Abstract
Objective
To compare the efficacy and safety of the treatment of vulvar lichen simplex chronicus (VLSC) using high-intensity focused ultrasound (HIFU) at different powers.
Methods
This retrospective study included 152 patients with VLSC. Among these patients, 70 were treated with HIFU at low power (level 2), and 82 were treated at normal power (level 3). The treatment responses, recurrence rates and intra- and postoperative complications were all compared.
Results
No statistically significant differences were found between the two groups in age, disease course, menopause status, lesion size and severity of symptoms. All patients received one session of HIFU therapy, and the treatment process was successful. No difference was found in the total response rate between the two groups at 1 (85.7% versus 87.8%, p = .35), 6 (80% versus 80.5%, p = .65) and 12 (80% versus 80.5%, p = .73) months after HIFU therapy. No significant difference was observed in the recurrence rate between the two groups at 6 (5.7% versus 9.8%, p = .36) and 12 (22.9% versus 26.8%, p = .57) months after HIFU treatment. Patients in the low-power group had a lower incidence of blisters (10% versus 23.3%, p = .04).
Conclusion
Based on our results, low-power HIFU treatment can achieve a therapeutic effect similar to normal power HFU treatment for VLSC, but its incidence of side effects is lower.
Introduction
Vulvar lichen simplex chronicus (VLSC) is a common, chronic, nonscarring inflammatory disease of the vulvar skin [Citation1] that develops predominantly in middle to late adult life. Chronic or intermittent pruritus accompanied by vigorous scratching or rubbing is the most common reported symptom of VLSC, and it can adversely affect sexual function and sense of well-being [Citation2]. Its true incidence and prevalence are unknown and difficult to estimate because affected patients may present to various specialists for their potentially embarrassing symptoms. In clinics devoted solely to the care of patients with vulvar disorders, VLSC accounts for 10%–35% of the patients evaluated [Citation3]. The clinical sign of LSC includes one or more erythematous, scaling, lichenified plaques with varying degrees of overlying excoriation, hyperpigmentation, hypopigmentation and lichenification. The typical clinical symptoms and signs make it easy to recognize on visual examination. However, due to the fact that very little is known about the cause and multifactorial nature of LSC, VLSC therapy is challenging.
Many medications or physical therapies have been used, often with unsatisfactory results and a high recurrence rate. The guidelines for the management of vulvar skin disorders recommend that treatment of lichen simplex chronicus is focused on vulvar self-care, control of itching and scratching, and treating inflammation with topical corticosteroids [Citation1]. Although improvement can be obtained with appropriate treatment, recurrences are common [Citation4]. At the same time, the side effects of excessive drug use should be given attention to. Reports have shown the effectivity of laser [Citation5], phototherapy [Citation6], mixed methylene blue compound injection [Citation7] and pimecrolimus cream [Citation8] in treating VLSC. The wearing of silk fabric underwear may also be effective in the management of VLSC [Citation9], but further research is needed.
High-intensity focused ultrasound (HIFU), as a non-surgical therapeutic treatment, has received increasing attention for the treatment of gynaecological diseases over the last 20 years [Citation10]. Several studies have shown that HIFU is safe and effective in treating patients with vulvar dermatoses [Citation11–14]. The power used in different studies on HIFU treatment protocol is different at level 3 power or above, and the treatment effects and adverse reactions are also different. However, to our knowledge, no study has yet compared the therapeutic effects and side effects of different powers of HIFU treatment for VLSC. Moreover, no study has investigated the effect of low-power HIFU on VLSC treatment. Theoretically, the lower the output power, the less severe the skin damage. However, it is not known whether low-power HIFU can achieve similar therapeutic effects as high-power HIFU for VLSC.
This study aimed to evaluate whether lower power is more favorable to the HIFU treatment of VLSC than high power by comparing efficacy and safety.
