Abstract
The majority of patients with metastatic colorectal cancer (CRC) to the liver are not amenable to curative-intent surgery or thermal ablation; there is a need for alternative locoregional therapies to control oligometastatic liver disease. Sequential lobar Yttrium-90 (Y90) radioembolization has demonstrated favorable results in the salvage setting for CRC patients with liver only or liver-dominant metastatic disease, but the role of Y90 in earlier-stage disease has not shown to be as promising. Recently, radiation segmentectomy, the super selective delivery of high (ablative) dose Y90 microspheres, has been introduced as a novel approach for patients with unresectable hepatocellular carcinoma. This review provides an overview of the current role of Y90 radioembolization for CRC patients with metastasis to the liver, with specific focus on the evolving application of radiation segmentectomy for patients with limited hepatic metastases from colorectal cancer.
Introduction
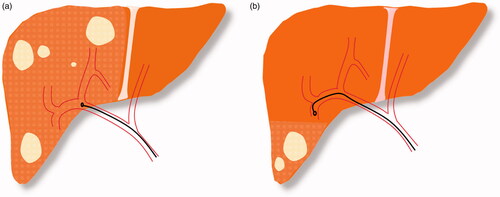
Due to hematogenous spread, the liver is the most frequent metastatic site in patients with colorectal cancer (CRC). Approximately 25% of CRC patients have hepatic involvement at the time of diagnosis and, eventually, 60% of patients develop liver metastases during the course of their disease [Citation1]. This review provides a brief discussion of local curative-intent treatment strategies for colorectal liver metastasis (CRLM) and highlights the evolving practice of Yttrium-90 (Y90) radioembolization, from a palliative sequential lobar therapy to an ablative segmental treatment. Radiation segmentectomy, the delivery of high (ablative) dose Y90 microspheres to ≤2 hepatic segments, has been established for patients with unresectable hepatocellular carcinoma (HCC); it is now being employed to treat select patients with oligometastatic CRLM. ().
Figure 1. (a) Lobar Y90 administration in multifocal lesions. (b) Segmental Y90 administration in lesions confined to two or fewer hepatic segments.
Local curative treatment options for colorectal liver metastasis
Surgical resection is considered curative for patients with CRLM; however, only 10–20% of these patients are appropriate candidates for surgical resection [Citation2]. The median overall survival (OS) for selected patients amenable to surgical resection is 3.6 years (median 5- and 10- year survival rate: 38 and 26%, respectively) [Citation3], with published scoring systems prognosticating best patients for surgical resection of CRLM [Citation4,Citation5].
Percutaneous thermal ablation options include radiofrequency ablation (RFA) and microwave ablation (MWA). There is not consistent data comparing the benefits of MWA and RFA in terms of local tumor progression (LTP) [Citation6,Citation7], but MWA does appear superior to RFA in perivascular tumors as it has minimal heat sink effect and hence does not create ablation zone distortions in proximity of large vasculature [Citation8]. Regardless of the ablation approach, achieving an ablative margin of 5–10 mm is of critical importance to lower the risk of local recurrence [Citation9–11]. Combining thermal ablation (RFA) and systemic chemotherapy, compared to chemotherapy alone, has been found to significantly prolong progression-free survival (PFS) and OS in CRLM patients with inoperable disease [Citation12,Citation13]. The survival benefits of local ablative treatments in unresectable CRLM patients has been confirmed through long-term outcomes in 119 patients [Citation14]. With a median follow-up of 9.7 years, patients who received a combination of aggressive local treatment and systemic chemotherapy had a significantly longer OS, compared to patients who received chemotherapy alone. Furthermore, five-year PFS rate in the combined-therapy arm was 24.2%, compared to 5.9% in chemotherapy arm [Citation14].
