Abstract
High-intensity focused ultrasound (HIFU) has been shown to be a valuable tool in the management of small liver tumors such as hepatocellular carcinoma (HCC). It has been shown to be a safe and effective means to ablate small HCC even in the presence of advanced cirrhosis. This review examines the challenges faced during HIFU ablation when the target tumors are located in difficult locations such as the liver dome, close to the rib cage, near large blood vessels or the heart, or adjacent to hollow viscera; and the special maneuvers employed to tackle such lesions.
Introduction
The majority of patients presenting with hepatocellular carcinoma (HCC) present with surgically unresectable disease. Despite advances in surgical technique, as few as 25% of HCC patients are amenable to curative surgical resection. The low resection rate for HCC has often been secondary to the presence of marginal liver functional reserve in the presence of cirrhosis of the liver with chronic liver disease [Citation1]. For patients with cirrhosis of the liver and small HCC, locoregional percutaneous ablation techniques such as radiofrequency ablation (RFA) have been increasingly attractive treatment options over the past two decades due to their effectiveness and relative simplicity [Citation2,Citation3]. Although the risk of serious complications relating to RFA is less than 0.5%, overall complication rates of RFA may approach 9% [Citation4,Citation5]. Complications of RFA include bleeding, thermal injury to surrounding structures, as well as direct tumor seeding associated with needle puncture into a major blood vessel. Advanced cirrhosis of a Child–Pugh score greater than 9 is considered a contraindication to RFA [Citation6], as the risks of bleeding complications and decompensation of liver function following such a procedure may outweigh the therapeutic benefits. Stereotactic body radiation therapy (SBRT) against HCC, by virtue of its transcutaneous nature, attempts to overcome the limitations of RFA; unfortunately, SBRT has a limited role in managing HCC in patients with Child-Pugh B disease, due to the markedly increased risk of radiation-induced liver disease [Citation7]. Although liver transplantation may be offered to patients with small HCC and advanced cirrhosis [Citation8], it may not be a viable option for many patients residing in regions suffering from a scarcity of suitable liver donors and long waiting lists for organ transplantation.
High-intensity focused ultrasound ablation (HIFU) has been shown to be a valuable tool in the management of small HCC, even in the presence of advanced cirrhosis and gross ascites [Citation9]. By raising the target tissue temperature to greater than 60 °C, its transcutaneous delivery of focused ultrasound energy results in thermal protein coagulation of the target tissue within the HCC, resulting in ablation of the target. The HIFU system utilizes a unique frequency of ultrasound of 0.8–3.5 MHz, which can be focused onto the target tissue by an externally-located transducer. The resultant heating of the target tumor tissue upon energy delivery is very localized, thereby sparing collateral thermal damage to the surrounding healthy tissue. Initial results have demonstrated that complete ablation rates of up to 28.5–68% after a single treatment can be achieved. It has also been shown that ascitic fluid can act as an effective acoustic medium during HIFU, sometimes aiding energy propagation toward the target tissue inside the liver; in practice, the procedure has been well-tolerated in patients with ascites [Citation9,Citation10]. HIFU, therefore, has a unique advantage where patients suffering from small HCC can undergo potentially curative ablation even in the presence of advanced cirrhosis.
The safe and effective delivery of HIFU energy to the HCC relies on the absence of any intervening tissue, such as air in the lungs, which may impair visualization of the target and delivery of energy to the target. HIFU ablation of HCC located in the liver dome, which forms parts of segments 7 and 8 situated close to the diaphragm, presents unique challenges. The liver dome is related to the anterior, lateral and posterior aspects of the diaphragm, the lung parenchyma surrounded by the pleura, and the rib cage. Medially the liver dome is related to the cardia and the inferior vena cava (IVC) anteriorly; the vertebral column posteriorly. The proximity of the liver dome to the lung may result in poor sonographic visualization of the target lesion due to the intervening air. The HIFU energy may also potentially injure the lung or the diaphragm in the absence of an acoustic fluid medium in the intervening tissue. The difficulties are compounded by the fact that the liver becomes increasingly subcostal in position in patients who are under general anesthesia, due to their relatively shallow respirations when anesthetized [Citation11]. Ribs are strong reflectors of ultrasound energy, and their presence in close proximity to the target tumor for HIFU may affect the deposition of ultrasound energy in the target, decreasing treatment efficacy, and may also increase the risk of adverse events [Citation12]. We, therefore, consider the liver dome to be a difficult location for HIFU ablation.
