Abstract
Objective
Percutaneous ethanol injection (PEI) and microwave ablation (MWA) are both important methods used in treating benign thyroid nodules. This study aimed to investigate the efficacy and safety of a modified PEI method combined with MWA for the treatment of symptomatic, predominantly cystic and benign thyroid nodules.
Materials and methods
This study included 201 patients who underwent treatment in our department between April 2015 and August 2018. Predominantly cystic thyroid nodules were treated by the modified PEI procedure, which included short-term boiling ethanol ablation (STBEA) and was combined with MWA. Complications, the volume reduction ratio (VRR), symptoms and cosmetic scores were recorded at 1, 3, 6 and 12 months after treatment and every 6 months thereafter.
Results
No major complications were observed during or after the treatment. Ten patients (4.8%) experienced temporary voice change, which resolved within 3 months. Of 200 (97.6%) out of 205 nodules showed significant volume reduction at the final follow-up. Recurrence occurred for only 5 (2.4%) nodules. The mean thyroid nodule volume decreased from 17.40 ± 3.21 mL at baseline to 1.17 ± 0.37 mL at 12 months. The greatest VRR was observed within the first 3 months after treatment.
Conclusions
The modified PEI method combined with MWA is safe and effective for the treatment of predominantly cystic benign thyroid nodules and provides a shorter operating time and lower recurrence rate than traditional treatments.
Introduction
Thyroid nodules are a common condition among the general population, with a reported prevalence ranging from 19% to 68% based on detection by high-resolution ultrasound (US) [Citation1,Citation2]. Approximately 15–25% of solitary thyroid nodules are cystic or predominantly cystic, and most of them are benign [Citation3,Citation4]. Hemorrhage and subsequent degeneration of pre-existing nodules are considered the common causes of most cystic lesions. Percutaneous ethanol injection (PEI) was first used for the treatment of autonomous thyroid nodules as an alternative to surgery and radioiodine in 1990 [Citation5] and then applied in the treatment of thyroid cysts in 1992 [Citation4]. With PEI, ethanol causes permanent tissue damage via the induction of cellular dehydration and protein denaturation, subsequently leading to necrosis, fibrosis and thrombosis of small blood vessels [Citation5]. Then granulation tissue replaces the nodules, appearing as scarring and progressively shrinking over time [Citation6]. In recent years, several studies have investigated the efficacy of PEI for the treatment of cystic thyroid nodules and demonstrated success rates of 75%–85% [Citation3,Citation7,Citation8]. A controlled randomized study involving 281 patients conducted by Roberto Valcavi et al. found with PEI method, the median 1-year volume reduction rate (VRR) of cystic thyroid nodules was 85.6%.
Generally, two types of simple PEI have been described: one that involves draining the cystic thyroid nodule first and then instilling ethanol slowly into the cyst where it is retained [Citation7–10]; the other type added a procedure in which fluid is evacuated after the ethanol has been in the cystic nodule for 2–25 min depending on the drainage volume [Citation3,Citation11,Citation12]. Although in 2005, Kim et al. found no significant difference in the success rates achieve with these two traditional methods, several other investigators have reported concerns about ethanol leakage or other potential complications from ethanol retention within the cysts [Citation3,Citation13,Citation14]. Furthermore, the second type involving evacuation has been less popular among physicians and patients, because the procedure time is nearly twice as long as that for PEI without aspiration [Citation15]. Therefore, a modified method that balances the concerns regarding procedure time and ethanol retention is urgently needed.