Current guidelines consider thermal ablation to be an alternative curative option for patients with oligometastatic disease [Citation15,Citation16], with survival outcomes approaching the results achieved with partial hepatectomy [Citation17]. However, resection is considered superior to ablation in those with multiple or larger tumors. An ongoing multi-institutional phase III randomized controlled trial (COLLISION) is comparing survival outcomes, complications, and cost-effectiveness of thermal ablation (either RFA or MWA) with resection in CRLM patients with resectable and ablatable disease ≤3 cm [Citation18]. The results of the trial are still pending. Clinically, patients not amenable to surgery or thermal ablation can be considered for other local liver treatments (chemoembolization, radioembolization, hepatic artery infusion and external beam radiation), but none have been considered curative.
Radioembolization in colorectal liver metastasis
In the latest version of National Comprehensive Cancer Network (NCCN®) guidelines, radioembolization is classified with uniform consensus as category 2 A for the treatment of liver-dominant, chemorefractory CRLM [Citation19]. Moreover, the European Society of Medical Oncology (ESMO) 2016 guidelines recommend that radioembolization should be considered for the treatment of patients with disease confined to the liver who have failed to respond to chemotherapeutic agents (level II-B recommendation) [Citation15].
There are two Y90 radioembolization devices that have been studied for patients with CRLM: resin (SIR-Spheres®, Sirtex Medical Ltd., North Sydney, Australia) and glass (TheraSphere®, Boston Scientific, Natick, Massachusetts). Per the U.S Food and Drug Administration (FDA), SIR-Spheres is indicated for the treatment of unresectable metastatic liver tumors from primary CRC with adjuvant intra-hepatic artery chemotherapy of floxuridine. Meanwhile, TheraSphere is approved by FDA for local tumor control of solitary tumors (1-8 cm in diameter), in patients with unresectable HCC, Child-Pugh Score A cirrhosis, well-compensated liver function, no macrovascular invasion, and good performance status. To this date, a direct prospective comparison of resin and glass microspheres has not been conducted in metastatic CRC. However, several studies employing these devices have yielded comparable survival outcomes [Citation20,Citation21].
Y90 radioembolization has historically been employed as a sequential lobar, salvage, palliative treatment in CRLM patients refractory to systemic therapy [Citation22]. In a multi-institutional retrospective review of 531 CRLM patients with unresectable chemorefractory disease who underwent salvage Y90 radioembolization using glass microspheres, Hickey et al. demonstrated an OS of 10.6 months from the first radioembolization session [Citation20]. In the largest series using resin microspheres, 606 pretreated CRLM patients who failed to respond to other treatments underwent SIR-Spheres radioembolization as 2nd-, 3rd-, or 4th- line of therapy (MORE study) [Citation23]. Patients had a median OS of 9.6 months. Published in 2017, Kennedy et al. performed an updated analysis of long-term results from the MORE study and reached a median OS of 10 months and 1-year survival of 45% [Citation24]. Several other studies have also found similar and consistent results (OS of 9.4–10.6 months) and have concluded that CRLM patients with chemorefractory disease can benefit from radioembolization in the salvage setting [Citation21,Citation22,Citation25–27].
There is growing interest in employing radioembolization to treat CRLM patients earlier in their disease course, combining local and systemic treatments to promote synergistic benefit. In a study on 74 CRLM patients with unresectable disease confined to the liver, patients were randomized to receive hepatic artery chemotherapy (floxuridine) alone or in combination with resin microsphere radioembolization [Citation28]. Patients in the radioembolization arm had a significantly greater complete/partial response rate and significantly longer median time to liver progression without increased toxicity [Citation28]. This was consistent with another study on CRLM patients who received systemic chemotherapy (fluorouracil/leucovorin) ± resin microsphere radioembolization [Citation29]. In addition to greater response rate and longer time-to-progression, patients in the combination-therapy group had a significantly longer median survival; although, they did experience more severe adverse events [Citation29]. The beneficial role of combination therapy in CRLM was further supported by a phase III trial on 46 patients with chemorefractory disease who underwent treatment with intravenous 5-fluorouracil ± resin microsphere radioembolization [Citation30]. It was shown that, despite no difference in median OS, patients undergoing combination therapy had a better time-to-liver-progression, time-to-tumor-progression, and disease control rate [Citation30]. In addition to traditional chemotherapy regimens mentioned in the studies above, the combination of radioembolization with contemporary chemotherapy regimens has been evaluated, demonstrating an acceptable safety profile [Citation31–33].