Thermal ablation of tumors carries the risk of injuring surrounding structures. The heart, the diaphragm, the rib cage; hollow structures such as the stomach, bowel, major blood vessels, the bile ducts and the gallbladder are particularly vulnerable to thermal injury if ablations are performed in close proximity [Citation13–16]. Especially vulnerable are relatively thin-walled hollow viscera such as the bowel and the gallbladder, since the thermal injury to these structures may result in peritonitis. Tumors close to such structures should also be considered as difficult locations for treatment by HIFU.
For the purposes of HIFU ablation, our center defines an HCC to be situated in a difficult location when it is located in the dome of the liver or partially obscured by the rib cage, or within 1 cm of the following structures: the stomach, bowel, gallbladder, the heart, major blood vessels or bile ducts. We shall describe our management strategies when faced with such lesions. This is followed by a literature review on the current evidence for safe and effective ablation of such lesions.
HIFU at the University of Hong Kong
Eligibility for HIFU
The diagnosis of HCC is based on two typical imaging findings, as well as any histology if previous surgery or biopsy had been performed. A lesion is considered to be HCC on magnetic resonance imaging (MRI) if it exhibits the typical vascular enhancement pattern of HCC which is hypervascular in the arterial phase, with hypovascular washout of contrast in the portovenous phase, as well as an increase in signal in T2W and diffusion-weighted images (DWI). A serum alpha-fetoprotein level of greater than 200 ng/ml or rising, with any of the above features on imaging, is also considered to be significantly diagnostic of HCC.
Following the diagnosis of HCC, the treatment plan is formulated. Surgical resection is offered to patients with suitable tumors and whose liver function, as judged by liver volumetry and indocyanine green retention test, allows. Percutaneous or open RFA is offered to patients with tumor sizes of under 5 cm, and if liver resection is deemed too risky. The approach (whether percutaneous or open) for RFA is decided in conjunction with a senior interventional radiologist. HCC patients with a poor liver function whose tumors are unresectable but who fulfill the UCSF criteria are considered for liver transplantation. HIFU ablation is offered to those who are unsuitable for liver resection or RFA, but whose general condition allows them to undergo general anesthesia. Contraindications to HIFU ablation include unsuitability for general anesthesia, tumor invasion into a major vascular structure, failure to locate the tumor on ultrasound examination, extrahepatic extension or metastasis, and patients who are receiving any treatment which may result in a skin complication.
HIFU screening procedure
HIFU ablation is the accepted standard treatment at our center for unresectable HCC if they are also considered unsuitable for RFA. The referral and application criteria for HIFU are sanctioned by the Central Technology Office of the Hospital Authority of Hong Kong. Our center utilizes the JC HIFU system (Chongqing Haifu Technology, Chongqing, China) for HCC ablations, which comprises a real-time ultrasound diagnostic imaging unit, a therapeutic unit, a degassed water circulation unit (including a water bath where the site to be treated is submerged), and a control computer console. The real-time diagnostic unit provides B-mode sonographic visualization of the tumor; the therapeutic unit contains an ultrasound energy transducer that focuses the ultrasound energy to a 12-cm focal point; the entire system is managed by the control computer.
All patients with HCC in difficult locations undergo pretreatment ultrasound screening using the diagnostic ultrasound unit within the HIFU system. This is to ensure that the HCC is located and visualized with the same machine used in ablation and to minimize the risk of injury to surrounding vital structures in the subsequent treatment session. Patients are excluded from HIFU treatment and offered alternative therapy options if there is any intervening bowel or omentum between the transducer and the tumor, or if the tumor is within 5 mm of a portal tract or the gallbladder or the stomach or a segment of the bowel. On the other hand, HIFU ablation is considered safe if the tumor only abuts a hepatic vein, or if it is located at least 5 mm from any of the hollow structures mentioned above. Pretreatment ultrasound screening has the added benefit in that it allows the operator to plan the patient’s positioning during the treatment session.
HIFU treatment session
Informed consent is obtained from patients who are scheduled to undergo HIFU ablation of HCC. They are subjected to general anesthesia for the procedure in order to minimize patient discomfort, as well as to allow for controlled periods of breath-holding during active ablation. Valsalva maneuver under general anesthesia is utilized intermittently to improve the exposure of the dome of the liver. For HCC located in the liver dome, artificial right pleural effusion is routinely employed to displace the lung parenchyma away from the tumor ablation site, to allow for better visualization of the tumor and surrounding structures, and to provide a satisfactory acoustic window for HIFU ablation. For tumors in close proximity to the bowel, the patient is placed on a fluid diet and prescribed mechanical bowel preparation on the day before the procedure in addition to the pre-anesthetic fasting.
