Abstract
Background
Symptomatic aseptic necrosis (SAN) followed by nodule rupture is a kind of severe complications after thermal ablation for benign thyroid nodules (BTN). No studies are available to evaluate its pathologic process, clinical manifestations, risk factors and effectiveness of therapies after microwave ablation (MWA).
Methods
From 2012 to 2019, 398 patients who received MWA for BTN were retrospectively reviewed. Clinical data included baseline patient characteristics, imaging features (internal vascularity and the proportion of the solid component), ablation power and time, complications and prognosis were collected and documented.
Results
Ten patients (2.51%) experienced post-MWA SAN, eight patients with nodule rupture and the other two without. The mean time from MWA to SAN symptom was 8.6 days and to rupture was 16.3 days. The initial symptoms of SAN patients were neck bulging, swelling and discomfort. Patients would go through nodule rupture once the nodule contents extended into the extrathyroidal area with the discontinuity of the anterior thyroid capsule, and fistula formed unavoidably in this condition. Incision drainage was effective for rupture and early treatment of non-steroidal anti-inflammatory drug might cure the early-stage SAN. Multivariate analysis showed sex (OR = 0.13; 95% CI: 0.03, 0.61; p=.03) was the risk factor leading to SAN and males were more vulnerable to SAN.
Conclusion
SAN after MWA came earlier and initially illustrated as neck bulging, swelling and discomfort. Early detection and early treatment might prevent the rupture of nodules. Once the breakdown of thyroid capsule occurred, rupture of ablated nodules out of skin was unavoidable and invasive procedures might be the most effective treatment.
Introduction
Minimally invasive ablation procedures such as microwave ablation (MWA), radiofrequency ablation (RFA), high intensity focused ultrasound (US) and laser ablation induced thermal therapy are increasingly used nowadays as an alternative to the thyroid surgery for benign thyroid nodules (BTN) [Citation1–6]. It enjoys popularity not only for its favorable outcomes but also for its cosmetic results. The mean volume reduction of BTN after thermal ablation reaches about 50–80% in 6-month follow-up [Citation7,Citation8]. Systematic reviews and meta-analyses have shown the low complication rate of thermal ablation lower than 5% [Citation9–11]. Complications encountered in thermal ablations for BTN include voice changes, skin burns, hematoma formation and transient hyperthyroidism [Citation9].
One kind of severe complications in thermal ablation for BTN is nodule rupture. Shin et al. reported three cases of nodule rupture in their multicenter study. Nodule rupture was detected at 9–60 days after RFA [Citation12]. Then, Chung et al. further reported 12 cases of thyroid nodule rupture after RFA at four Korean thyroid centers [Citation13]. They classified nodule rupture into three types based on its localization. Patients complained of neck bulging and pain when nodule rupture occurred, some experienced fistula and flow-out of necrosis. Some risk factors and clinical features show before the nodule rupture occurs. Early detection and early treatment of this severe complications are very important.
MWA is another type of thermal ablation and has been used as a safe minimal invasive method in thyroid ablation [Citation14–17]. Its theory of thermal ablation is different from RFA [Citation18] as electromagnetic methods of inducing tumor destruction by rotation of dipole molecules [Citation19]. Rupture also happens after MWA as a severe complication. There are some differences in clinical course of nodule rupture after MWA compared to RFA. However, no related studies have illustrated this severe complication after MWA. We found that the symptomatic aseptic necrosis (SAN) of BTN is the early pathological process of nodule rupture after MWA. Patients might experience nodule rupture as the progression of disease. We call this phenomenon SAN rather than rupture. Thus, we proposed the definition of SAN as: aseptic inflammation of necrosis in thyroid lesions after thermal ablation; laboratory tests and the necrosis culture result show no evidence of inflammation; neck bulging, swelling and discomfort are first symptoms, nodule would rupture and fistula might occur when liquefactive necrosis forms.
In this study, we evaluated the imaging features, clinical manifestations, risk factors and effectiveness of treatment therapies with SAN after MWA.
Materials and methods
Patients
The study protocol was approved by the institutional review board of our hospital and informed consent for each procedure was obtained from all patients before the procedure.