SIRFLOX was a randomized phase III trial on 530 patients with isolated liver or liver-dominant disease, exploring the combination of lobar resin microsphere radioembolization with a contemporary (FOLFOX) chemotherapy regimen as first-line of treatment in CRLM [Citation34]. This study was a negative trial, failing to meet the primary endpoint of improved PFS; however, it did reveal that the combination of radioembolization and FOLFOX significantly delays local disease progression in the liver [Citation34]. While SIRFLOX failed to show any difference in post-treatment resectability, a secondary analysis on the trial suggested that a significantly greater number of patients in the combination-therapy arm, compared to the chemotherapy arm, were technically resectable [Citation35]. When the results of SIRFLOX were combined with two other similar trials (FOXFIRE and FOXFIRE-Global [Citation36]), it confirmed that the combined treatment of radioembolization and systemic therapy in the first line achieves better local disease control in the liver with a higher rate of imaging response compared to chemotherapy alone, without benefit to OS or overall PFS [Citation37]. EPOCH is a prospective, multicenter, phase III trial, studying the efficacy and safety of chemotherapy combined with glass micrsosphere radioembolization in the second-line, including those who have failed to respond to first-line systemic treatment [Citation38]. The primary endpoints of the study are PFS and liver PFS. Patient enrollment has ended in October 2018 with results pending.
Radiation segmentectomy
As in metastatic CRC, radioembolization has been historically employed as a lobar treatment in the salvage setting for patients with HCC not amenable to surgery, ablation or chemoembolization [Citation39–41]. Advancements in patient selection, imaging and dosimetry have helped transition the practice of radioembolization from a lobar salvage treatment to a segmental curative-intent option in HCC, especially for those patients with preserved liver function and limited disease burden (solitary tumors up to 5 cm) [Citation42]. Radiation segmentectomy, also known as segmental ablative radioembolization, is a highly selective radioembolization approach to deliver high doses of Y90 (>190 Gray [Gy]) to targeted areas in order to achieve tumor ablation, while sparing normal liver parenchyma [Citation43]. With this approach, radioembolization is typically confined to two Couinaud hepatic segments, lowering the risk of radiation-associated complications [Citation44]. With curative intent, it can be employed to treat patients who are not suitable candidates for surgical resection or ablative therapies, either due to underlying patient comorbidity, increasing tumor size, or challenging tumor location [Citation45].
The radiation segmentectomy literature in HCC focuses on glass microspheres. Glass and resin radioembolic devices have distinct characteristics, with resin microspheres being slightly larger (20–60 µm vs. 15–35 µm for glass microspheres), and resin microspheres having a lower specific activity/microsphere at calibration (resin microspheres are 50 becquerel/sphere vs. 2500 becquerel/sphere for glass microspheres). These differences currently allow for higher segmental radiation dose treatments with glass microspheres. Radiation segmentectomy provides high imaging response rates, reported between 83 and 92%, with a sustained duration of response as evidenced by long time-to-progression (greater than 26 months) and time to second tumor therapy [Citation46–48]. Correlating imaging response to actual pathologic changes, radiology-pathology studies have revealed a target dose radiation threshold required to achieve complete pathologic necrosis (CPN). In a study by Vouche et al. explant pathologic evaluation of 33 patients downstaged to surgery with radiation segmentectomy revealed greater than 90% necrosis in all patients (with CPN in 52% of this population); the segmental radiation dose of 190 Gy was found to provide a statistically significant benefit to achieve CPN [Citation45]. This was validated by a subsequent study, where segmental Y90 radiation dose >190 Gy yielded CPN in more than 80% of tumors [Citation49]. More recently, Gabr et al. observed that an absorbed radiation dose > 190 Gy yielded CPN in 86% of tumors, and an absorbed radiation dose >400 Gy achieved CPN in 100% of tumors, recommending a threshold for tumor absorbed dose in radiation segmentectomy to exhibit CPN [Citation50].