Following induction of general anesthesia and endotracheal intubation, the patient’s skin is degassed and degreased, and a urinary catheter is inserted. For HIFU ablation of liver tumors in segments 7 and 8, we perform an artificial right pleural effusion (). During infusion of pleural effusion, the puncture site and the surrounding chest wall are monitored for any sign of edema which would signify an incorrect needle position. Upon completion of infusion, the patient is re-positioned in the right-lateral position in the water bath containing degassed water.
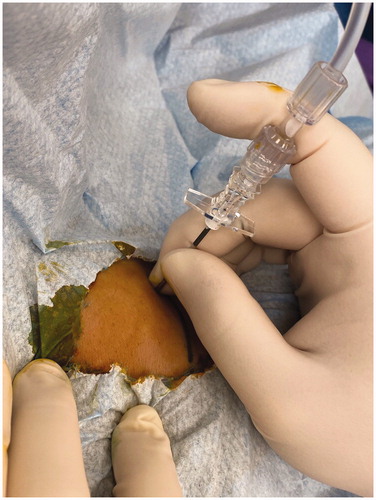
Figure 1. Artificial right pleural effusion technique. The patient is placed in the left-lateral position, and pleural puncture is performed under aseptic technique through the right fourth intercostal space along the mid-axillary line using a 16-gauge epidural needle with Tuohy bevel (B., Braun Melsungen AG, Melsungen, Germany). Ventilation is suspended in the expiratory phase with an open adjustable pressure limit valve when the needle is introduced into the pleural cavity. Correct placement is confirmed when there is a passive loss of resistance to a falling column of fluid. Approximately 800 ml of warm normal saline is infused into the right pleural cavity.
For HCC located in the left liver, a nasogastric tube is inserted to decompress the stomach in order to improve visualization of the liver tumor and to minimize the risk of thermal injury to the stomach. The patient is then repositioned in the prone position in the water bath. Extra attention is paid to tumors in segment 2 in close proximity to the heart, as the ultrasound energy transducer must be tilted toward the cephalad direction in order to minimize the risk of cardiac injury by the ultrasound path.
HIFU treatment is performed under real-time ultrasound guidance, where target tumors are localized by a 3.6 MHz diagnostic ultrasound probe (Philips) incorporated in the transducer therapeutic unit. Planning is performed according to tumor size and location, as shown on the computer monitoring module, and synchronized with the real-time diagnostic imaging probe. Parallel 5 mm slices of the target tumor are scanned and then ablated slice-by-slice with focused ultrasound energy produced by the transducer at 0.8 MHz. The tissue of each tumor slice is completely ablated from deep to the superficial with successive sweeps of the treatment transducer (sonication). During sonication, gray-scale changes in the ablation zone would represent effective ablation.
General anesthesia is reversed upon the conclusion of HIFU ablation. The patient’s vital signs including blood pressure, pulse oximetry and urine output are routinely monitored. Routine blood tests for complete blood picture, liver and renal function and prothrombin time are taken daily until discharge from hospital. A chest radiograph is taken to rule out the presence of any significant pneumothorax. The artificial pleural effusion is not routinely drained. The patient is closely monitored for any potential complications arising from the HIFU procedure and artificial right pleural effusion according to an established protocol.
Tumor response to HIFU is assessed by contrast MRI which is performed 1 month after the procedure. Complete tumor ablation is indicated by the complete absence of hyperintensity signal in T2W images as well as the complete absence of contrast enhancement within the region of the target tumor.
Safe and complete HIFU ablation of tumors in the liver dome
HIFU ablation has been shown to be a safe treatment modality against liver tumors, even in the presence of advanced cirrhosis [Citation9]. However, the challenge lies in the effective ablation of target tissue in difficult locations without damaging the surrounding tissue. A number of strategies have been proposed to achieve this goal.
Positioning the ultrasound transducer
The patient under treatment for a tumor in the liver dome is typically placed in the right lateral decubitus position, with the ultrasound transducer located underneath the patient and submerged in the water bath. The act of angulating the HIFU transducer, along with the in-built diagnostic ultrasound probe, toward the cephalad direction is the basic first step to improving sonographic visualization and access to the liver dome [Citation17] (). Experienced operators in the percutaneous drainage of liver abscesses had described the ideal subcostal approach [Citation18], but unfortunately due to the design limitations of the current HIFU transducer and the importance of not allowing the transducer to be in direct contact with the skin in order to avoid burns, the only viable route would instead be the intercostal approach.