Materials and methods
Ethical considerations
The study protocol was approved by the institutional review board of the Third Xiangya Hospital of Central South University (No.21002), and informed consent was obtained from the patients.
Patients
This work is a retrospective cohort study performed in the Department of Obstetrics and Gynecology, Third Xiangya Hospital of Central South University, Changsha, China, between January 2019 and December 2019. Patients were identified by searching available electronic medical records. The inclusion criteria are as follows: (1) women aged 20–70 years old; (2) patients diagnosed according to the diagnosis standard of VLSC; (3) lesions in the mons pubis, labia majora, labia minora, nympholabial furrow, praeputium clitoridis and perineum; (4) patients who did not receive physicotherapeutics or glucocorticoids in the last three months before HIFU treatment; and (5) patients treated with focused ultrasound at our hospital with complete follow-up data. The exclusion criteria are as follows: (1) patients who were contradicted to general and local anesthesia or sedatives; (2) biopsy of the vulva indicating neoplastic epithelial disorders of the vulva; (3) patients in menstrual period, pregnancy or lactation; and (4) patients with uncontrolled diabetes. Data on age, gravidity, parity, menstruation, clinical manifestations, previous treatments, physical examination findings, pathological results of vulva biopsy, treatment parameters, therapeutic effect and side effects were collected from a review of electronic medical records and telephone follow-up.
HIFU therapy
The treatment was performed under local infiltration anesthesia using 10–20 mL 0.5% lidocaine, with the patient placed in a dorsal lithotomy position. HIFU treatment was performed with a model CZF-focused ultrasound therapeutic device (Chongqing Haifu Medical Technology, Chongqing, China). Its output power has six levels with the following power: 2.5, 8.4, 9.2, 10.6, 11.4 and 12.1 W, respectively. Patients in the low-power group were treated with an output power of level 2, and those in the control group were treated with an output power of level 3, which is the power commonly used in previous literature. The other treatment parameters were a frequency of 10.4 MHz and a pulse of 1,000 Hz. During the treatment, the probe was in close contact with the skin above the lesion through an ultrasound couplant. Linear scans were performed over the lesion and healthy tissues 5 mm around that area at a speed of 10–20 mm/s until the treatment area showed mild congestion, swelling and hyperthermia. The treatment duration lasted for 10–42.5 min. After the treatment, ice packs were intermittently applied on the treated area for 12–24 h. Moist burn ointment was applied locally for three days to two weeks, depending on the specific circumstances of skin burns.
Follow-up
All patients were followed up once a week for the first month, at the sixth month, at the twelve month and then once per year after the treatment. The treatment responses were classified as curative (no symptoms remained), effective (the symptoms were relieved), or persistent (the symptoms remained the same) based on the patients’ responses to the therapy. The total response rate was calculated as a percentage as follows: (No. of patients curative + No. of patients effective)/Total No. of patients who underwent HIFU treatment × 100%. Recurrence was defined as a recurrence after a complete remission of symptoms or a worsening of symptoms after a period of improvement. Intra- and postoperative complications were recorded. Potential complications included urinary tract injury, blisters, ulcer, scleroma and infection during or after the procedure.
Statistical analysis
The Statistical Program for the Social Sciences (SPSS Inc., Chicago, IL, USA) was used to conduct statistical analysis. The normally distributed continuous data were presented as the mean ± standard deviation, and the mean differences were analyzed using Student’s t-test. The abnormally distributed continuous data were presented as the median and interquartile range and then analyzed using the Mann-Whitney U test. The categorical data were presented as the frequency and percentage per study group and analyzed using the chi-square test.
Results
Baseline characteristics
In this study, 70 patients were identified in the low-power group and treated with HIFU at power level 2, and 82 patients were identified in the control group and treated with HIFU at power level 3. presents the demographic data. No significant differences were found in the baseline patient characteristics between the two groups. Specifically, no significant differences were observed in age, disease course, menopause status, lesion size and severity of symptoms, which are possible factors associated with efficacy according to previous studies. Fifty-two women in the low-power group and 58 women in the control group complained of severe pruritus, while only four patients in the low-power group and two patients in the control group had mild pruritus as the main complaint.