Radiation segmentectomy in colorectal liver metastasis
Although promising outcomes of radiation segmentectomy in treatment of early-stage HCC are now established [Citation42,Citation45–47,Citation51,Citation52], available data on efficacy of such treatment in patients with CRLM is limited, with this concept now being explored for patients with oligometastatic disease. () There are challenges in utilizing Y90 radioembolization in the treatment of CRLM patients. Since most of these patients have been heavily pretreated with multiple chemotherapeutic regimens, their hepatic vasculature flow can be profoundly altered [Citation53]. This can limit the ability to perform super-selective catheterization required for this approach. Further, CRLMs are often hypovascular, making tumor targeting more challenging. This can mostly be overcome with the application of intra-procedure cone-beam computed tomography (CT).
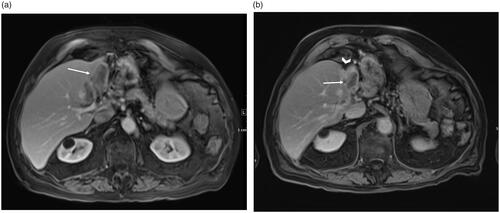
Figure 2. A 86 year-old male CRC patient with metastatic lesion in the left lobe (a) Pretreatment MRI showing baseline low-attenuation lesion in segment II/III measuring 6.3 × 3.2 cm (arrow). Radiation segmentectomy was performed for the patient and a dose of 235 Gy was administered. (b) MRI at one-year follow-up shows a shrunken non-enhancing tumor (3.6 × 1.9 cm, arrow) with retraction and atrophy of the treated segments (arrowhead).
In a retrospective study to assess the efficacy of radiation segmentectomy in the treatment of hepatic metastases, Meiers et al. included 10 patients, 7 patients with primary origin of colorectal cancer, with inoperable metastasis confined to ≤2 liver segments [Citation54]. Each patient underwent 1–3 outpatient radiation segmentectomy sessions. Tumor response to treatment was assessed based on Response Evaluation Criteria in Solid Tumors (RECIST), modified RECIST (mRECIST) and Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) [Citation54]. Among patients with colorectal origin, radiation segmentectomy was technically unsuccessful in one patient due to altered vasculature, who developed progressive disease. Following successful procedures in 6 metastatic CRC patients, all initially showed either a complete/partial response or stable disease. Of these 6 patients, four had complete response or stable disease in their last follow-up imaging (ranging 1–14 months). However, 2/6 patients showed disease progression after 7 and 18 months (after complete response at 4 and 16 months, respectively). Mean PFS for the entire cohort was 7.1 months and none of the patients experienced a significant post-procedure adverse event [Citation54].
In another investigation on 36 patients with hepatic metastasis originating from different sources, Padia et al. enrolled 11 patients with primary CRC [Citation55]. None of these patients were amenable to resection or thermal ablation. The survival and response results were not stratified by primary cancer origin in this study. After undergoing radiation segmentectomy, tumor response to treatment was evaluated using RECIST and mRECIST. In the entire cohort, 92% of the patients showed either partial response or stable disease in initial follow-up imaging and only 3 patients had progressive disease based on RECIST criteria [Citation55]. Patients with hypervascular tumor had either complete or partial response to treatment based on mRECIST. Survival rate after 6 and 12 months was 96 and 83%, respectively [Citation55]. Eventually, over a median follow-up of 12 months (range: 3–72 months), up to 30% of the patients developed disease progression. The authors opined that the observed progression rate might be due to a selection bias of including patients with advanced disease [Citation55]. This post-treatment progression is not isolated to radioembolization; tumor progression is also observed in patients with liver metastasis who undergo other treatment modalities such as RFA. For example, one study of percutaneous RFA for CRLM found a LTP rate of 33% within the first year after treatment [Citation56]. The most common adverse events in the Padia et al. study were fatigue (19%) and post-embolization syndrome (17%). No gastrointestinal ulceration was experienced following the treatment [Citation55].
