?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Local thermal ablation, a minimally invasive technique, has been widely used in clinical treatment of lung cancer. This study aimed to discuss the clinical efficacy of systemic chemotherapy combined with radiofrequency ablation (RFA) versus systemic chemotherapy combined with microwave ablation (MWA) in treating lung cancer.
Methods
A retrospective analysis involving 124 lung cancer patients, who received RFA (n = 68) and MWA (n = 56) combined with systemic chemotherapy in Cangzhou People’s Hospital from August 2017 to December 2019, was conducted. Before comparative analysis for therapeutic efficacy, the two groups of patients were matched with propensity score matching method at a ratio of 1:1. Indicators including progression-free survival (PFS), overall survival (OS), short-term efficacy, tumor marker level, local tumor control rate, and postoperative complications were comparatively analyzed.
Results
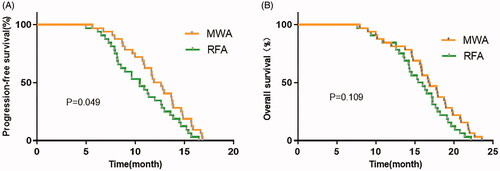
There was no statistical difference in disease control rate and objective response rate (90.6% and 78.1% vs 93.8% and 84.4%) between RFA group and MWA group. The incidence of complications was 12.5% in RFA group and 18.8% in MWA group with no statistically significant difference. In addition, the local tumor control rate in MWA group (90.6%) was significantly higher than that in RFA group (78.1%). Regarding survival, a statistically significant difference was observed in median PFS of RFA and MWA groups (9.2 months vs 10.4 months, p < 0.05), while OS in two groups slightly varied.
Conclusion
MWA was superior to RFA over local tumor control rate and PFS and showed great potential in lung cancer ablation treatment.
Introduction
Lung cancer is one of the most common malignant tumors in the world and is also one of the most common causes of death, with its incidence and mortality being on the rise [Citation1]. Lung cancer is mainly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC is the main type of disease, accounting for about 85%. Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are more common in NSCLC [Citation2]. Surgical resection is still the main treatment method for patients with early stage of lung cancer, but most patients are in middle and advanced stages when they are diagnosed with lung cancer, missing the best time for surgical treatment [Citation3]. Systemic chemotherapy is the main adjuvant means that not only brings great pain to patients, but also results in a limited survival time after treatment [Citation4]. Therefore, it is necessary for us to adopt new treatment methods to maximize the survival rate of patients.
Thermal ablation, a minimally invasive technique, has good safety and effectiveness that can improve the clinical treatment effect and prolong the survival time of patients. It has been widely used in the clinical treatment of lung cancer [Citation5]. At present, the most common thermal ablation techniques include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, laser ablation (LA), etc. [Citation6]. RFA has been widely used in the past decade as a standard treatment for patients with early-stage lung cancer, and it shows better anticancer effect and greater survival benefits than chemical ablation or cryoablation [Citation7]. MWA is a recently developed thermal ablation technique that opens up a new prospect for the selection of optimal ablation method for local treatment of early-stage lung cancer [Citation8]. By now, RFA and MWA have been comparatively analyzed for their therapeutic effect in a variety of cancers. For example, An et al. conducted a study devoted to research RFA and MWA as first-line treatments for patients with small single perivascular hepatocellular carcinoma and found that MWA contributes to better tumor control than RFA [Citation9]. In addition, another study also mentioned that MWA combined with chemotherapy is superior to chemotherapy alone in progression-free survival (PFS) and overall survival (OS) [Citation10]. Although there have been a lot of comparisons between RFA and MWA in different treatment schemes and different tumor types, the clinical efficacy of thermal ablation combined with systemic chemotherapy in the treatment of lung cancer is still uncertain [Citation11]. Therefore, we aimed to study the clinical efficacy of thermal ablation (RFA and MWA) combined with systemic chemotherapy in treating patients with lung cancer and to provide a reference for the clinical application of thermal ablation techniques in lung cancer treatment.