Furthermore, the use of simple PEI as first-line treatment for predominantly cystic thyroid nodules (cystic component >50%) is debated, because for nodules in which the solid component is greater than 20%, the recurrence rate after PEI treatment is over 50% [Citation11,Citation16]. Many studies have concluded that the solid component of cysts is an important impediment to the efficacy of simple PEI, mainly because the ethanol will be distributed unevenly in the solid parts and the vascularity within the solid component could cause the loss of ethanol [Citation11,Citation17]. To avoid the disadvantages of PEI for treatment of predominantly cystic thyroid nodules, different local thermal ablation methods, such as laser ablation, radiofrequency ablation and microwave ablation (MWA), have been applied instead and tend to improve outcomes while reducing the recurrence rate [Citation18–24]. Two randomized clinical trials explored the efficacy of RFA ablation compared to PEI for treating predominantly cystic thyroid nodules. Both studies drew a conclusion that the therapeutic efficacy of RFA is equal to EA and EA is less expensive [Citation25,Citation26]. There are several studies investigated the effects of MWA on benign thyroid nodules showed that the VRR was up to 84.67%–90% and the treatment efficacy was effected by the different internal characteristics [Citation17,Citation27–29]. Unfortunately, there are few comparative studies on EA and MWA for treatment of predominantly cystic thyroid nodules.
Considering the effectiveness and limitations of current treatment methods, we sought to develop a modified PEI method that combines PEI and MWA for the treatment of predominantly cystic thyroid nodules in order to achieve improved efficacy for these nodules, shorten the operation time, and avoid the effects of alcohol retention in the nodules. For this modified PEI procedure, we developed a skillful approach called short-term boiling ethanol ablation (STBEA) that can achieve the efficacy of traditional PEI without leaving ethanol in the lesion for too long. In this study, we retrospectively evaluated the efficacy, safety and operative time of this modified PEI method (STEABA + MWA) for the treatment of predominantly cystic thyroid nodules.
Materials and methods
Patients
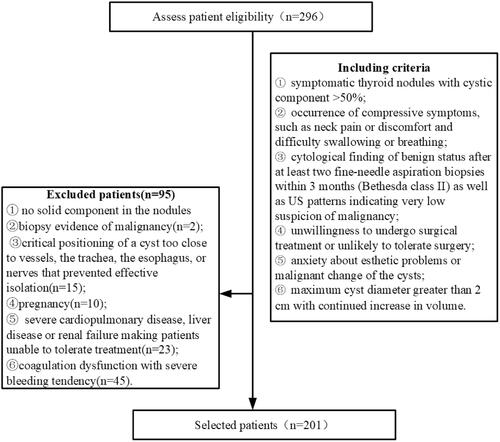
This retrospective analysis included a total of 201 patients who were examined and treated at Beijing Friendship Hospital between April 2015 and August 2018. This trial was approved by the ethics committee of Beijing Friendship Hospital with the approval ID of BJFH-EC/2014-027. All the patients signed written consent before treatment and they were well informed of the technical details of modified PEI method combined with MWA as well as the possibility of their clinical data being used in further retrospective researches. Patients were included only if they met the following criteria ():
symptomatic thyroid cysts or mainly cystic nodules observed on US (cystic component > 50%);
occurrence of compressive symptoms, such as neck pain or discomfort and difficulty swallowing or breathing;
cytological finding of benign status after at least two fine-needle aspiration biopsies within 3 months (Bethesda class II) [Citation30] as well as US patterns indicating very low suspicion of malignancy;
unwillingness to undergo surgical treatment or unlikely to tolerate surgery due to clinical conditions or esthetic needs;
anxiety about esthetic problems caused by the cysts or malignant change of the cysts;
maximum cyst diameter greater than 2 cm with continued increase in volume [Citation31].
The exclusion criteria were:
no solid component in the nodules
biopsy evidence of malignancy;
critical positioning of a cyst too close to vessels, the trachea, the esophagus, or nerves that prevented effective isolation;
pregnancy;
severe cardiopulmonary disease, liver disease or renal failure making patients unable to tolerate treatment;
coagulation dysfunction with severe bleeding tendency.
Preoperative assessment and MWA equipment
The following data were recorded before treatment: three diameters of the nodules (largest diameter, d1 and two perpendicular diameters, d2 and d3); the composition of the nodules (assessed by US examiner subjectively); the cosmetic grading score (CGS; 1: no palpable mass, 2: invisible but palpable mass, 3: visible mass visible only to experienced clinician’s eyes, and 4: easily visible mass) [Citation32]; and the symptomatic score (SS; visual analog scale, 0–10) indicating the distress of patients. The volume (V) of the nodules was calculated using the following equation: V = d1 × d2 × d3 × π/6.