Most recently, Kurilova et al. evaluated the safety and efficacy of radiation segmentectomy with glass microspheres in patients with limited hepatic metastases [Citation57]. Ten patients with metastatic liver disease deemed not candidates for surgery or thermal ablation, including 2 patients with colorectal origin of metastasis, were included. These patients were treated with ablative tumor targeting (190 Gy), with actual delivered radiation dose to the tumor (MIRD dosimetry) ranging from 163 to 1303 Gy. Based on RECIST 1.1 and Choi criteria, 44 and 100% of patients had partial or complete tumor response, respectively. With a median follow-up of 17.8 months, overall LTP was 21% and the rate of disease progression in the treated segment was 33%. Of two CRLM patients, one had LTP at 4.9 months [Citation57]. The other patient received two treatment sessions and had no tumor progression on the last follow-up visit at 35 months. The one-, two-, and three-year LTP-free survival (LTPFS) was 83, 83, and 69%, respectively, and the median LTPFS was not reached. The median OS was 41.5 months in the entire cohort and three-year OS rate was 74%. Adverse events, reported in 50% of the procedures, were mainly self-limiting. One major complication was observed in a patient with history of prior Whipple surgery who developed biloma and liver abscess 6.5 months after undergoing radiation segmentectomy, requiring hospitalization and abscess aspiration [Citation57].
These early reports on the application of radiation segmentectomy for select patients with hepatic metastatic disease have limitations, being retrospective with small sample size, heterogeneous patient populations, limited long-term outcomes and no pathologic specimen to substantiate imaging findings. Details of the above trials are outlined in . However, based on these recent studies, radiation segmentectomy appears to offer local disease control with acceptable toxicity for the treatment of patients with CRLM, particularly with tumors in challenging anatomic locations or with comorbidities making them not amenable to resection or thermal ablation.
Table 1. Outcomes of trials evaluating the efficacy of radiation segmentectomy in colorectal liver metastasis.
Future directions
Areas of further study are required to optimize the application of radiation segmentectomy for patients with CRLM, including patient selection and tumor response assessment. From historical salvage radioembolization studies, factors such as tumor differentiation, number of extrahepatic disease sites, lymphovascular invasion of the primary tumor, the sum of the two largest lesion diameters at the treatment site, baseline carcinoembryonic antigen (CEA) and alanine transaminase (ALT) levels and albumin level have been shown to predict the survival outcomes of CRLM patients after radioembolization [Citation58,Citation59]. More recently, genetic mutations have played a larger role in determining the sensitivity of diseases to varying treatment options. For instance, radioembolization offers more benefit to unresectable CRLM patients with wild-type Kirsten rat sarcoma viral oncogene homolog (KRAS), compared to those with KRAS mutation [Citation60]. Presence of RAS mutation has also been associated with decreased local PFS after thermal ablation [Citation61,Citation62]. In this evolving space, phosphatidylinositol 3-kinase (PI3K) pathway mutation has also been studied, being a strong predictor of longer time-to-local-progression in CRLM patients following radioembolization [Citation63]. Ideally, these baseline patient data are taken into consideration when selecting most appropriate local liver therapy.
Assessing the response to local tumor therapies has evolved to include tumor enhancement and/or necrosis rather than simply the anatomic size of the treated tumor(s); however, this is limited in patients with CRLM because of the hypovascular nature of these tumors. While RECIST has been widely adopted to evaluate the response to radioembolization, it was recently shown that metabolic response assessment using modalities such as 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT can be used as a surrogate biomarker, possibly superior to other imaging modalities in early investigation of response to the treatment and predicting PFS [Citation64,Citation65]. PERCIST criteria, which assesses metabolic response, is shown to be more accurate than RECIST when evaluating response to treatment in CRLM patients [Citation66]. Among different parameters measured by PERCIST, metabolic tumor volume and total lesion glycolysis have been shown to be the most significant predictors of OS after radioembolization in CRLM patients [Citation67]. Besides guidelines that investigate metabolic response, some CT morphologic criteria such as Choi, which utilizes a combination of tumor attenuation and diameter, was shown to be more reliable than RECIST for predicting liver PFS in CRLM patients [Citation68].