Table 1. Demographic characteristics of patients with VLSC.
Results of HIFU therapy
All patients in both groups successfully completed one session of HIFU treatment (). The sonication time was 21.0 ± 6.0 min in the low-power group and 18.2 ± 5.7 min in the control group (p = .04). The total treatment energy in the low-power group was 4,022.4 ± 1,188.8 J, which is similar to that in the control group (4,435.4 ± 1,615.6, p = .23).
Table 2. Comparison of treatment parameters and short-term complications between the two groups.
Complications
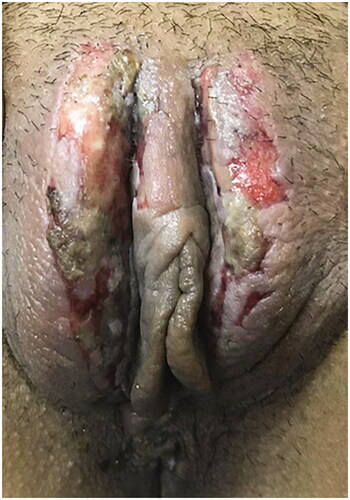
Immediately after treatment, the skin of the treated region of all patients in both groups appeared slightly red and swollen but free of pain. In the low-power group, seven patients developed blisters after HIFU treatment, and one patient developed vulval ulcers (). Blisters developed in 19 patients in the control group, four patients developed ulcers, and one patient had scleroma in the treatment lesion. shows one patient in the control group with a severe vulvar ulcer. The patient developed multiple blisters during HIFU treatment, and gradually developed ulcer 1 week after HIFU treatment. The patient was treated with moisture exposed burn ointment and recombinant human epidermal growth factor for six weeks after the procedure. The incidence of blisters in the low-power group was significantly lower than that in the control group (10% versus 23.3%, p = .04). No bowel injury, urinary tract injury, or infection occurred during or after the procedure in either group.
Follow-up results
The efficacy results of the two groups after HIFU therapy are shown in . One month after treatment, pruritus was absent in 46 patients and improved in 14 patients in the low-power group. Ten patients complained of pruritus as before. The total response rate was 85.7% in the low-power group. For the patients in the control group, pruritus was cured in 62 patients and relieved in 10 patients. HIFU therapy was found to be ineffective in 10 patients in the control group. The total response rate was 87.8% in the control group, similar to that of the low-power group (p = .35).
Table 3. Comparison of efficacy between the two groups after HIFU therapy.
Six months after treatment, symptoms recurred in four (5.7%) patients in the low-power group, and the severity of symptoms was consistent with that before treatment. In the control group, eight (9.8%) patients had pruritus again, among which two patients had mild pruritus compared with that before treatment, and six patients had the same pruritus as that before treatment. No significant difference in recurrence rate was observed between the two groups (p = .36). The total response rate decreased in both groups compared with that one month after the procedure.
Twelve months after treatment, the total response rate was 80.0% in the low-power group and 80.5% in the control group. No significant difference was found between the two groups (p = .73). Symptoms recurred in 16 patients in the low-power group and in 22 patients in the control group. However, their pruritus was milder than that before treatment. The recurrence rate was 22.9% in the low-power group and 26.8% in the control group. No significant difference in the recurrence rate was found between the two groups (p = .57).
Discussion
VLSC is an eczematous disease characterized by unremitting itching and scratching. Although common, the actual incidence of VLSC is unknown. Histopathologically, VLSC lesions have epidermal thickening, hyperkeratosis, spongiosis and acanthosis. The mucosa of patients with VLSC may become microscopically indistinguishable from skin due to the aberrant development of a granular layer and keratinization. Notably, VLSC usually lacks vertical fibrosis, which is seen in extragenital LSC and has prominent fibroblasts [Citation15,Citation16].