US examination of thyroid nodules was performed before treatment and at each follow-up (1, 3, 6 and 12 months post-operatively and every 6 months thereafter) with a Hitachi Ascendus instrument (L75-type high-frequency linear array, frequency range: 5–18 MHz). The MWA therapy instrument used was a KY-2000 (Kangyou Medical, Nanjing, China) that consists of a microwave generator, a flexible low-loss coaxial cable and a cooled 16-G shaft antenna (a 10-cm shaft coated with polytetrafluoroethylene and a 3-mm narrow radiating segment 3 mm from the tip). The generator can produce 1–100 W of power at 2450 MHz; the energy we used for thyroid ablation was between 30 and 50 W. Cooled MWA was applied to reduce the occurrence of complications [Citation19,Citation33,Citation34].
Operative procedure
Pre-PEI
The patient was placed in the supine position with the neck fully exposed, and then local disinfection and subcutaneous local anesthesia with 1% lidocaine were applied after determination of the best puncture angle by US. Venous access for contrast-enhanced US and intraoperative rescue was also opened. To protect the recurrent laryngeal nerve or large blood vessels, a liquid isolation region around the thyroid capsule was created with the injection of approximately 30 mL cold saline (9%, 4 °C) depending on the patient’s tolerance.
Repeated irrigation
The cystic and mucous material inside the nodule was aspirated to cause the capsule collapse. Aspiration was stopped when only 1–2 mL of liquid remained inside the capsule. The tip of the needle could still be seen as well as a visible cystic separation between the tip and the wall, which helped to avoid hemorrhage due to accidental puncture. If the cystic fluid was too thick to be aspirated, this step was skipped. The needle was kept stably inside the capsule while the syringe was loaded with 50 mL normal saline. Then the cyst was irrigated repeatedly until the fluid in the tube was almost colorless and clean. With each cycle, the volume of the irrigated fluid was less than half of that inside the cyst. Fresh normal saline was applied after 3–5 aspiration steps when the liquid was well mixed between the fluid in the tube and in the cyst.
Next, the repeated irrigation process was continued using 99.9% ethanol according to the patient’s tolerance. Because of the chemical effect between the alcohol and the epithelial wall, the fluid became turbid again at the beginning of the ethanol irrigation process. After 3–5 irrigation cycles, when the fluid in the syringe was totally colorless and clean again, this step ended. Based on the volumes of the initial cyst, the aspirated fluid, and the alcohol injected, we estimated the concentration of alcohol needed to exceed 80% alcohol in solution to have the intended effect inside the cyst [Citation35]. If intra-capsular hemorrhage occurred, we sprayed the site immediately and repeatedly with alcohol to stop it or applied MWA to the site.
Short-term boiling ethanol ablation (STBEA)
After the irrigation, 1–2 mL ethanol was left in the capsule to keep it open. A 1–2-mm skin incision was made, and the ablation antenna was positioned under sonographic monitoring. For MWA, a power output of 30–50 W and frequency of 2450 MHz were used. Under US guidance, we positioned the needle tip in the ethanol and applied the STBEA method, which causes the ethanol to boil for about 3 s to consolidate the efficacy.
Microwave ablation procedure (MWA)
For mainly cystic thyroid nodules, we conducted MWA treatment after the above procedures. The tip of the microwave needle was stabbed into the solid component, with a power output of 30–50 W at 2450 MHz. The ‘leverage and pry-off method’ and moving-shot technique were used during the ablation process [Citation17]. The ablation was ceased until the solid part converted to a hyperechoic zone completely. If the patient could not tolerate the pain or discomfort during the procedure, we paused for a while and reduced the power if necessary. The procedure was completed when both color Doppler flow imaging (CDFI) and contrast-enhanced US no longer showed any evidence of the solid component. Patients remained under observation for 2 h with compression on the neck for at least 30 min to prevent bleeding and were examined again before discharge. The treatment time, irrigation volume and immediate complications were recorded.