From March 2012 to September 2019, 398 patients who received MWA for BTN were retrospectively reviewed. BTNs were confirmed by core needle biopsy and no malignant US findings were detected according to the guidelines of the KSThR [Citation20,Citation21]. Patients presented with one of the following indications were included: (1) greater than 2.0 cm in largest diameter, progressive growth and solid component more than 20%; (2) symptomatic problems such as neck discomfort, foreign body sensation or compressive symptoms; (3) cosmetic problems; (4) refused or were ineligible for surgery.
Before MWA, laboratory examinations including blood routine and thyroid function were routinely performed. Clinical data included baseline patient characteristics, imaging features (echogenic features, internal vascularity and the proportion of the solid component of each nodule on US), ablation power and time, complications and prognosis were collected and documented.
MWA procedure
All MWA procedures were performed percutaneously under US guidance. The MWA system was the KY-2000 microwave system (Kangyou Medical, Nanjing, China). A 16-gauge, internal-cooled microwave antenna with a 3-mm active tip was used in this study. Local anesthesia consisting of 1% lidocaine was injected into the puncture site and perithyroidal area. If BTN contained fluid, the fluid was aspirated before MWA procedure. If nodules adjacent to vital structures such as the vagus nerve, trachea or esophagus, hydrodissection technique was performed to protect important surrounding structures. A 21-gauge PTC needle was introduced percutaneously between the thyroid capsule near the index nodule and its surrounding structures, then normal saline was injected to separate the thyroid capsule from the surrounding structures. Trans-isthmic approach and moving shot technique were applied. The antenna tip was positioned in the deepest and farthest area of the index nodule and then pulled back when a transient hyperechoic zone appeared within 5–10 s after turning on the microwave power. The output power of 20–50 W was used in different conditions. For nodules with rich vascularity, high power or prolonged ablation time was applied to block the blood flow. The MWA procedure was stopped when the entire visualized area of the nodule had become a transient hyperechoic zone. Contrast enhanced ultrasound (CEUS) immediately after MWA was performed to detect residual tumors and guide the additional ablation of residual tumors. Complications during or after MWA were identified and documented.
Definition
The nodular volume (V) was estimated by the ellipsoid formulation V=πabc/6, where a is the maximal diameter; b and c were the two orthogonal diameters. According to the US presentation of the internal nodule on color Doppler flow imaging (CDFI), the nodular vascular scores were classified into four grades: (1) no color signal in the nodule; (2) color signals in <25% of the nodule; (3) color signals in 25–50% of the nodule; and (4) color signals in >50% of the nodule [Citation22]. The internal content of a nodule is categorized in terms of the ratio of the cystic portion to the solid portion in the nodule: solid (no obvious cystic content), predominantly solid (≤50% of the cystic portion), predominantly cystic (>50% but ≤80% of the cystic portion).
Diagnostic criteria
According to our experience, we developed the diagnostic criteria for SAN and nodule rupture as:
SAN
(1) Received thermal ablation for thyroid lesions; (2) occurrence of neck bulging, swelling and discomfort typically within 1 month after thermal ablation; (3) laboratory tests and the necrosis culture result indicate no evidence of inflammation; (4) US shows the enlarged ablated lesions and fluid sonolucent area around the ablated nodule; CDFI shows increased vascularity in the early stage. (5) Nodule rupture and fistula formation might occur with disease progression.
SAN with nodule rupture
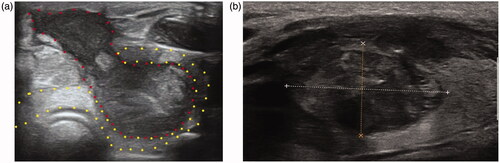
The continuity of the lesion capsular is interrupted. The liquefactive necrotic contents of ablated lesions ooze out into the surrounding structures such as thyroid capsule, muscle or the skin. SAN with nodule rupture or without nodule rupture could be clearly identified on US image ().
