Abstract
Purpose
To assess the capability of ultrasound-computed tomography (US-CT) fusion imaging to guide a precise targeting of renal tumors invisible or poorly visible with US
Materials and methods
From 2016 renal tumors poorly visible or inconspicuous/invisible at US were treated at our institution with the guidance of US/CT fusion in a room equipped with CT scanner. Feasibility of the procedure, accuracy of targeting, complications, and technique efficacy were evaluated.
Results
Of 227 patients treated from 2016 to March 2020, 91 patients (65 males and 26 females, mean age 68.5 ± 10.1 years) with 97 renal lesions (mean maximum diameter 21.6 ± 9.4 mm) inconspicuous/invisible (29/97, 29.9%) or poorly visible (68/97, 70.1%) at US underwent treatment under US-CT fusion guidance. US-CT fusion imaging guidance was always technically feasible and enabled correct targeting in 97/97/(100%) of cases. Technical success was achieved in 93/97 lesions (95.9%). Three lesions were retreated during the same ablative session, while 1 was retreated in a subsequent session. Thus, primary efficacy was achieved in one session in 96/97 (98.9%) cases and secondary efficacy in 97/97 (100%) cases
Conclusion
US-CT image fusion guidance allows for a correct tumor targeting of renal tumors poorly visible or inconspicuous/invisible with US alone, with a high rate of technical success and technique efficacy.
Introduction
Image-guided thermal ablations are increasingly used in the treatment of patients with renal cancers, in particular in patients that are unfit for surgery or with previous ipsi- or contralateral renal surgeries [Citation1–3]. Several different ablative modalities, including cryoablation, radiofrequency (RF), microwave (MW) and laser ablation have been successfully used in the treatment of small renal tumors, with sustained oncological results and low complications rate [Citation4–7]. Regardless of the ablative technique applied, precise and reliable imaging is crucial in the guidance of renal thermal ablation, and for a precise early assessment of the result [Citation8–10].
Thanks to recent technological development, it is nowadays possible to fuse datasets deriving from different imaging modalities, and thus to perform ablation under the guidance of fusion imaging [Citation11–13]. This technology is nowadays more and more applied in the guidance of thermal ablations, particularly in the liver, while experiences in the kidney are still limited [Citation14–17]. Particularly, fusion imaging might be particularly useful when the target lesion is completely invisible or poorly visible at ultrasound (US) [Citation15,Citation18], as in the case of a small renal lesion with the same echogenicity of surrounding renal parenchyma. In the case of a lesion that is not visible at US, device precise placement can be extremely difficult under US, and other methods of guidance like computed tomography (CT) or magnetic resonance (MR) could be needed [Citation19–21]. However, CT lack real-time device visualization during the procedure and is burdened by a not negligible radiation dose, and MR interventional suites are rare and require a complex operatory environment [Citation19–22].
The aim of the present work was to evaluate the feasibility and capability of US-CT image fusion to guide a correct targeting of renal tumors invisible or poorly visible with US in patients undergoing thermal ablation.
Materials and methods
Institutional Review Board approval was obtained (European Institute of Oncology, IRCCS, number of registration 2382), and patient's informed consent was waived for this retrospective study. Patients treated with image-guided thermal ablation for a renal tumor from 2016 to March 2020 were retrospectively reviewed. Indication to image-guided thermal ablation was established during a multidisciplinary discussion involving urologists, radiologists, oncologists and radiation therapists. All procedures were performed by a team of two interventional radiologists, at least one with more than 10 years of experience. Before ablation, US evaluation was always performed and all tumors underwent percutaneous biopsy as a separate procedure before treatment. Patients with inconspicuous/invisible lesions and poorly visible lesions at US were treated with US/CT fusion imaging guidance.