The primary symptom of VLSC is itching, often intractable and uncontrollable, sometimes developing into burning sensation and pain. Symptoms can be intermittent or chronic and may be present for weeks, months or even years. Thus far, little is known about the cause of LSC. The common triggers for the development of the disease include environmental factors, dermatological diseases and psychological stressors [Citation2]. The multifactorial nature of LSC makes its management difficult. The management of VLSC is mainly divided into four categories: identification of any underlying disease, breakup of the itch-scratch cycle, repair of the barrier layer function and reduction of local inflammation [Citation1].
HIFU therapy, as a recently developed noninvasive technique, has been used to treat solid tumors and vulvar lesions. An ultrasonic beam can be directed to the target in the human body, resulting in temperature rising, which leads to coagulative necrosis of the tissue in the focal region. Ultrasonic exposure can cause cell proliferation stimulation, increased synthesis of collagen protein and vascularization [Citation17]. After HIFU treatment, vulvar tissue structures recovered their normal pigmentation, the count of micro-vessels increased, their lumens recovered and nerve endings and fibroblast counts in the lower layer of the dermis increased [Citation14]. HIFU may inhibit superficial collagen fibrosis in the dermis by affecting the expression of notch1, c-fos and TGF-b3 in vulvar skin tissue, consequently reducing the recurrence rate of VLSC [Citation18]. Several studies demonstrated the efficacy of HIFU for non-neoplastic epithelial disorders of the vulva, including VLSC and vulva lichen sclerosus [Citation11,Citation12,Citation19]. The power they used in these previous studies ranged from level 3 to 4. Thus far, no study has compared the efficacy and safety of HIFU at different powers in the treatment of VLSC or reported the effect of power level 2 on VLSC.
In this study, we treated 70 patients with HIFU at power level 2 and 82 patients at power level 3. No significant difference in the total response rate was found between the two groups after HIFU treatment. The cure rate at six months after HIFU treatment was 60% and 65.8% in each group, similar to previous research. Li et al. treated 76 patients with squamous hyperplasia and lichen sclerosus using HIFU and found that 64.47% of the patients were cured after a two-year follow-up [Citation14]. Ye et al. found that HIFU, with a cure rate of 42.2%, was effective in alleviating symptoms and improving vulva signs [Citation12]. However, the current study found that some patients had recurrent symptoms six months after treatment and that the recurrence rate further increased one year after treatment. No significant difference was found in the recurrence rate when the patients were grouped by power.
Although HIFU treatment is relatively safe, the possible skin burns that it causes should be given attention to. If used improperly, it may cause severe skin damage or coagulative necrosis. It may also cause vulva scar formation. Skin burn is the main side effect of HIFU treatment, mainly manifested as blisters or superficial ulcers. Skin burn is considered related to the rapidly elevating temperature due to the excessive ultrasound energy accumulation on the focal skin surface. Among the patients, 10% in the low-power group and 23.3% in the control group had blisters after the treatment. The results of this study found that the higher the power used in the treatment, the higher the probability of skin burns. Although the close monitoring of morphological changes during HIFU treatment can reduce the incidence of skin burns, this is sometimes difficult to master. Reducing the power output may help reduce the incidence of skin burns.
There are several other measures that can be used to reduce the risk of skin burns. First, during treatment, the probe should be moved continuously, and the speed of probe movement should be accelerated to 10–20 mm/s. Holding the probe in the same area for more than 2 s can cause skin damage. Second, during the treatment process, the skin in the treatment area should be kept as smooth as possible to avoid excessive energy deposition due to the formation of interface reflection caused by the presence of air on the skin surface due to the deep skin folds. Third, skin damage can be reduced by applying ice to physically cool the area and by using moist burn cream on the treatment area.