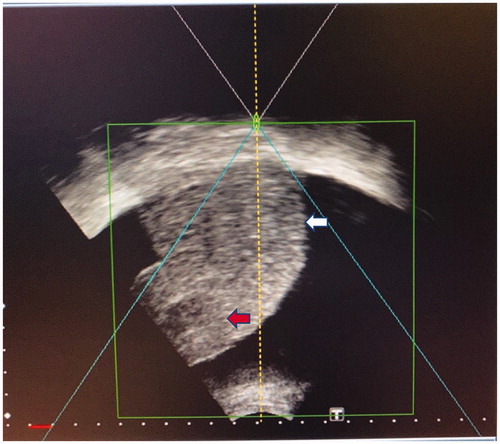
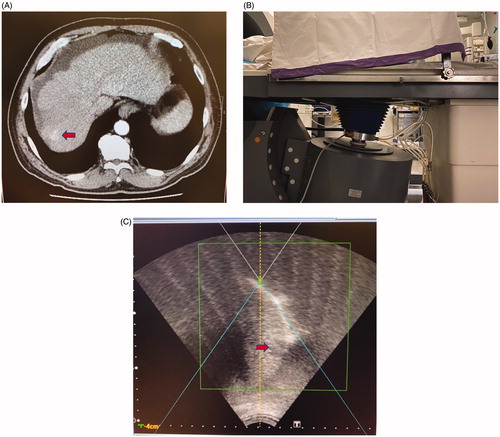
Figure 2. This patient with cirrhosis and HCC located in segment 7 of the liver being treated with HIFU. (A) CT image showing the location of the tumor (red arrow) which was in the liver dome and close to the diaphragm. (B) Tilting the HIFU transducer probe toward the cephalad direction, which is the right-hand side of this photo, in order to obtain an optimal view of the liver dome and the tumor. The patient is lying in the water bath in a right-lateral position. (C) HIFU sonographic image of the liver dome and the tumor (red arrow) obtained by this maneuver.
Following the same principle, tumors situated high in segment 2 and in close proximity to the heart have been safely ablated in our center by tilting the HIFU transducer in the cephalad direction after the patient is placed in a prone position. Our center has never experienced any cardiac injury following HIFU [Citation9].
Sonographic localization of the liver tumor
There are occasions where real-time sonographic examination during the HIFU procedure may not readily visualize the entire target tumor as depicted by the pre-procedural contrast CT or MR scans, despite optimal positioning of the transducer. This may be secondary to the limitations due to the positioning of the transducer in the HIFU system when viewing the liver dome, as well as the reduced sensitivity of detecting small tumors with ultrasound in a cirrhotic liver. Careful examination of the procedural CT or MR images enables the operator to gain a thorough understanding of the target tumor’s relationships with the surrounding blood vessels, calcifications and bones. Extrapolation of the tumor position using readily-identified internal reference anatomical landmarks such as the hepatic veins, the inferior vena cava, the portal pedicles, the diaphragm and the chest or abdominal wall was found to be highly accurate, up to 98%, in percutaneous interventional procedures on the liver [Citation19]. However, this study by Sainani et al. [Citation19] was based on CT-guided procedures, and its value in ultrasound detection of smaller lesions may be hampered by a cirrhotic liver.
Means to alter the acoustic environment of the liver tumor include the administration of an iodized oil emulsion by transarterial chemoembolization (TACE) 1–2 weeks prior to HIFU ablation, or the injection of a microbubble contrast agent such as SonoVue (Bracco, Italy, 8 µl sulfur hexafluoride microbubbles per milliliter) during HIFU ablation [Citation20,Citation21]. The changes in echogenicity in the target tumor, in contrast with the surrounding non-tumor liver, following administration of these agents may help in identifying the lesions in difficult-to-image areas in the liver such as the dome. Huang et al. [Citation20] proposed that such agents may not only aid localization but also have the benefit of enhancing the ablation effects of HIFU. Additionally, HIFU aided by SonoVue enabled a single attendance for definitive treatment, without the need for pretreatment with TACE [Citation20].