All procedures were performed in one of two dedicated angio suites. One room is equipped with a C-arm (Ziehm Vision RFD Hybrid Edition, Ziehm Vision, Nuremberg, Germany), a computed tomography (CT) (GE Lightspeed, GE Healthcare, Chicago, USA) and an ultrasound (US) machine (GE E9, GE Healthcare, Chicago, USA) equipped with a dedicated fusion imaging software. The other room is equipped with a hybrid angiography CT solution (ALPHENIX 4 D CT, Canon Medical Systems Corporation, Kanagawa, Japan) and a US machine (GE E9, GE Healthcare, Chicago, USA) equipped with dedicated fusion imaging software. Dedicated convex probes with embedded electromagnetic sensors were used. All procedures were performed under general anesthesia. Patients’ decubitus was established to achieve a better path to the target lesion and to lower the risk of complications. For centrally located tumors retrograde pyeloperfusion was performed through an endoscopically placed 6 Fr single ureteral stent. Hydrodissection with sterile water was used to displace the surrounding structure on the basis of operator judgement. A contrast enhanced CT (CECT) scan was then performed to evaluate the lesion and allow for fusion imaging with US images. CECT was always performed in expiratory phase, in order to achieve a reproducible breathing depth. A dedicated active patient tracer (omniTRAX, CIVCO, Coralville, Iowa, US) was placed on the patient and included in the field of view. This active tracer placed on the patient can be automatically identified in the CT volume by the navigation system, and thus can be used to correlate the CT volume dataset with the US data in the space. An electromagnetic transmitter is placed near the area of interest. The fusion system can then identify the relative position of the active tracer placed on the patient (and thus of the CT dataset volume position in space) and of the ultrasound probe (and thus of the US images) thanks to its embedded electromagnetic sensor, and thus to correlate the CT and US images in space. Fusion of the CECT data set with real-time US images was automatically achieved by the system. After fusion imaging, the feasibility of the procedure was established by the operator performing the treatment. In case even with adjunctive maneuver like hydrodissection, and with the application of fusion imaging ablation was considered to be at risk of complication, the treatment was not performed. The device was inserted under the guidance of US/CT fusion imaging, aiming at an ideal point established by the operator on the preoperative CT. After device insertion, a CT was performed to confirm the correct placement of the device itself.
Ablation was performed using RF (LeVeen CoAcces RFA, Boston Scientific, Marlborough, MA, USA needle electrode) or microwave MW (Emprint, Medtronic, Minneapolis, MN, USA). Power and time were established according to manufacturer indications, and according to size and shape of the lesion to be treated.
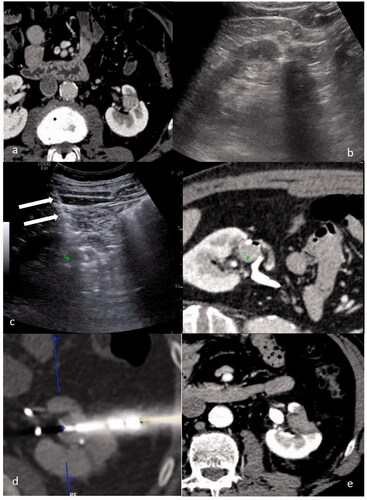
After ablation, a CECT was performed to evaluate possible immediate complications and to check for the immediate result of the ablation. In case of suspicious persistence of pathological tissue, device repositioning and retreatment was performed in the same manner as previously described. A CECT within 24 h was performed, to evaluate treatment outcomes and possible complications. Patients were then followed according to standard protocol with a CECT and a clinical visit at 6 weeks, 3, 6, 12, 18 and 24 months, and yearly afterwards. A case is shown in .
Figure 1. A case of a 52 y.o. patient with a single 12 mm RCC relapse after robotic assisted partial nephrectomy: (a) CT scan showing a 12 mm enhancing lesion in the left kidney. (b) US evaluation performed before treatment didn’t allow for clear identification of the tumor. (c) US/CT fusion imaging obtained during the treatment allowed to place a marker (green marker) on the tumor on the CT images to identify the target on US, and thus to provide guidance for needle insertion (white arrows). (d) CT scan performed immediately after needle insertion confirmed the correct targeting of the tumor (blue marker). (e) CT performed 24 h after the procedure showing a complete ablation.
4,1 Variables analysis
Feasibility of the procedure was evaluated. It was defined as the possibility of performing ablation safely according to the judgment of the operator.
Accuracy of targeting was evaluated at the CT performed after device insertion. Tumor was considered correctly targeted if the tip of the ablative device was located within 5-mm range from the ideal target point preoperatively established by the operator [Citation18,Citation23]. Tumor was considered successfully ablated when the non-enhancing ablative area covered the whole tumor.
Complications were evaluated and classified according to standard definition [Citation22].
Primary technique efficacy was defined as complete ablation at 6-weeks CECT control, while secondary technique efficacy was defined as complete ablation at 6-weeks CECT control after a course of treatment of two ablations.
Local tumor progression was defined as the appearance of a tumor foci at the edge of the ablation zone after a follow-up beyond that performed for the assessment of technique efficacy.