Conclusion
Y90 radioembolization is a versatile therapy. It has historically been employed for patients with metastatic CRC as a salvage therapy, delivered in lobar-fashion. The natural evolution of this radio-embolic device is the segmental delivery of high-dose, ablative, radiation to oligometastatic CRLM, potentially expanding the curative intent of local therapies for these patients beyond resection or ablation. Future studies should aim to determine tumor radiation-dose thresholds for complete pathologic response in CRLM, as they are likely different than those revealed for HCC. Moreover, as the combination of systemic chemotherapy with other local treatments such as RFA has already shown to be effective in CRLM, future prospective studies exploring the impact of combining radioembolization with systemic therapies for patients with oligometastatic disease can provide valuable insight.
Disclosure statement
R.S. is a consultant for Boston Scientific and Sirtex Medical. R.J.L is a consultant for Boston Scientific. No potential conflict of interest was reported by the author(s).
References
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9.
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–1410.
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318.
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77(7):1254–1262.
- Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21(13):4278–4283.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. JVIR. 2018;29(2):268–275.e1.
- Gruber-Rouh T, Marko C, Thalhammer A, et al. Current strategies in interventional oncology of colorectal liver metastases. BJR. 2016;89(1064):20151060.
- Han K, Kim JH, Yang SG, et al. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology. 2021;298(1):212–218.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorect Cancer. 2020;S1533-0028(20)30134-1. DOI:10.1016/j.clcc.2020.09.005
- Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23(10):2619–2626.
- Ruers T, Punt CJ, van Coevorden F, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). Am Soc Clin Oncol. 2015;33:3501–3501.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Instit. 2017;109(9). DOI:10.1093/jnci/djx015
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION Trial Group. Cancers. 2020;12(7):1779.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Puijk RS, Ruarus AH, Vroomen L, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) – a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821.
- Benson AB, 3rd, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. JNCCN. 2017;15(3):370–398.
- Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016;57(5):665–671.
- Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65(2):412–425.
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. JVIR. 2005;16(12):1641–1651.
- Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol. 2015;6(2):134–142.
- Kennedy A, Cohn M, Coldwell DM, et al. Updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases. J Gastrointest Oncol. 2017;8(4):614–624.
- Jakobs TF, Paprottka KJ, Raeßler F, et al. Robust evidence for long-term survival with (90)Y radioembolization in chemorefractory liver-predominant metastatic colorectal cancer. Eur Radiol. 2017;27(1):113–119.
- Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(10):1861–1869.
- Saxena A, Meteling B, Kapoor J, et al. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann SurgOncol. 2015;22(3):794–802.
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711–1720.
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(2):78–85.
- Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28(23):3687–3694.
- Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. JVIR. 2011;22(6):780–786.
- Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(9):1099–1106.
- van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27(25):4089–4095.
- van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(15):1723–1731.
- Garlipp B, Gibbs P, Van Hazel GA, et al. Secondary technical resectability of colorectal cancer liver metastases after chemotherapy with or without selective internal radiotherapy in the randomized SIRFLOX trial. British J Surg. 2019;106(13):1837–1846.
- Dutton SJ, Kenealy N, Love SB, et al. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer. 2014;14:497.
- Wasan HS, Gibbs P, Sharma NK, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159–1171.
- Chauhan N, Mulcahy MF, Salem R, et al. TheraSphere Yttrium-90 glass microspheres combined with chemotherapy versus chemotherapy alone in second-line treatment of patients with metastatic colorectal carcinoma of the liver: protocol for the EPOCH phase 3 randomized clinical trial. JMIR Res Protoc. 2019;8(1):e11545.
- Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium‐90 radioembolization for intermediate‐advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826–1837.