Ascites
The presence of ascites improves visualization of the liver dome since the accumulated fluid acts as an acoustic medium separating a portion of the dome from the diaphragm (). By displacing the liver from part of the diaphragm, fluid in the peritoneal cavity may also insulate the diaphragm from thermal injury during ablation; the technique of artificially-induced ascites has been described to safely ablate liver dome tumors by percutaneous RFA [Citation22–25]. Cheung et al. [Citation9] noted that the presence of underlying gross ascites did not increase the complication rate of HIFU, and also reported that the ascites not only provided clear images for the diagnostic ultrasound probe, but it also acted as a good medium for energy transfer to the target tissue during sonication. For patients without underlying ascites, however, artificial ascites for HIFU may carry additional risks due to needle puncture. The prolonged treatment of HIFU, sometimes exceeding three hours in duration, would require frequent re-infusion due to the constant dissipation of fluid around the peritoneal cavity. Moreover, the hydro dissection effect may not be effective in the presence of peritoneal adhesions from previous surgical resection, TACE or thermal ablation [Citation17].
Artificial pleural effusion
The creation of an artificial pleural effusion to improve visualization of the liver dome by means of instillation of fluid into the right pleural cavity was described by Shibata et al. [Citation26] in 2002 when performing percutaneous RFA. Fluid in the right pleural cavity provides an acoustic window where even the bare area of the liver, which is in direct contact with the diaphragm and not lined with peritoneum, can be visualized by ultrasound via the intercostal approach. When performing radiofrequency ablations on a series of 25 liver dome tumors, Koda et al. found that artificial pleural effusion allowed the operator to visualize the whole tumor on gray-scale sonography in 22 lesions that were not detectable or were poorly visible, and to obtain a safer and easier puncture line in 14 lesions [Citation27]. A large series of percutaneous RFA of liver dome lesions assisted by artificial pleural effusion on 587 patients demonstrated that hydrothorax could safely be achieved without any increase in major complication rate [Citation28].
Dubinsky et al. [Citation29] suggested that during HIFU, reflections of the ablating ultrasound energy by the air within the lung parenchyma and bowel gas could result in burns to the abdominal wall. By displacing the lung parenchyma with fluid, an artificial right pleural effusion may therefore be protective against burns, in addition to providing an acoustic window for safe HIFU. An animal study conducted with MR-guided HIFU of the liver dome, assisted by artificial pleural effusion, confirmed good temperature increases within the target liver tissue without any macroscopic or microscopic injury to surrounding vital structures such as the diaphragm, lung or pleura [Citation30].
When examining a series of 49 patients undergoing ultrasound-guided HIFU for HCC less than 3 cm in diameter in various locations within the liver [Citation31], the complete ablation rate by HIFU, as determined by MRI assessment after treatment, was 90.6%. For larger tumors, the complete ablation rate was 58.8%. These remarkable results included 31 patients who received artificial pleural effusion during HIFU, as well as 18 others who did not require this adjunct. The same authors also demonstrated that the use of artificial pleural effusion did not compromise the complete ablation rate [Citation31]. Within the series, for the 31 patients with liver dome HCC undergoing HIFU aided by artificial pleural effusion, one (3.2%) developed bruising over the chest wall secondary to the needle puncture injuring an intercostal vessel but there was no pulmonary or diaphragmatic injury in any patient [Citation31].
Complications following HIFU with artificial pleural effusion were uncommon. A series of 53 patients who underwent the procedure reported that 38 patients had asymptomatic pleural effusions which persisted for at least 2 weeks after HIFU, but no intervention was required; there was the inadvertent introduction of saline into the lung parenchyma in one case, and one patient suffered from a pneumothorax after treatment [Citation32]. A rare delayed complication of a diaphragmatic rupture was reported 2 years after curative treatment of a liver dome HCC, which resulted in herniation of the colon through the defect [Citation32]. In another center that routinely employed artificial pleural effusion for ablations of tumors in segments 7 and 8, Cheung et al. found no increase in complication rates for HIFU of lesions in those segments [Citation9].
Artificial pleural effusion results in alveolar collapse and some intrapulmonary shunting to a modest degree; it is not considered to be overtly detrimental due to the relatively small volume of infused fluid [Citation33]. Drainage is not routinely required after the HIFU procedure since the intrapleural fluid is readily absorbed from the chest cavity due to the increased lymphatic drainage in response to the additional fluid [Citation34]. Unpublished data from our hospital found that in a recent series of 19 patients who underwent artificial pleural effusion for HIFU, of median age 66 (range 47–79 years), the median length of hospital stay after HIFU was 1 day (range 1–9 days). One patient developed symptomatic right hydro-pneumothorax a few hours after HIFU, which required drainage by a chest tube and a 9-day hospital stay for monitoring after the procedure, but all the other patients made uneventful recoveries after HIFU. There were no hospital re-admissions due to complications from HIFU with artificial pleural effusion. The current clinical evidence therefore strongly suggests that artificial pleural effusion is an invaluable adjunct to safe and precise HIFU ablation of tumors situated in the liver dome ( and ).