Results
A total of 227 patients were treated for a renal tumor from 2016 to March 2020. Overall, 91/227(40.1%) patients (65 males and 26 females, mean age 68.5 ± 10.1 years) had 97 lesions that were classified as inconspicuous/invisible (29/97, 29.9%) or poorly visible (68/97, 70.1%) at US. Mean lesion maximum diameter was 21.6 ± 9.4 mm. Tumor histovariants were clear cell in 58/97 (59.7%), papillary in 17/97 (17.5%), not-defined in 15/97 (15.4%), chromophobe in 2/97 (2%), and benign in 5/97 (5.1%). 6/97 (6.1%) lesions were treated with RFA, while 91/97 (93.9) lesions were treated using MWA. According to RENAL nephrometry score lesions were categorized at low complexity (4–6) in 11/97 (11.3%) cases, moderate complexity (7–9) in 47/97 (48.5%) cases, and at high complexity (10–12) in 39/97 (40.2%) cases. Mean hospital stay was 2.1 ± 0.5 days.
Real-time fusion imaging was technically feasible and enabled to perform ablations successfully in all cases. Correct targeting was achieved in 97/97 (100%) cases. In 4/97 (4.1%) of cases, immediate post-ablation CECT demonstrated persistence of vital unablated tissue (technical success 95.9%). In 3 cases the ablative device was immediately reinserted, and subsequent ablation performed in the same operatory session, while in one case, due to the presence of a pericapsular hematoma, the procedure was stopped, and a second successful ablation was performed in a subsequent ablative session. Thus, primary efficacy was finally achieved in one session in 96/97 (98.9%) cases and secondary efficacy in 97/97 (100%) cases. Overall, complications occurred in 14/91 (15.3%) patients, 13 being subcapsular or pericapsular hematoma that did not require interventional management nor blood transfusion (minor complications) while in 1 case a hemothorax occurred. In this last patient, due to the presence of a large amount of blood into the pleural cavity, surgical management was required. At a mean follow-up time of 19.2 ± 13.6 months, local tumor progression occurred in 3/97 (3.1%) of the treated lesion. Due to old age and severe comorbidities, the three patients were kept under active surveillance. At last follow-up 90/91 (98.9%) of patients were alive, while one patient with a completely ablated tumor died for unrelated reasons.
Discussion
Image-guided thermal ablations are increasingly used in the treatment of a variety of diseases, including benign and malignant tumors, due to the minimal invasiveness of the treatment and excellent clinical results, particularly in those patients that are not suitable for surgical management [Citation1–6,Citation24]. Imaging plays a pivotal role in these procedures, being the ‘eyes’ of the operator during the procedure. US is the most widely used imaging modality guidance for ablations in the abdomen, particularly in Europe and in middle and far East, as it widely available and allow for real-time visualization, that is particularly useful when dealing with organs that move with breathing or can be displaced during the ablation device insertion [Citation9,Citation25]. On the other hand, CT, particularly with the application of contrast media, can provide higher contrast resolution and better identification of small lesions, and is also often used as a guidance method for ablations, particularly in the United States [Citation26,Citation27]. Sometimes tumors easily seen with CT maybe not well visible or inconspicuous/invisible with the conventional US. In these cases, the availability in the operatory theater of both modalities can be of great help to the operator to successfully and safely complete the procedure [Citation7]. In the present series, 29.9% of evaluated lesions resulted to be invisible or poorly visible in US and, particularly, 12.7% (29/227) resulted to be not suitable for ablation with the only guidance of US. In the last years, there has been a growing interest in methods to guide ablations automatically combining the advantages of different imaging modalities. Systems for real-time image fusion of US and CT have been commercially available for some years, and have been applied in the abdomen, particularly in the treatment of liver tumors, and seems to be particularly useful in the treatment of difficult lesions or not well visible lesions [Citation15,Citation18,Citation28]. Limited reports are present in the literature regarding their application in the guidance of ablation of renal lesions [Citation16,Citation17]. In 2012 Amalou et al., reported the first case of a patient with Von Hippel Lindau disease and recurrent renal tumor successfully treated with percutaneous ablation guided by image fusion [Citation17]. One possible advantage arising from the application of fusion imaging in the renal ablation scenario would be the possibility to reduce the number of CT scans that would be otherwise required to correctly deploy the needle in the target lesion with the CT alone as guidance. Further studies are necessary to evaluate this potential advantage of the application of fusion imaging.