Post-procedural observation and follow-up
Patients underwent follow-up US examinations at 1, 3, 6 and 12 months postoperatively and every 6 months thereafter. Three diameters of the nodules, the CGS, and the SS were recorded at each follow-up. The volume reduction ratio (VRR) was calculated by the following formula: VRR (%) = (initial volume − follow-up volume)/initial volume × 100%. The treatment endpoints included: 1) the VRR was more than 90% by the final follow-up; 2) the VRR was more than 50% after 1 year from the treatment [Citation22,Citation36]. If the VRR after 1-year follow-up was less than 50%, the treatment should be repeated. To evaluate the safety of the treatment, complications were carefully monitored. According to the international working group on image-guided tumor ablation, major complications include permanent voice change or dysphonia, and minor complications include recoverable voice change or discomfort during the procedure [Citation37].
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 20.0 (IBM, Armonk, NY). A p value less than .05 was considered indicative of a statistical significance. Variables are expressed as mean ± standard deviation (SD). According to homogeneity test results, Analysis of variance for repeated measurement was used to analyze the differences among volume and VRR at different follow-up time points. Tukey honestly significant difference test and Tamhane test were used to compare data among different follow-up groups. The safety outcome was reported using the number of cases and percentage. SS and CGS data were compared by Wilcoxon’s signed-rank test.
Results
Patients’ characteristics
This retrospective study included 201 patients (145 females and 56 males) who were treated using the modified PEI method with MWA in our hospital from April 2015 to August 2018. The demographic and clinical details of the patients are presented in . The mean SS for all patients was 3.69 ± 1.29 before treatment, and the mean CGS was 3.70 ± 0.55 before treatment. The mean length of follow-up was 13.30 ± 9.25 months. Of the 201 included patients, 10 (4.8%) patients underwent a second ablation (4 due to cyst recurrence and 6 to achieve better reduction of nodule size) and 1 (0.5%) patient with recurrence underwent a third ablation procedure.
Table 1. Baseline and clinical characteristics of patients.
Safety evaluation
None of the patients experienced pain that required pausing of the procedure, and none had major complications. Ten (4.8%) patients experienced temporary voice change, which resolved within 3 months without any permanent voice change. In addition, no patients had scar formation ().
Nodule characteristics
Among the 201 patients, 205 thyroid nodules were treated with the modified PEI and MWA approach. The characteristics of the nodules are presented in . The median values for the largest nodule diameter and volume were 3.75 ± 1.28 cm and 17.40 ± 3.21 mL. The included nodules were predominantly cystic. The mean procedure time was 15.02 ± 5.66 min, and the mean irrigation volume was 17.51 ± 17.67 mL. Among the 205 treated nodules, 18 (8.7%) nodules had disappeared by the 12-month follow-up, and 200 (97.6%) nodules showed significant volume reduction by the final follow-up. Only 5 (2.4%) nodules recurred (final follow-up volume was not less than the initial volume).
Table 2. Characteristics and clinical details for treated nodules.
Efficacy evaluation
During the post-operative follow-up, the mean thyroid nodule volume decreased over time from 17.40 ± 3.21 mL at baseline to 4.48 ± 1.21 mL at 1 month (66.57 ± 16.10%, p < .05), 2.37 ± 0.76 mL at 3 months (83.07 ± 8.23%, p < .05), 1.79 ± 0.59 mL at 6 months (89.52 ± 7.65%, p < .05), 1.17 ± 0.37 mL at 12 months (93.34 ± 7.38%, p < .05), and 1.48 ± 0.18 mL at the final follow-up (90.20 ± 8.18%, p < .05; ).
Table 3. Size and features of nodules before treatment and at each follow-up.
Compared with the baseline values, significant improvements in the SS and CGS were observed at each follow-up (). The mean SS and CGS both decreased in 12 months follow-up.