Figure 1. US images of SAN with or without nodule rupture. (a) SAN with nodule rupture, the continuity of thyroid capsular is interrupted and the necrotic contents of the ablated lesion (areas marked by red dots) oozed out of the thyroid (areas marked by yellow dots); (b) SAN without nodule rupture. The shape of ablated lesion was regular and the necrosis was confined in the ablated lesions. SAN: symptomatic aseptic necrosis.
Patients’ follow-up
All patients received CEUS after MWA within three days to assess the ablation area. Then patients underwent regular US/CEUS and thyroid function test at 3 months, 6 months and 12 months during the first 1 years, and every year thereafter. Regarding blood tests and the thyroid function indexes (thyroid stimulating hormone (TSH), triiodothyronine (T3) and free thyroxine (fT4)) were performed at every reported follow-up. Complications were recorded according to the reporting standards [Citation23,Citation24]. For patients suspected of SAN, careful US evaluation and blood routine were performed. Regular and careful follow-up of those patients were performed to follow the dynamic progress of SAN. Patients with nodule rupture or fistula formation also received secretion culture and regular dressing.
Statistical analysis
Values for quantitative variables were expressed as the mean ± SD and for categorical variables were expressed as percentage. For comparison of categorical variables, χ2 tests and Fisher’s exact tests were used, while for continuous variables, the Mann–Whitney U test was used. Outcomes along with SAN between different variables were assessed using binary logistic regression. A forward, stepwise multivariate logistic regression was performed to identify significant confounding factors. Variables with p<.20 in the univariate analysis were entered into the multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each result. All statistical analyses were performed by using SPSS, version 18.0 (SPSS, Chicago, IL). p<.05 was considered to indicate a statistically significant difference.
Results
Of the included 398 patients, 10 patients (2.51%) experienced post-MWA SAN, five men and five women. The mean age was 41 years (range, 24–56 years). Baseline demographics are shown in as patients with or without SAN. The mean volume of the index nodule was 21.94 ± 27.12 ml, which was significantly larger than patients without SAN (p= .03) (). No significant differences were found in the maximum ablation power, mean ablation time and energy per nodule () between the two groups. Other characteristics such as age and tumor number were similar between the two groups. The detailed information of SAN patients is shown in . The mean time from MWA to SAN symptom was 8.6 days (range, 3–20 days) and to rupture was 16.3 days (range, 8–24 days) (, ). The initial symptoms of SAN patients in our study were neck bulging, swelling and discomfort. US image showed the enlarged ablated nodule and fluid sonolucent area around the ablated nodule (). The CDFI showed increased vascularity. Following the development of symptoms, patients might go through nodule rupture as the nodule contents of liquefactive necrosis extended into the extrathyroidal area with the discontinuity of the anterior thyroid capsule. In our study, eight SAN patients experienced nodule rupture and nodules ruptured into the cervical strap muscles and finally formed fistula (). The necrotic contents of ablated tumor oozed out of the skin through the needle tract. Culture of oozed contents showed no bacterial growth. Two patients experienced low fever at the initial of symptoms but the WBC and NG tests were normal.
Figure 2. Comparison of variables in thyroid lesions with SAN, rupture, without SAN or without rupture. (a) Time to symptom or to rupture in patients with SAN; (b) tumor volume between nodules with rupture and SAN without rupture; (c) energy applied per volume between nodules with rupture and SAN without rupture; (d) tumor max diameter between nodules with rupture and without rupture in all included patients. SAN: symptomatic aseptic necrosis.
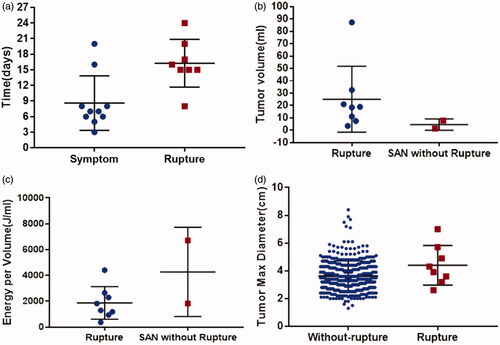
Figure 3. A female patient experienced SAN without nodule rupture after MWA. (a) The nodule was in the left thyroid lobe and shown as hyperechoic lesion on US image. The pathology result was adenoma and the tumor size was 3.8 × 2.3 × 1.7 cm; (b) CEUS before MWA showed hyper-enhancement; (c) 20 days after MWA, this patient complained of neck bulging and swelling. US image showed the enlarged ablated nodule and the fluid sonolucent area around the ablated nodule (red arrow) in the thyroid; (d) six months later, this patient recovered and the US image showed the debulking of the ablated nodule in transverse section. (e) US image showed the debulking of the ablated nodule in vertical section. SAN: symptomatic aseptic necrosis; MWA: microwave ablation; CEUS: contrast enhanced ultrasound; US: ultrasound.