Materials and methods
Patient enrollment
From August 2017 to December 2019, patients with lung cancer who received systemic chemotherapy combined with thermal ablation (RFA or MWA) in Cangzhou People’s Hospital were recruited, and 124 patients were finally included. This study was approved by the Medical Ethics Committee of Cangzhou People’s Hospital (the ethics review report was provided in the attachment). Criteria for inclusion: aged ≥18; considered to be unsuitable for surgical operation; with no response to standard chemotherapy or radiotherapy; with surgical contraindications of a high incidence: severe pharmacologically uncontrollable arrhythmia, myocardial infarction less than 3 months, heart failure, emphysema, cirrhosis, chronic renal insufficiency, other existing tumors, multiple sclerosis, aortic aneurysm, low “ECOG performance status”; the risk of death due to coexisting diseases lower than the risk of death caused by tumors; maximum diameter of primary lesion ≤5 cm. Criteria for exclusion: with mental disorders and unable to cooperate with treatment; with severe heart, liver, kidney and other organ dysfunction or failure, with aplastic anemia, leukemia and other blood diseases and with an allergic history of drugs used in the study; complicated with malignant tumors and treated with anticoagulants such as aspirin, heparin sodium or heparin calcium in recent 3 months. The general information of the included patients is shown in .
Table 1. Comparison of the general data of the two groups (before propensity score matching).
Therapeutic schemes
Preoperative examinations included blood routine, liver and kidney function, tumor marker level, chest computed tomography (CT) scanning, single-photon emission computed tomography (SPECT) or positron emission computed tomography (PET), abdominal B-ultrasound, bone scanning, and head MRI. The position and distance of the central plane of the tumor were measured according to CT images to determine the position, angle and depth of needle insertion. Then, CT scanning was repeated to make the insertion away from important structures such as great vessels, bronchi and diaphragms. Both RFA and MWA procedures were performed by two clinicians with at least 5 years of experience in tumor interventional radiology. RFA Group: under the guidance of CT, an internal refrigerant electrode (Cool-Tip RF ablation system; Medtronic, Minneapolis, MN) or LeVeen electrode (Boston Scientific, Natick, MA) was placed percutaneously into the lung cancer lesions, and then the multi-needle electrode was spread out and the automatic output power was selected. The treatment time depended on the size of the tumor. For lesions with a diameter <3 cm, the needle was inserted into the central part of the lesions, and RFA was performed. For lesions with a diameter > 3 cm, the site for RFA was altered to completely ablate the lesions to a range of 0.5 cm away from the mass. For participants with multiple lung tumors, RFA was performed multiple times within 8 weeks to reduce the risk of complications. MWA Group: MWA was performed with ECO-100A1 system (ECO Medical Instrument Co., Ltd.). The frequency was set to 2450 ± 50 MHz, and the output power of the frequency-modulated continuous wave was from 0 to 100 W. Under the guidance of CT, the electrode needle was placed into the lung cancer lesions, the puncture needle was fixed, the water circulation system was opened, and the microwave therapeutic apparatus was connected. The output power of 60–80 W was applied for 6–8 min to completely ablate the lesions to a range of 0.5–1 cm away from the mass. For lesions larger than 3.5 cm, two ablation antennas were applied in combination.
CT scanning was performed again after operation to observe the changes of tumor and surrounding tissue structure, and whether there are complications such as pneumothorax or hemorrhage. Since no abnormality was confirmed, the patients were sent back towards for 2 h of repose. Fever, expectoration with blood and other symptoms may happen after operation, and preventive antibiotics, hemostasis and other symptomatic treatments should be given.
Both groups of patients received systemic chemotherapy at least 7 days after surgery. Paclitaxel injection (specification: 5 ml:30 mg; production batch number: 20140303, Haikou Qili Pharmaceutical Co., Ltd.) was given by intravenous drip at 135 mg/m2 on the first and eighth days of each treatment cycle. Cisplatin injection (specification: 50 ml:50 mg; production batch number: 20130930, Australia Hospira Australia Pty Ltd) at 60 mg/m2 along with 0.9% sodium chloride injection (a total of 500 ml) was given by intravenous drip on the first day of each cycle. Totally, 4 cycles of treatment (one cycle: 21 days) were set, combined with nutrition support therapy and immunotherapy.