Detailed VRR in different follow-up time points are shown in . The greatest VRR was observed within the first 3 months after treatment. Statistical analysis showed that the VRRs at 3rd month after treatment were significantly larger than that at 1 month (p < .05), and the mean VRRs at 1 month, 3rd month, 6th month and 12th month were increasing.
Table 4. Changes of VRR after treatment in the follow-ups.
Discussion
The aim of this study was to retrospectively evaluate the efficacy, safety and operation time of the modified PEI method. In this clinical study, we found that the volume of all thyroid nodules decreased significantly after the treatment. Our clinical results showed the success rate of the therapy was 97.6% (200/205 nodules) as defined by a VRR exceeding 90% at the final follow-up or a VRR > 50% on US along with symptom control by 6 months after treatment. For 5 of the 205 nodules (2.4%), the final follow-up volume was not less than the initial volume. No major complications occurred during the procedure or follow-up, and 10 (4.8%) patients experienced minor complications, which resolved within 3 months.
Our findings are consistent with previous studies showing that PEI is safe and effective for the treatment of predominantly cystic thyroid nodules. Among the studies that assessed the efficacy and safety of PEI, the reported success rates for this procedure ranged from 64% to 95% [Citation7,Citation38,Citation39]. Most recently, a 2018 study in Turkey [Citation10] treated 55 cystic and mixed nodules in 53 patients and achieved volume reduction percentages of 80.7% at 6 months and 82.1% at 12 months after treatment without any serious complications.
Deandrea et al. have conducted an important study regarding the long-term efficacy of PEI treatment of thyroid nodules that included different percentages of cystic composition. The results showed that with PEI, the more cystic components, the higher VRR. It can be understood from this study that PEI can cause damage to the cyst wall; it cannot have a good effect on the solid components in the nodules. Therefore, for nodules with less cystic components (less than 50%), the new method we developed will be better, because it can damage both the cyst wall and the solid components at the same time.
As discussed, many studies have confirmed that PEI is effective for nodule volume reduction, but ethanol injection has side effects related to the leakage of ethanol and is associated with an unsatisfactory rate of nodule recurrence [Citation20]. Previous studies have reported recurrence rates of 26%–33% with simple PEI [Citation11,Citation16]. In a study in Korea [Citation11], 38.3% (41/107) of patients with predominantly cystic nodules experienced recurrence after ethanol ablation. Jang et al. [Citation40] and Kim et al. [Citation15] suggested that the presence of a solid component within the cyst was an independent factor reducing the efficacy of ethanol ablation. We developed a modified PEI method that combines simple PEI with MWA, and the results of this study show that this modified PEI method reduced the recurrence rate to 2.4%. Moreover, the modified PEI method was completed in a treatment time of 15.02 ± 5.66 min, which is less than the 25-min treatment time reported to be necessary for the effectiveness of simple PEI with evacuation [Citation12].
The modified PEI method that we developed and tested in this study applied STBEA with the goal of achieving the efficacy of traditional PEI while avoiding the negative effects of extended retention of ethanol within the lesion. Ethanol can permanently tissue damage by inducing cellular dehydration and protein denaturation that eventually causes tissue necrosis, fibrosis and thrombosis of small blood vessels [Citation41,Citation42]. The results showed that boiling of 80% ethanol for 3–5 s via STBEA caused the formation of a necrotic region with the largest depth, which was significantly greater than that achieved with retaining ethanol within the cyst for 10 min (p < .05). Notably, no difference between was observed with the use of 99.90% versus 80% ethanol. However, as the ethanol concentration decreased below 80%, the depth of the necrotic region decreased. In the modified PEI method applied in this study, 99.90% ethanol was used to complete the irrigation procedure to ensure the ethanol concentration within the capsule reached more than 80%. The irrigation procedure was repeated to achieve complete washing out of the colloid substance in the nodules to cause more damage. These experimental results indicated that in the application of ethanol ablation, the final concentration of ethanol should be more than 80%, which is consistent with the finding of a previous study [Citation35]. Moreover, boiling ethanol for 3–5 s offers better effectiveness than simply keeping it inside the cyst for some time while also reducing the risk of leakage [Citation19,Citation43].