This study has several limitations. First, the study has a retrospective design. Second, the follow-up time was relatively short. More work such as evaluation of the degree of skin keratosis before operation and preconditioning and longer observation times are needed to verify whether there are differences in long-term efficacy between different powers.
Conclusion
This study demonstrates that HIFU at low power can achieve a therapeutic effect similar to normal power HFU treatment. Compared with that in the control group, the incidence of blisters in the low-power group was significantly lower.
Authors’ contributions
LJ Li: Data collection, manuscript writing. SL He: Data analysis. JF Jiang: Protocol development, manuscript editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Diagnosis and Management of Vulvar Skin Disorders: ACOG Practice Bulletin, Number 224. Obstet Gynecol. 2020; 136(1):e1–e14.
- Ringel NE, Iglesia C. Common benign chronic vulvar disorders. Am Fam Physician. 2020;102(9):550–557.
- Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther. 2004;17(1):8–19.
- Thorstensen KA, Birenbaum DL. Recognition and management of vulvar dermatologic conditions: lichen sclerosus, lichen planus, and lichen simplex chronicus. J Midwifery Womens Health. 2012;57(3):260–275.
- Zhang L, Lai Y, Wan T, et al. A randomized clinical study of the treatment of white lesions of the vulva with a fractional ultrapulsed CO2 laser. Ann Palliat Med. 2020;9(4):2229–2236.
- Virgili A, Minghetti S, Borghi A, et al. Phototherapy for vulvar lichen simplex chronicus: an ‘off-label use’ of a comb light device. Photodermatol Photoimmunol Photomed. 2014;30(6):332–334.
- Li Y, Shi J, Tan W, et al. Prospective observational study of the efficacy of mixed methylene blue compound injection for treatment of vulvar non-neoplastic epithelial disorders. Int J Gynaecol Obstet. 2020;148(2):157–161.
- Kelekci˙ HK, Uncu HG, Yi˙lmaz B, et al. Pimecrolimus 1% cream for pruritus in postmenopausal diabetic women with vulvar lichen simplex chronicus: a prospective non-controlled case series. J Dermatolog Treat. 2008;19(5):274–278.
- Corazza M, Borghi A, Minghetti S, et al. Effectiveness of silk fabric underwear as an adjuvant tool in the management of vulvar lichen simplex chronicus: results of a double-blind randomized controlled trial. Menopause. 2015;22(8):850–856.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Wu C, Zou M, Xiong Y, et al. Short- and long-term efficacy of focused ultrasound therapy for non-neoplastic epithelial disorders of the vulva. Int J Obstet Gy. 2017;124(Suppl 3):87–92.
- Ye M, Deng X, Mao S, et al. High intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva: factors affecting effectiveness and recurrence. Int J Hyperthermia. 2015;31(7):771–776.
- Ruan L, Xie Z, Wang H, et al. High-intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva. Int J Gynaecol Obstet. 2010;109(2):167–170.
- Li C, Bian D, Chen W, et al. Focused ultrasound therapy of vulvar dystrophies: a feasibility study. Obstet Gynecol. 2004;104(5 Pt 1):915–921.
- Burrows LJ, Shaw HA, Goldstein AT. The vulvar dermatoses. J Sex Med. 2008;5(2):276–283.
- Chan MP, Zimarowski MJ. Vulvar dermatoses: a histopathologic review and classification of 183 cases. J Cutan Pathol. 2015;42(8):510–518.
- Skyba DM, Price RJ, Linka AZ, et al. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98(4):290–293.
- Liu Y, Fan Y, Tang H, et al. Therapeutic effects of focused ultrasound on expression of Notch1, C-Fos and transforming growth factor-β3 in vulvar skin of SD rats with vulvar lichen simplex chronicus. Ultrasound Med Biol. 2020;46(9):2311–2321.
- Zhou W, Zhu L, Zhou H, et al. The efficacy of high-intensity, focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva. Cell Mol Biol. 2016;62(4):111–115.