treatment and disappeared at six months following-up (). No more abnormal findings were observed on follow-up MRI.
Figure 2. A 42-year-old woman with a left broad ligament uterine fibroid and a small submucous fibroid. The fibroid was hypo-intense on T2WI MRI (A) and hypervascular on CE-MRI before treatment (B). There was localized edema and swelling of abdominal muscles (white arrow) (C) and the NPV ratio of the treated fibroid was 91.3% immediately after treatment (D). No abnormal MRI finding related to focused ultrasound ablation was observed (E) and the fibroid volume decreased by 55.5% at 6-month follow-up (F).
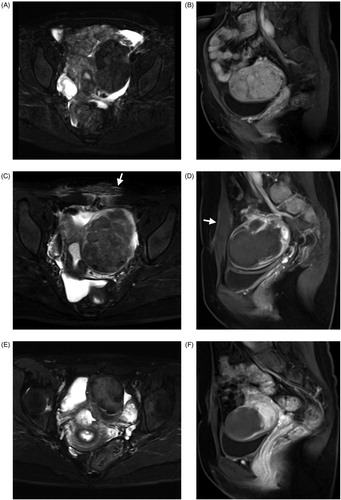
Table 2. The symptom changes at 6 months after treatment (n = 12).
Discussion
The subserous uterine fibroid is not a contraindication for HIFU ablation unless it has a narrow base, which could present potential thermal risks to surrounding organs during HIFU ablation. As a specific type of subserous uterine fibroid, the broad ligament fibroid occurred in 5.1% (12/236) of the cases in our study, which was higher than 0.4–0.8% of all uterine fibroids in a previous report [Citation3].
The broad ligament fibroid originates from the uterine sidewall, growing between the anterior and posterior peritoneum of the broad ligament. It might push against the blood vessels over the lateral side of the uterus so that the larger pelvic vessels move closer to the fibroid (). Thus, both laparoscopic and open myomectomy can easily lead to vascular damage, causing intraoperative bleeding. Hemostasis following myomectomy may be difficult because of limited surgical access. A ureter can also be accidentally injured in operation if a broad ligament fibroid is in an unexpected location just next to it or oppresses it, resulting in its displacement. As a noninvasive treatment, HIFU ablation has the advantages of safety and effectiveness with minimal or no damage to surrounding tissues and organs. Therefore, HIFU ablation becomes a new option for treating broad ligament fibroids.
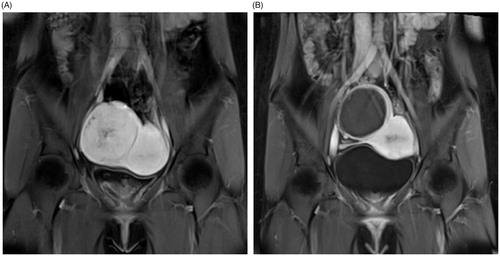
Figure 3. A 24-year-old woman with a right broad ligament uterine fibroid. The CE-MRI showed a right broad ligament uterine fibroid oppressing the right iliac vessels (A) and the non-perfused area of ablated fibroid (97.8% of NPV ratio) with intact serosal layer after HIFU ablation (B).
A safe ultrasound field was a prerequisite to ensure HIFU ablation’s success during the treatment of broad ligament fibroids. The surrounding structures of broad ligament fibroid are complex, and its periphery is often adjacent to the ureter, nerves, pelvic vessels, and especially the intestinal tract. Previous studies had reported that one of the serious complications of focused ultrasound ablation was intestinal perforation, even if its incidence was low [Citation8]. During HIFU ablation, the intestinal tract must be pushed away from the acoustic pathway. A distended bladder can achieve this, and the use of a water balloon can also push the bowel away out of the acoustic pathway; thus, the bowels are not damaged [Citation9]. As it grows, a board ligament fibroid often becomes displaced, changes position, and compresses pelvic blood vessels, ureters and nerves, making surgical treatment difficult. Although the pelvic nerves are located behind the broad ligament, the focused ultrasound beam may affect the post-focal ablation field, causing nerve impairment. Therefore, the focal target point should apply at least a 1.5 cm margin away from the posterior edge of the broad ligament fibroid, and sonication must stop once the patient reports leg numbness or pain. This is to ensure nerves are not harmed. Compared to the nerves, a ureter located at the posterior or lateral side of the broad ligament fibroid has a reduced chance of thermal injury during HIFU ablation because the urine flowing in its lumen will carry away any diffused heat. HIFU ablation also seldom has a thermal effect on the large pelvic vessels because of the vascular cooling effect [Citation10] (). Even so, ultrasound-guided HIFU ablation can always avoid hitting them under the real-time guidance of color Doppler imaging. Our results showed that ultrasound-guided HIFU ablation for treating broad ligament fibroids was technically feasible.