Because the adjacent structure of the thyroid gland is physiologically important and complex, complications can be caused by improper operation or the heating process, especially when a lesion is large or located in the lower back side [Citation34]. To protect the recurrent laryngeal nerve or large blood vessels, a liquid isolation region around the thyroid capsule was created in our study. The rates of side effects and complication rates with the combined approach applied in this study (PEI plus MWA) were no more than those reported for MWA [Citation19,Citation44]. In another study [Citation17], our team treated 435 patients using US-guided MWA, and the results also showed no major complications occurred after treatment. Huynh et al. [Citation45] conducted a retrospective study on 42 large benign thyroid nodules (≥3 cm) treated with MWA at 30–50 W power, and as in this study, none of the patients experienced pain at a level that required pausing during the ablation procedure.
Notably, studies of cyst treatments have rarely included more than 100 patients. In 2004, Valcavi et al. [Citation8] reported a randomized controlled trial in which 143 patients underwent cyst drainage plus PEI and 138 patients were treated with only simple cyst drainage. They found that after 1 year the volume reduction in the PEI group was 85.6% versus 7.3% in simple drainage group (p < .001). Most studies have included numbers of patients ranging from 10 [Citation7] to 98 [Citation39].
Our study still has several limitations. First, it had a retrospective design; further prospective studies are required to verify the findings of this study. Second, the follow-up period was short, and thus, a further study with long-term follow-up is needed. In addition, our patients came from all over China, and loss of patients at different follow-up times occurred due to missed appointments related to geographical difficulties. Therefore, complete data for all laboratory results could not be obtained for the whole follow-up period. For those patients who could not be followed up strictly, we emphasized that the final follow-up must be done. Last but not list, the modified PEI method need to combine with MWA, and it cost more than simple PEI. For the predominantly thyroid cysts, the new method can reduce the recurrence rate significantly, but for the patients who have limited financial resources, doctors should weigh the pros and cons.
In summary, our study results indicate that the modified PEI method combined with MWA is an effective and safe method for treating symptomatic, predominantly cystic, benign thyroid nodules based on a 97.6% success rate and 2.4% recurrence rate. More studies are necessary to compare the feasibility, safety and efficacy of the modified PEI method with other less invasive procedures for cystic benign thyroid nodules.
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008;13(2):105–112.
- Bennedbaek FN, HegedüS L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88(12):5773–5777.
- Yasuda K, Ozaki O, Sugino K, et al. Treatment of cystic lesions of the thyroid by ethanol instillation. World J Surg. 1992;16(5):958–961.
- Livraghi T, Paracchi A, Ferrari C, et al. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Radiology. 1990;175:827–829.
- Schrut GC, Miasaki FY, Paz-Filho G, et al. Changes associated with percutaneous ethanol injection in the treatment of thyroid nodules. Endocr Pathol. 2011;22(2):79–85.
- Verde G, Papini E, Pacella CM, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clin Endocrinol. 1994;41:719–724.
- Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocrine Practice. 2004;10(3):269–275.
- Reverter JL, Alonso N, Avila M, et al. Evaluation of efficacy, safety, pain perception and health-related quality of life of percutaneous ethanol injection as first-line treatment in symptomatic thyroid cysts. BMC Endocr Disord. 2015;15(1):73.
- Ozderya A, Aydin K, Gokkaya N, et al. Percutaneous ethanol injection for benign cystic and mixed thyroid nodules. Endocr Pract. 2018;24(6):548–555.
- Suh CH, Baek JH, Ha EJ, et al. Ethanol ablation of predominantly cystic thyroid nodules: evaluation of recurrence rate and factors related to recurrence. Clin Radiol. 2015;70(1):42–47.
- Iniguez-Ariza NM, Lee RA, Singh-Ospina NM, et al. Ethanol ablation for the treatment of cystic and predominantly cystic thyroid nodules. Mayo Clin Proc. 2018;93(8):1009–1017.
- Monzani F, Lippi F, Goletti O, et al. Percutaneous aspiration and ethanol sclerotherapy for thyroid cysts. J Clin Endocrinol Metab. 1994;78:800–802.