In our center, ablations were initially performed with both US and CT, which were both available in the operatory theater. US was used for real-time monitoring of needle insertion, and CT for targeting confirmation and CECT for immediate post-ablation evaluation [Citation7]. Since 2015, a system for US-CT image fusion in the guidance of ablations in the abdomen was introduced. The availability of a CT in the operatory theater, and the systematic application of general anesthesia, allowed to perform pre-ablation CT with the patient already in the desired decubitus, and in a fixed expiratory phase. This allowed for the ideal setting for a precise fusion of US and CT, as discrepancies between the virtual CT volume and the virtual US volume were kept to the minimum. One of the main issues for the application of fusion imaging to the guidance of thermal ablation is the reliability of the fusion itself, as an inaccurate overlay of the CT and US volumes might determine a wrong targeting of the tumor. This can occur particularly when the CT dataset is acquired before the operative session, with the patient in a different decubitus and not under general anesthesia. In these cases, often registration is performed using internal anatomical landmark, which can be quite time-consuming, and the result largely depends on operator experience. In a large clinical series on the application of image fusion to liver lesions invisible of poorly visible at US, correct targeting whit manual registration was achieved in 95.6% of cases [Citation18]. Thanks to this setting, with the patient in general anesthesia and availability of a dedicated CT in the operatory room, it was possible to correctly perform an automatic fusion imaging, and to perform the planned ablation in all cases, even when the tumor was completely invisible at US. Furthermore, correct targeting was achieved in all tumors. After ablation, in 4.1% of cases, residual viable tissue was evident at CT, even if the targeting was considered correct. This is in line with results of the literature regarding renal tumor ablation [Citation4,Citation5,Citation7]. Notably, the availability of a CT in the operatory theater allowed us for an immediate post-ablative assessment, and thus to immediately retreat those patients, in the same operatory session, sparing patients the need of a subsequent further ablation session under general anesthesia. This further highlight the importance of a precise and reliable imaging modality for immediate result assessment [Citation10,Citation29]. Finally, primary effectiveness was achieved in 98.9% of cases.
An inadequate visualization of the device during its insertion might determine the unintended damage of surrounding structures, or the misplacement of the device itself, with consequent damage of surrounding structures (like vessels or collecting system) during ablation. For this reason, a CT control of the precise device placement was always performed and is highly suggested when performing renal ablations [Citation8]. In our series, complications occurred in 15.3% of cases, being mainly represented by minor complications like subcapsular or pericapsular hematomas. Only one major complication occurred, which is in line with the previously reported complications rate [Citation4,Citation5,Citation7].
Some limitations of the present study should be considered. First, this is a retrospective analysis of a limited number of cases, performed at a single center. However, to the authors’ knowledge, this represents the largest available series on the treatment on renal tumors inconspicuous/invisible or poorly visible at US treated with the guidance of fusion imaging. Second, the patients were treated at a high-volume referral center, by a dedicated team of interventional radiologists, with two dedicated angio-suites. Thus, results can be difficult to be generalized and replicate in different scenarios. Third, the main aim of the present study was to evaluate the feasibility and capability of US-CT image fusion to guide a correct targeting of renal tumors invisible or poorly visible with US. For this reason, also recently treated tumors were included in the analysis, and medium- and long-term results of ablation were not analyzed. Further studies on larger series with a multicentric setting and prospective design are needed to better investigate the role of fusion imaging in the guidance of renal tumor ablation, possibly with longer follow-up. Due to the low number of patients treated with RFA, and the low number of events, a statistical comparison of results achieved with RFA and MWA was not performed.
In conclusion, US-CT image fusion is a feasible and effective image guidance modality, which allowed the successful targeting and treatment of renal tumors invisible or poorly visible with US. This technology holds the potential of expanding the application of image-guided thermal ablation to a larger patients’ population, and to improve results of treatment in difficult cases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ginzburg S, Tomaszewski JJ, Kutikov A. Focal ablation therapy for renal cancer in the era of active surveillance and minimally invasive partial nephrectomy. Nat Rev Urol. 2017;14:669–682.
- Filippiadis D, Mauri G, Marra P, et al. Percutaneous ablation techniques for renal cell carcinoma: current status and future trends. Int J Hyperth. 2019;36:21–30.
- Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–259.
- Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: Comparative local control and complications after radiofrequency ablation and cryoablation. Am J Roentgenol. 2013;200:461–466.