Figure 4. HIFU sonographic image of a small HCC in segment 8 (red arrow) being treated, after the instillation of 800 ml of normal saline into the right pleural cavity as artificial right pleural effusion (area denoted by ‘P’). A rib (denoted by ‘R’) is partially obscuring the liver in this intercostal view, therefore breath-holding is mandatory during sonication.
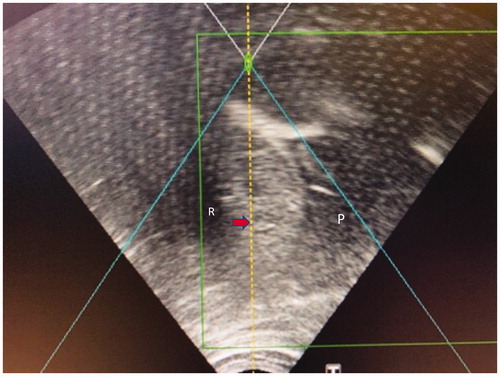
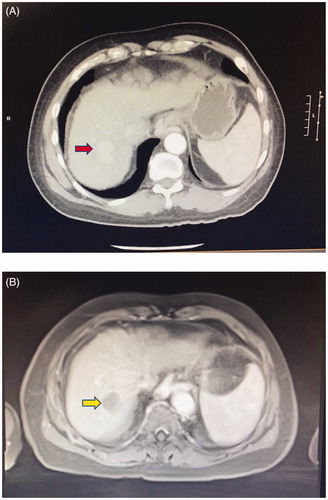
Figure 5. A 66-year-old man with cirrhosis and HCC in segment 7 of the liver. (A) Contrast CT scan showing the hypervascular tumor (red arrow). The patient subsequently underwent HIFU ablation of the tumor, facilitated by artificial right pleural effusion. (B) Contrast MRI taken 1 month after treatment shows no arterial enhancement in the tumor (yellow arrow), which is in keeping with good treatment response.
Additional strategies to ablate liver tumors partially obscured by the rib cage
Much of the right liver is covered by ribs. HIFU of target tumors in close proximity to the rib cage may result in ineffective energy transfer due to the ribs acting as reflectors of ultrasound energy. Moreover, the ribs may in turn reflect the energy to the overlying skin, leading to excessive heating of the skin and subcutaneous tissue which in some cases may even lead to skin burns [Citation21,Citation35].
Intermittent breath holding
Temporarily halting respirations during sonication stops the movement of the liver, allowing smaller lesions to be safely ablated by HIFU energy applied in between the intercostal spaces [Citation9,Citation31]. The intermittent breath-holding can be performed during conventional ventilation through a single-lumen endotracheal tube, with the breath-holding performed under sustained airway pressures during sonication [Citation33]. Intermittent breath-holding (up to 30 s each time) should be performed with greater caution in any patient with compromised cerebral circulation such as in the elderly, since these short periods of high airway pressure may have a detrimental effect on cerebral blood flow [Citation36,Citation37]. Propofol-based total intravenous anesthesia (TIVA) is the preferred anesthetic regime in order to provide for uninterrupted anesthesia during frequent breath-holds [Citation33], and it has the added advantage of attenuating the detrimental effects of breath-holds on cerebral perfusion [Citation38].
Surgical rib removal
Owing to the inherent physical barrier of the ribs, and the technical limitations of the HIFU transducers, HIFU of larger HCC partially obscured by the rib cage presents a unique challenge. In order to provide an unimpeded acoustic pathway for HIFU ablation, Zhu et al. [Citation12] removed part of the rib cages of 16 patients, 2 weeks prior to HIFU for HCC. The rib removal procedures were performed under general anesthesia, where most patients underwent partial removal of the 7th to 9th ribs between the right mid-clavicular line and the posterior axillary line, while their skin flaps were preserved. Apart from one case of wound seroma following rib removal, and one case of skin burn after HIFU in a patient who previously underwent rib removal, no other serious complications were encountered. None of the patients who underwent partial rib resection developed any impairment in their lung function. In these 16 patients, there were 23 tumors treated with HIFU, with a mean tumor diameter of 7.0 ± 2.1 cm (range 5–10 cm), and a complete ablation rate of 69.6% following one session of HIFU. Despite such reports, the practice of rib removal has fallen into decline due to recent improvements in less-invasive localization techniques as mentioned earlier, along with treatment adjuncts such as TACE with HIFU which have achieved satisfactory ablation results without ever resorting to an invasive procedure such as surgery.