- Bennedbaek FN, Karstrup S, Hegedus L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol. 1997;136(3):240–250.
- Kim DW, Rho MH, Kim HJ, et al. Percutaneous ethanol injection for benign cystic thyroid nodules: is aspiration of ethanol-mixed fluid advantageous? AJR Am J Neuroradiol. 2005;26:2122–2127.
- Basu N, Dutta D, Maisnam I, et al. Percutaneous ethanol ablation in managing predominantly cystic thyroid nodules: an eastern India perspective. Indian J Endocrinol Metab. 2014;18:662–668.
- Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med (Maywood). 2017;242(15):1515–1523.
- Kim JH, Lee HK, Lee JH, et al. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol. 2003;180:1723–1726.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–16–e16.
- Yoon HM, Baek JH, Lee JH, et al. Combination therapy consisting of ethanol and radiofrequency ablation for predominantly cystic thyroid nodules. AJNR Am J Neuroradiol. 2014;35(3):582–586.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label trial (Lara trial). Thyroid. 2020;30(6):847–856.
- Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(1):37–44.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Baek JH, Ha EJ, Choi YJ, et al. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015;16(6):1332–1340.
- Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269(1):293–300.
- Wu W, Gong X, Zhou Q, et al. Ultrasound-guided percutaneous microwave ablation for solid benign thyroid nodules: comparison of MWA versus control group. Int J Endocrinol. 2017;2017:1–7.
- Mo HS, Wei L, Ye H, et al. Microwave ablation of visible benign thyroid nodules with different internal characteristics: a comparative study with follow-up results. J Invest Surg. 2020;1–7. DOI:https://doi.org/10.1080/08941939.2020.1854903
- Deandrea M, Trimboli P, Creanza A, et al. Long-term follow-up of cystic thyroid nodules treated with percutaneous ethanol injection (PEI) using two different approaches. Eur J Endocrinol. 2020;183(5):489–495.
- Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665.
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13(2):117–125.
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010;194(4):1137–1142.
- Korkusuz Y, Mader OM, Kromen W, et al. Cooled microwave ablation of thyroid nodules: Initial experience. Eur J Radiol. 2016;85(11):2127–2132.
- Zheng BW, Wang JF, Ju JX, et al. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine. 2018;62(2):307–317.
- Yan-Hong F, Lin-Xue Q, Hai-Ma G, et al. Sclerotherapy of simple hepatic cysts by repeated aspiration and alcohol instillation. Turk J Gastroenterol. 2012;23(4):359–365.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol. e1694;25(11):1691–1705.
- Cho YS, Lee HK, Ahn IM, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR Am J Roentgenol. 2000;174(1):213–216.
- Del Prete S, Caraglia M, Russo D, et al. Percutaneous ethanol injection efficacy in the treatment of large symptomatic thyroid cystic nodules: ten-year follow-up of a large series. Thyroid. 2002;12(9):815–821.
- Jang SW, Baek JH, Kim JK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012;81(5):905–910.
- Pomorski L, Bartos M. Histologic changes in thyroid nodules after percutaneous ethanol injection in patients subsequently operated on due to new focal thyroid lesions. APMIS. 2002;110(2):172–176.
- Crescenzi A, Papini E, Pacella CRR, et al. Morphological changes in a hyperfunctioning thyroid adenoma after percutaneous ethanol injection: histological, enzymatic and sub-microscopical alterations. J Endocrinol Invest. 1996;19(6):371–376.
- Felicio JS, Conceicao AM, Santos FM, et al. Ultrasound-guided percutaneous ethanol injection protocol to treat solid and mixed thyroid nodules. Front Endocrinol (Lausanne). 2016;7:52.
- Zhi X, Zhao N, Liu Y, et al. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34(5):644–652.
- Khanh HQ, Hung NQ, Vinh VH, et al. Efficacy of microwave ablation in the treatment of large (>/=3 cm) benign thyroid nodules. World J Surg. 2020;44:2272–2279.