In this study, the average NPV ratio of BLUFs was 84.1 ± 9.4%, which was similar to 84.3 ± 15.7% of our previous report on MRgHIFU ablation for Non-BLUFs [Citation11]. The volume reduction rate of the broad ligament fibroids was 56.2 ± 9.0% at six months follow-up, which was also close to 59.1% ± 9.0% of our previous study [Citation11]; however, it was higher than that of Funaki’s report (36.5%) on focused ultrasound ablation for intermural myoma with T2WI hypo-intense signal [Citation12]. The previous research showed that the greater the percentage of the treated fibroid destroyed by the focused ultrasound ablation, the better and more longer-lasting the symptomatic relief [Citation13,Citation14]. The symptoms related to the broad ligament fibroids were relieved over six months in the preliminary study. However, the main issue, the safety of ultrasound-guided HIFU, was to be addressed, with efficacy measurements being a secondary consideration.
Therapeutic safety was highly prioritized throughout the process of HIFU ablation of this unusual fibroid. Technically, it is generally more challenging to use the ultrasound-guided HIFU procedure to ablate broad ligament fibroids. We had adopted some measures described above to avoid damage to the organs and tissues surrounding the tumor. The sonication strategy was that the initial focal spot should target the fibroid’s deep layer with lower acoustic power. When the coagulation necrosis was induced in this deeper layer, sonication with higher acoustic power was moved from sub-deep to the superficial layer of fibroid. This was carried out to reduce the ultrasonic energy diffusing through the focal area to the posterior field, where it could otherwise cause injury to the bowel, nerves, and vessels. This strategy also helps avoid early coagulation necrosis at the superficial layers, which could be a barrier to ultrasound energy delivery to the fibroid’s deeper layer. Consequently, only mild adverse events were encountered after HIFU treatment, which did not require any subsequent treatment.
This study’s limitations are (a) a small sample size from just one hospital and (b) the presence of other intramural or submucosal fibroids in some patients, thus influencing the efficacy measurements on follow-up visits. Therefore, any symptom improvement was not used for effectiveness evaluation in this study as the uterine fibroid symptom severity score [Citation15]. Yet, these patients had benefited from this noninvasive treatment of their fibroids without other known surgical risks.
In conclusion, this preliminary study demonstrated that ultrasound-guided HIFU was feasible, effective, and safe in treating broad ligament uterine fibroids. Yet, further investigation of HIFU ablation in a larger group of patients should be performed to confirm the safety and long-term efficacy of board ligament fibroid treatment.
Disclosure statement
The authors have no interest to declare.
References
- Christiansen JK. The facts about fibroids: presentation and latest management options. Postgrad Med. 1993;94(3):129–137.
- Dixon D, Parrott EC, Segars JH, et al. NIH congress: uterine fibroid research. Fertil Steril. 2006;86(4):800–806.
- Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298.
- Chapman A, ter Haar G. Thermal ablation of uterine fibroids using MR-guided focused ultrasound: a truly non-invasive treatment modality. Eur Radiol. 2007;17:2505–2511.
- Chen J, Li Y, Wang Z, Committee of the Clinical Trial of HIFU versus Surgical Treatment for Fibroids, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG: Int J Obstet Gy. 2018;125(3):354–364.
- Wang Y, Wang Z, Xu Y. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19(4):724–732.
- Zhao WP, Chen JY, Zhang L, et al. feasibility of ultrasound-guided high-intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82:e43–49.
- Chen J, Chen W, Zhang L, et al. safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: A review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Zhang L, Chen WZ, Liu Y, et al. Feasibility of magnetic resonance imaging–guided high-intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73(2):396–403.
- Lehmann KS, Poch FG, Rieder C, et al. Minimal vascular flows cause strong heat sink effects in hepatic radiofrequency ablation ex vivo. J Hepatobiliary Pancreat Sci. 2016;23:508–516.
- Xu Y, Fu Z, Yang L, et al. Feasibility, safety, and efficacy of accurate uterine fibroid ablation using magnetic resonance imaging–guided high-intensity focused ultrasound with shot sonication. J Ultrasound Med. 2015;34(12):2293–2303.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–589.
- Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a non-invasive soma ablative technique. Am J Obstet Gynecol. 2003;189(1):48–54.
- Taran FA, Tempany CMC, Regan L, for the MRgFUS Group, et al. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol. 2009;34:572–578. ;.
- Wang Y, Wang Z, Xu Y. Efficacy, Efficiency, and Safety of MR-guided High-intensity Focused Ultrasound for Ablation of Uterine Fibroids: Comparison with Ultrasound-guided Method. Korean J Radiol. 2018;19(4):724–732.
- Jain N, Singh S. Fibroid Uterus: Surgical Challenges in Minimal Access Surgery. Chapter 12. Broad Ligament Fibroids. Rooma Sinha, Arnold Advincula, Kurian Joseph (eds). Boca Raton, FL: CRC Press; 2020. 90–96.