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64.
- Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 1: technical and methodologic considerations. J Vasc Interven Radiol. 2006;17(8):1251–1278.
- Lewandowski RJ, Gabr A, Abouchaleh N, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287(3):1050–1058.
- Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79(1):163–171.
- Mora RA, Ali R, Gabr A, et al. Pictorial essay: imaging findings following Y90 radiation segmentectomy for hepatocellular carcinoma. Abdom Radiol. 2018;43(7):1723–1738.
- Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60(1):192–201.
- Biederman DM, Titano JJ, Bishay VL, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology. 2017;283(3):895–905.
- Padia SA, Johnson GE, Horton KJ, et al. Segmental Yttrium-90 radioembolization versus segmental chemoembolization for localized hepatocellular carcinoma: results of a single-center, retrospective, propensity score-matched study. JVIR. 2017;28(6):777–785.e1.
- Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163.e2.
- Ahmed AF, Samreen N, Grajo JR, et al. Angiosomal radiopathologic analysis of transarterial radioembolization for the treatment of hepatocellular carcinoma. Abdom Radiol. 2018;43(7):1825–1836.
- Gabr A, Riaz A, Johnson GE, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48(2):580–583.
- Biederman DM, Titano JJ, Korff RA, et al. Radiation segmentectomy versus selective chemoembolization in the treatment of early-stage hepatocellular carcinoma. JVIR. 2018;29(1):30–37.e2.
- Padia SA, Kwan SW, Roudsari B, et al. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. JVIR. 2014;25(7):1067–1073.
- Hickey R, Mulcahy MF, Lewandowski RJ, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys. 2014;88(5):1025–1031.
- Meiers C, Taylor A, Geller B, et al. Safety and initial efficacy of radiation segmentectomy for the treatment of hepatic metastases. J Gastrointest Oncol. 2018;9(2):311–315.
- Padia SA, Johnson GE, Agopian VG, et al. Yttrium-90 radiation segmentectomy for hepatic metastases: a multi-institutional study of safety and efficacy. J Surg Oncol. 2021;123(1):172–178.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Kurilova I, Bendet A, Fung EK, et al. Radiation segmentectomy of hepatic metastases with Y-90 glass microspheres. Abdomin Radiol. 2021. DOI: 10.1007/s00261-021-02956-6
- Kurilova I, Beets-Tan RGH, Flynn J, et al. Factors affecting oncologic outcomes of 90Y radioembolization of heavily pre-treated patients with colon cancer liver metastases. Clin Colorect Cancer. 2019;18(1):8–18.
- Sofocleous CT, Violari EG, Sotirchos VS, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorect Cancer. 2015;14(4):296–305.
- Lahti SJ, Xing M, Zhang D, et al. KRAS status as an independent prognostic factor for survival after Yttrium-90 radioembolization therapy for unresectable colorectal cancer liver metastases. JVIR. 2015;26(8):1102–1111.
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–2734.
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. British J Surg. 2017;104(6):760–768.
- Ziv E, Bergen M, Yarmohammadi H, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget. 2017;8(14):23529–23538.
- Jongen JMJ, Rosenbaum C, Braat M, et al. Anatomic versus metabolic tumor response assessment after radioembolization treatment. JVIR. 2018;29(2):244–253.e2.
- Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39(9):1391–1399.
- Sager S, Akgün E, Uslu-Beşli L, et al. Comparison of PERCIST and RECIST criteria for evaluation of therapy response after yttrium-90 microsphere therapy in patients with hepatocellular carcinoma and those with metastatic colorectal carcinoma. Nucl Med Commun. 2019;40(5):461–468.
- Shady W, Kishore S, Gavane S, et al. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after (90)Y radioembolization of colorectal liver metastases: a comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol. 2016;85(6):1224–1231.
- Shady W, Sotirchos VS, Do RK, et al. Surrogate imaging biomarkers of response of colorectal liver metastases after salvage radioembolization using 90Y-loaded resin microspheres. AJR. 2016;207(3):661–670.