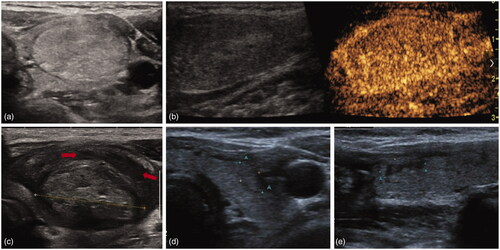
Figure 4. A male patient experienced SAN with nodule rupture after MWA. (a) The nodule was in the right thyroid lobe and shown as heterogeneous hypoechoic lesion on US image. The pathology result was nodular goiter and the tumor size was 2.6 × 1.6 × 1.6 cm; (b) MWA procedure of the thyroid lesion; (c) breakdown of the thyroid capsule (blue arrow) and the necrosis ruptured into the cervical strap muscles (red arrow). (d) Color Doppler of the ruptured lesion and normal thyroid tissue; (e) the skin appearance at the time of US image c acquired in this figure; (f) the skin appearance before incision drainage. SAN: symptomatic aseptic necrosis; MWA: microwave ablation; US: ultrasound.
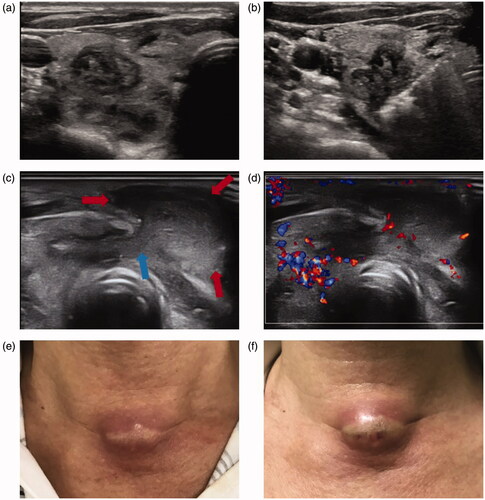
Table 1. Baseline patient characteristics.
Table 2. Baseline characteristics of the 10 patients with SAN after MWA.
Table 3. Clinical and imaging manifestations in patients with SAN after MWA.
All 10 patients were initially treated conservatively, with close observation or oral drugs. Oral drugs included antibiotics and non-steroidal anti-inflammatory (NSAI) drug ibuprofen. Two patients without rupture were treated with ibuprofen as soon as they complained of neck bulging and swelling and typical US images were detected, as the following laboratory tests showed no evidence of inflammation. Those two patients finally recovered without rupture. Other eight patients were finally treated with aspiration or incision drainage as conservative treatments could not alleviate the symptoms. Once the breakdown of the anterior thyroid capsule and the communication between intra- and extrathyroidal lesions at the MWA site occurred, rupture could not be avoidable and conservative treatments were ineffective. All those eight patients recovered after invasive treatments.