HIFU of tumors close to other vital structures
Proximity to blood vessels
Traditional methods of liver ablation such as those employing RFA, microwave ablation, cryoablation, ethanol ablation, have been shown to offer local tumor control in the liver. However, if the liver tumor to be treated lies in close proximity to a vascular structure, the effectiveness of such techniques may be limited by the possibility of thermal vascular injury or injury due to the puncture; moreover, the presence of a fast-flowing blood vessel adjacent to the ablation target tissue may reduce the efficacy of ablation due to the heat-sink effect [Citation39]. By performing a 3-dimensional finite element method (FEM) simulation, Zhang et al. [Citation40] found that the presence of large blood vessels did not have a significant effect on temperature distribution and thermal dose profile in HIFU ablation of a simulated tumor, provided that the sonication of the tissue adjacent to the vessel lasted less than 2 s or where the distance of the large blood vessel from the tumor was greater than 2 mm. Animal studies also drew the conclusion regarding the safety of HIFU for juxtavascular tumors and also demonstrated that the heat-sink effect may be less significant in HIFU when compared with other ablation modalities [Citation41–43].
Zhang et al. [Citation44] reported a series of HIFU for juxtavascular HCC, where the distance between the tumor and a major blood vessel (the inferior vena cava, the main hepatic vein branches, or the portal vein and its branches) was less than 1 cm. In these 39 patients with 42 tumors, the average tumor size was 7.36 ± 4.25 cm (range of 1.5–22 cm); the complete tumor necrosis rate following initial ablation was 50%. Lesions with residual active tumor as seen on imaging 2 weeks after initial ablation were treated with the second session of HIFU. Apart from 7 patients who developed mild skin burns or blisters after HIFU, there was no other significant complication. No injuries to bile ducts or blood vessels were reported. The authors demonstrated the safety of HIFU on juxtavascular HCC, and that HIFU can safely achieve virtually complete necrosis of tumors close to major blood vessels. The seemingly inferior rate of complete ablation may be accounted for by the relatively large tumors in this particular series, as well as the cooling heat sink effect of the larger blood vessels on these tumors.
Proximity to other organs and structures
Orsi et al. [Citation45] performed HIFU on 30 liver tumors in 23 patients, of which 6 tumors were HCC and 24 tumors were metastases to the liver. All tumors were located within 1 cm of a major blood vessel, the stomach, the heart, bile duct, the gallbladder, or the bowel. Careful bowel preparations were made for 3 days prior to the treatment of lesions close to the stomach or bowel, including placing the patient on a liquid diet (without milk), fasting for 12 h prior to treatment, and an enema early in the morning of treatment. The average energy delivered to the liver lesions (2.2 ± 1.0 cm in diameter) was 570,558 ± 496,776 J, which resulted in complete ablation of 22 out of 24 liver metastatic lesions and all of the HCC. Clinical observations after the procedures, and post-procedural CT or MRI evaluations, recorded no complications. After a median of 12 months (range 1–18 months) follow-up, local recurrence was detected in 6 out of 17 patients with liver metastases, but no recurrence was seen in those who underwent treatment for HCC.
Conclusion
HIFU is a promising treatment for HCC. Its application on tumors situated in the liver dome, close to the rib cage, or in close proximity to the stomach, bowel, gallbladder, heart, major blood vessels or bile ducts presents unique challenges. However, meticulous pre-procedural preparation and special maneuvers during HIFU can achieve complete ablation with few complications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Fan ST. Surgical therapy of hepatocellular carcinoma in the cirrhotic liver. Swiss Surg. 1999;5(3):107–110.
- Poon RT, Fan ST, Tsang FH, et al. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon’s perspective. Ann Surg. 2002;235(4):466–486.
- Cheung TT, Ng KK, Chok KS, et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol. 2010;16(24):3056–3062.
- Cheung TT, Ng KK, Poon RT, et al. Tolerance of radiofrequency ablation by patients of hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16(5):655–660.
- Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89(10):1206–1222.
- Maeda M, Saeki I, Sakaida I, et al. Complications after radiofrequency ablation for hepatocellular carcinoma: a multicenter study involving 9,411 Japanese patients. Liver Cancer. 2020;9(1):50–62.
- Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111(3):412–417.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699.
- Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg. 2012;36(10):2420–2427.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11(3–4):149–154.