- Buy X, Lang H, Garnon J, et al. Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. Am J Roentgenol. 2013;201:1353–1361.
- Sartori S, Mauri G, Tombesi P, et al. Ultrasound-guided percutaneous laser ablation is safe and effective in the treatment of small renal tumors in patients at increased bleeding risk. Int J Hyperth off J Eur Soc Hyperthermic Oncol North Am Hyperth Gr. 2018;35(1):19–25.
- Mauri G, Mistretta FA, Bonomo G, et al. Long-term follow‐up outcomes after percutaneous us/ct‐guided radiofrequency ablation for ct1a‐b renal masses: Experience from single high‐volume referral center. Cancers (Basel). 2020;12(5):1183.
- Krokidis ME, Orsi F, Katsanos K, et al. CIRSE Guidelines on Percutaneous Ablation of Small Renal Cell Carcinoma. Cardiovasc Intervent Radiol. 2017;40:177–191.
- Mauri G, Nicosia L, Varano GM, et al. Tips and tricks for a safe and effective image-guided percutaneous renal tumour ablation. Insights Imaging. 2017;8:357–363.
- Mauri G, Solbiati L, Orsi F, et al. Thermal ablation of liver tumours: the crucial role of 3D imaging. Cardiovasc Intervent Radiol. 2020;43:1416–1417.
- Wood BJ, Locklin JK, Viswanathan A, et al. Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future. J Vasc Interv Radiol. 2007;18:9–24.
- Ward TJ, Goldman RE, Weintraub JL. Electromagnetic navigation with multimodality image fusion for image-guided percutaneous interventions. Tech Vasc Interv Radiol. 2013;16:177–181.
- Mauri G, De Beni S, Forzoni L, et al. Virtual navigator automatic registration technology in abdominal application. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:5570–5574.
- Mauri G. Expanding role of virtual navigation and fusion imaging in percutaneous biopsies and ablation. Abdom Imaging. 2015;40:3238–3239.
- Liu F-Y, Yu X-L, Liang P, et al. Microwave ablation assisted by a real-time virtual navigation system for hepatocellular carcinoma undetectable by conventional ultrasonography. Eur J Radiol. 2012;81:1455–1459.
- Monfardini L, Gennaro N, Della Vigna P, et al. Cone-beam CT-assisted ablation of renal tumors: preliminary results. Cardiovasc Intervent Radiol. 2019;42(12):1718–1725.
- Amalou H, Wood BJ. Multimodality fusion with MRI, CT, and ultrasound contrast for ablation of renal cell carcinoma. Case Rep Urol. 2012;2012:1–5.
- Mauri G, Cova L, De Beni S, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–151.
- Eisenberg JD, Gervais DA, Singh S, et al. Radiation exposure from CT-guided ablation of renal masses: effects on life expectancy. Am J Roentgenol. 2015;204:335–342.
- Arellano RS, Garcia RG, Gervais D. a, et al. Percutaneous CT-guided radiofrequency ablation of renal cell carcinoma: efficacy of organ displacement by injection of 5% dextrose in water into the retroperitoneum. Am J Roentgenol. 2009;193(6):1686–1690.
- Silverman SG, Tuncali K, Morrison PR. MR Imaging-guided percutaneous tumor ablation. Acad Radiol. 2005;12:1100–1109.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Monfardini L, Orsi F, Caserta R, et al. Ultrasound and cone beam CT fusion for liver ablation: technical note. Int J Hyperth. 2018;0:1–5.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an italian experience. Front Endocrinol (Lausanne). 2021;11:1.
- Lyrdal D, Andersson M, Hellström M, et al. Ultrasound-guided percutaneous radiofrequency ablation of small renal tumors: clinical results and radiological evolution during follow-up. Acta Radiol. 2010;51(7):808–818.
- Kim HJ, Park BK, Park JJ, et al. CT-guided radiofrequency ablation of T1a renal cell carcinoma in Korea: mid-term outcomes. Korean J Radiol. 2016;17(5):763–770.
- Zagoria RJ, Traver MA, Werle DM, et al. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. Am J Roentgenol. 2007;189(2):429–436.
- Long Y, Xu E, Zeng Q. 2020; Intra-procedural real-time ultrasound fusion imaging improves the therapeutic effect and safety of liver tumor ablation in difficult cases. Am J Cancer Res. 10:2174–2184.
- Mauri G, Porazzi E, Cova L, et al. 2014; Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. (2)5:209–216.