In univariate analysis, sex (OR = 0.24; 95% CI: 0.07, 0.85; p=.03), location (OR = 0.39; 95% CI: 0.10, 1.46; p=.16), power (OR = 1.07; 95% CI: 0.99, 1.16; p=.06), fT4 (OR = 1.13; 95% CI: 0.96, 0.61; p = 1.34) and TSH (OR = 0.47; 95% CI: 0.19, 1.21; p=.12) were factors associated with SAN. In multivariate analysis, only sex (OR = 0.13; 95% CI: 0.03, 0.61; p=.03) was the significant factor leading to SAN ). As for thyroid tumor rupture, sex (OR = 0.24; 95% CI: 0.06, 0.99; p=.03), size (OR = 1.65; 95% CI: 0.99, 2.72; p=.05), tumor volume (OR = 1.02; 95% CI: 0.99, 1.05; p=.07), location (OR = 0.31; 95% CI: 0.06, 1.48; p=.14) and max power (OR = 1.07; 95% CI: 0.98, 1.16; p=.12) were factors associated with rupture in univariate analysis. In multivariate analysis, only sex (OR = 0.18; 95% CI: 0.03, 0.97; p=.04) was the significant factor leading to rupture (). Male patients were more vulnerable to SAN in our current study.
Table 4. Univariate regression analysis of factors leading to SAN.
Table 5. Univariate regression analysis of factors leading to rupture.
Discussion
Nodular rupture is a severe complication encountered in thermal ablation of BTN. Related studies mainly focused on RFA and no studies of MWA concerning this severe complication have been reported to the best of our knowledge. Our study initially reported this severe complication after MWA for BTN from its initial occurrence. We define it as SAN because no infection has been found and pathology results showed necrosis, also nodules might not go through rupture after active early treatment. We speculate the pathogenesis of SAN might be that the necrosis after MWA could not be absorbed effectively and timely, and following inflammation stimulated the surround thyroid tissues and caused exudation. With the progression, the necrosis would break down the thyroid capsule to the anterior cervical area through the needle tract. If the breakdown of thyroid capsule occurs, fistula could not be avoided observed in our study. Two patients without rupture in our study had the SAN confined in thyroid gland and no invasion of thyroid capsule.
The time to symptom and rupture after MWA were significantly shorter than that after RFA. According to Chung et al.’s study, the mean time from RFA to nodule rupture was 54.6 days (range, 11–156 days) [Citation13]. In our study, the mean time from MWA to symptom was 8.6 days (range, 3–20 days) and to rupture was 16.3 days (range, 8–24 days). MWA had higher thermal efficacy than RFA and could achieve higher temperature in shorter time [Citation18]. Tissues go through more complete coagulation necrosis during MWA than during RFA. The inflammatory reaction was less acute during the MWA according to previous studies [Citation25]. Besides, MWA is less sensitive to heat-sink effect and could achieve more complete blood vessel coagulation than RFA. Hence, the absorption of coagulation necrosis might be slower than that of RFA. Previous studies also have validated the better volume reduction rate of BTN after RFA than after MWA [Citation26,Citation27]. The above analysis might be the reasons why symptom and rupture appeared earlier after MWA than after RFA.
We found that sex was a risk factor for SAN and rupture. Male patients were more susceptible to this severe complication in our study. Thyroid nodules are more popular in female patients [Citation28]. Whether the hormone level has an influence on this severe complication has not been illustrated and further studies are still in need to analyze this phenomenon. Logistic regression analysis showed that the maximum power used during the MWA was the risk factor for SAN and rupture, through the difference was not significant. Higher power could coagulate thyroid nodules more quickly in higher temperature, which could induce more carbonization in the ablated area. Carbonization would impede the absorption of thyroid nodules [Citation26]. Tumor size was a risk factor for rupture in univariate analysis but the difference was not significant in multivariate analysis. Larger tumors typically represent larger tumor volume, but the cystic portion would influence the solid volume that needs to be ablated, which might be attributed to the reason why tumor volume was not a risk factor. For larger tumors, interval ablation might be a good choice to reduce complications. Interval ablation could reduce the thermal energy in one session and favor the absorption of ablated thyroid nodules. In our study, larger tumors (typical larger than 5 cm) with interval ablation did not show SAN.