- Drummond GB, Allan PL, Logan MR. Changes in diaphragmatic position in association with the induction of anaesthesia. Br J Anaesth. 1986;58(11):1246–1251.
- Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol. 2009;72(1):160–166.
- Koda M, Ueki M, Maeda N, et al. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180(6):1561–1562.
- Ishiko T, Beppu T, Sugiyama S, et al. Radiofrequency ablation with hand-assisted laparoscopic surgery for the treatment of hepatocellular carcinoma in the caudate lobe. Surg Laparosc Endosc Percutan Tech. 2008;18(3):272–276.
- Kim YS, Rhim H, Sung JH, et al. Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Interv Radiol. 2005;16(3):407–410.
- Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25(1):S57–S68.
- Kambadakone A, Baliyan V, Kordbacheh H, et al. Imaging guided percutaneous interventions in hepatic dome lesions: tips and tricks. World J Hepatol. 2017;9(19):840–849.
- Johnson RD, Mueller PR, Ferrucci JT, Jr, et al. Percutaneous drainage of pyogenic liver abscesses. AJR Am J Roentgenol. 1985;144(3):463–467.
- Sainani NI, Schlett CL, Hahn PF, et al. Computed tomography-guided percutaneous biopsy of isoattenuating focal liver lesions. Abdom Imaging. 2014;39(3):633–644.
- Huang L, Zhou K, Zhang J, et al. Efficacy and safety of high-intensity focused ultrasound ablation for hepatocellular carcinoma by changing the acoustic environment: microbubble contrast agent (SonoVue) and transcatheter arterial chemoembolization. Int J Hyperthermia. 2019;36(1):244–252.
- Wu F, Wang ZB, Chen WZ, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235(2):659–667.
- Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196(4):907–913.
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19(11):2630–2640.
- Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009;34(3):371–380.
- Rhim H, Lim HK, Kim YS, et al. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008;190(1):91–98.
- Shibata T, Iimuro Y, Ikai I, et al. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002;13(3):313–315.
- Koda M, Ueki M, Maeda Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183(3):583–588.
- Kondo Y, Yoshida H, Tateishi R, et al. Percutaneous radiofrequency ablation of liver cancer in the hepatic dome using the intrapleural fluid infusion technique. Br J Surg. 2008;95(8):996–1004.
- Dubinsky TJ, Cuevas C, Dighe MK, et al. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190(1):191–199.
- Wijlemans JW, de Greef M, Schubert G, et al. Intrapleural fluid infusion for MR-guided high-intensity focused ultrasound ablation in the liver dome. Acad Radiol. 2014;21(12):1597–1602.
- Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253(5):981–987.
- Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36(2):185–195.
- Yao CL, Trinh T, Wong GT, et al. Anaesthesia for high intensity focused ultrasound (HIFU) therapy. Anaesthesia. 2008;63(8):865–872.
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10(1):219–225.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11(12):1061–1069.
- Prabhakar H, Bithal PK, Suri A, et al. Intracranial pressure changes during Valsalva manoeuvre in patients undergoing a neuroendoscopic procedure. Minim Invasive Neurosurg. 2007;50(2):98–101.
- Tiecks FP, Lam AM, Matta BF, et al. Effects of the valsalva maneuver on cerebral circulation in healthy adults. A transcranial Doppler Study. Stroke. 1995;26(8):1386–1392.
- Marval PD, Perrin ME, Hancock SM, et al. The effects of propofol or sevoflurane on the estimated cerebral perfusion pressure and zero flow pressure. Anesth Analg. 2005;100(3):835–840.
- Bertot LC, Sato M, Tateishi R, et al. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21(12):2584–2596.
- Zhang CX, Zhang Z, Zhang Z, et al. Effects of Large Blood Vessel Locations during High Intensity Focused Ultrasound Therapy for Hepatic Tumors: a finite element study. Conf Proc IEEE Eng Med Biol Soc. 2005;2006:209–212.
- Carling U, Barkhatov L, Reims HM, et al. Can we ablate liver lesions close to large portal and hepatic veins with MR-guided HIFU? An experimental study in a porcine model. Eur Radiol. 2019;29(9):5013–5021.
- Kopelman D, Inbar Y, Hanannel A, et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS): ablation of liver tissue in a porcine model. Eur J Radiol. 2006;59(2):157–162.
- Solomon SB, Nicol TL, Chan DY, et al. Histologic evolution of high-intensity focused ultrasound in rabbit muscle. Invest Radiol. 2003;38(5):293–301.
- Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19(2):437–445.
- Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245–W252.