Initial treatment for SAN is conservative treatment. We found NSAI drug was effective in early treatment for patients with initial symptom. The symptom was initial swelling of the treated nodules while the thyroid capsule was intact. The surrounding of ablated nodule experienced inflammatory exudation shown as fluid sonolucent area on the US image. If the breakdown of the thyroid capsule occurred, the rupture was unavoidable and incision and drainage were the most effective methods to alleviate the symptom. Beak’s group showed that antibiotic was effective in treating rupture [Citation13], but the culture of oozed contents in our study showed no bacterial growth and laboratory test showed on inflammatory. Treatments for SAN are still in exploratory stage and more studies are needed to illustrate it. Jung et al. emphasized the conservative treatment in patients with nodule ruptures after RFA [Citation2], while we tended to advise incision and drainage to alleviate symptom and shorten the duration of symptoms. More complete necrosis was achieved after MWA in thyroid lesions and fistula almost could not be avoided once rupture of thyroid lesion occurred in our study.
Our study has several limitations. First, our retrospective study design may have led to selection bias. A possibility of uncontrolled confounding factors cannot be avoided. Second, only 10 patients with SAN were detected and included in this study. As a rare complication, more cases are needed to further illustrate its pathology, clinical manifestations and treatment therapy. Third, CT results were lacking. US is convenient and advantageous in thyroid disease, but CT could give overall presentation of the neck structure. Forth, this was a single-center study. Thus, our results might not be consistently reproducible in other settings.
In conclusion, SAN after MWA came earlier and initially illustrated as neck bulging, swelling and discomfort. Early detection and early treatment might prevent the rupture of nodules. Once the breakdown of thyroid capsule occurred, rupture of ablated nodules out of skin was unavoidable and invasive procedures might be the most effective treatment.
Disclosure statement
All authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- Korkusuz Y, Gröner D, Raczynski N, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol. 2018;28(3):929–935.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Monpeyssen H, Ben Hamou A, Hegedüs L, et al. High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules: a 3-year retrospective multicenter follow-up study. Int J Hyperthermia. 2020;37(1):1301–1309.
- Trimboli P, Pelloni F, Bini F, et al. High-intensity focused ultrasound (HIFU) for benign thyroid nodules: 2-year follow-up results. Endocrine. 2019;65(2):312–317.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Pacella CM, Papini E. Thermal ablation procedures: the need of careful appraisal. Endocrine. 2020;67(1):268–269.
- Vorländer C, David Kohlhase K, Korkusuz Y, et al. Comparison between microwave ablation and bipolar radiofrequency ablation in benign thyroid nodules: differences in energy transmission, duration of application and applied shots. Int J Hyperthermia. 2018;35(1):216–225.
- Mainini AP, Monaco C, Pescatori LC, et al. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound. 2017;20(1):11–22.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Wang JF, Wu T, Hu KP, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J (Engl). 2017;130(11):1361–1370.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Shin JH, Jung SL, Baek JH, et al. Rupture of benign thyroid tumors after radio-frequency ablation. AJNR Am J Neuroradiol. 2011;32(11):2165–2169.
- Chung SR, Baek JH, Sung JY, et al. Revisiting rupture of benign thyroid nodules after radiofrequency ablation: various types and imaging features. Endocrinol Metab (Seoul). 2019;34(4):415–421.
- Khanh HQ, Hung NQ, Vinh VH, et al. Efficacy of microwave ablation in the treatment of large (≥3 cm) benign thyroid nodules. World J Surg. 2020;44(7):2272–2279.
- Feng B, Liang P, Cheng Z, et al. Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol. 2012;166(6):1031–1037.
- Yue W, Wang S, Wang B, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. 2013;82(1):e11–e16.
- Dong P, Wu XL, Sui GQ, et al. The efficacy and safety of microwave ablation versus lobectomy for the treatment of benign thyroid nodules greater than 4 cm. Endocrine. 2021;71(1):113–121.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(5):317–325.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007;72(1):124–131.
- Moon WJ, Baek JH, Jung SL, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12(1):1–14.
- Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation – multicenter retrospective study. Radiology. 2008;247(3):762–770.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Velez E, Goldberg SN, Kumar G, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology. 2016;281(3):782–792.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Hu K, Wu J, Dong Y, et al. Comparison between ultrasound-guided percutaneous radiofrequency and microwave ablation in benign thyroid nodules. J Cancer Res Ther. 2019;15(7):1535–1540.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