Observation indexes and efficacy evaluation criteria
After treatment, 5 ml venous blood from the left elbow was collected at 8:00 am the next day, and centrifuged at 2000 r/min for 10 min. The supernatant was put into an EP tube and then separated with serum collected and stored in a freezer at −70 °C. The levels of serum tumor markers including neuron specific enolase (NSE), squamous cell carcinoma antigen (SCCA), cytokeratin fragment 21-1 (CYFRA21-1) and carcinoembryonic antigen (CEA) were detected by ELISA method. The adverse reactions and complications during hospitalization were recorded. Therapeutic effect was evaluated according to the enhanced CT scanning and various physical and chemical examinations before and after treatment. According to the Response Evaluation Criteria in Solid Tumors (RECIST) [Citation12], the efficacy was divided into the following four levels: (1) Complete response (CR): all non-target lesions disappear and tumor markers return to normal; (2) Partial response (PR): one or more non-target lesions and/or levels of tumor markers continuously higher than normal; (3) Stable disease (SD): one or more non-target lesions and/or levels of tumor markers persistently higher than normal, yet no new lesions and/or clear progression of existing non-target lesions; (4) Progressive disease (PD): one or more new lesions and/or clearly progressive existing non-target lesions. The presence of ground glass in the treated area larger than the periphery of the primary site after ablation is considered a sign of complete ablation. Local tumor control rate was assessed in accordance with the report of the Chinese Expert Consensus Symposium: Guidelines for Thermal Ablation of Primary and Metastatic Lung Tumors: 2015 Edition [Citation13]. Increased local soft tissue density (>15 HU) was considered as a sign of residual or recurrent disease, while thin symmetrical edges with increased density (<5 mm wide) observed within 6 months after ablation was considered as a sign of peripheral enhancement of benign tumors [Citation14].
Follow-up visit
In order to further study the long-term efficacy of the thermal ablation techniques toward patients, all patients were followed up from the day of hospitalization, including telephone follow-up and hospital follow-up. Progression free survival (PFS) refers to the time from the beginning of treatment to the progression or recurrence of the disease, while the overall survival (OS) refers to the time from the beginning of treatment to the end of death for any reason or loss to follow-up. As of December 2019, 64 patients were followed up for 2-24 months, and no patients were lost to follow-up.
Statistical methods
SPSS 23.0 software was used for statistical analysis. The measurement data were expressed as mean ± standard deviation (± s), and the comparison between groups was conducted by t-test; the enumeration data were expressed as rate (%), and the comparison between groups was performed by χ2 test or Fisher exact test. Kaplan-Meier method was used to calculate the survival, and Log-rank test was used for analyzing the comparison between groups. The PSM process was implemented by the PSM extension program of SPSS, and 1:1 nearest neighbor matching method (RFA Group as the reference group) was used for group matching. The matched covariates included sex, age, smoking history and other important clinical factors. The difference was statistically significant when p < 0.05 in two-sided test.
Results
General information of the two groups after matching
Before matching, the average age of RFA group was (61.34 ± 7.63) years old, and that of MWA group was (59.79 ± 8.65) years old. Clinical data showed that there were significant differences in smoking history, tumor size and pathological classification between the two groups, and the distribution was uneven between the two groups (p < 0.05), as shown in . In order to reduce the influence of confounding factors, we used the following factors for propensity score matching analysis (1:1): age, gender, smoking history, tumor diameter, ECOG performance status, disease stage, histological type. Following that, 32 cases were included in each group. The average age of the patients was (60.56 ± 8.14) years old, and the tumor diameter was (3.2 ± 0.8) cm. At this time, the general data of the two groups were not statistically significant (p > 0.05) but were clinically comparable. See for detailed information.
Table 2. Comparison of general data between the two groups (after propensity score matching).
Comparison of short-term efficacy between the two groups
As shown in , the disease control rate (DCR) and objective response rate (ORR) of RFA group were 90.6% and 78.1%, respectively, and those of MWA group were 93.8% and 84.4%, respectively. There was no statistically significant difference between the two groups (p > 0.05), indicating that RFA () and MWA () have the similar short-term efficacy in the treatment of lung cancer.
Figure 1. Axial CT images of the 65-year-old men with lung cancer treated with RFA. Upper left: CT prior to RFA treatment shows peripheral carcinomas in the left lung (arrow); Upper right: CT findings during RFA treatment, the ablation site is covered with ground glass; Lower left: CT image 3 months after RFA treatment shows increased appearance and decreased density of the treated area; Lower right: CT image 6 months after RFA treatment shows ground-glass changes in the treated area, cavitation changes in the primary focus, and surrounding fibrotic scarring with signs of inflammation.
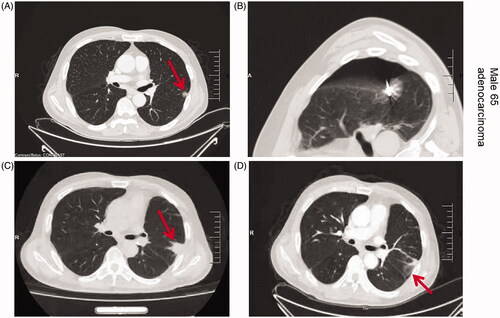
Figure 2. Axial CT images of the 66-year-old women with lung cancer treated with MWA. Upper left: CT prior to MWA treatment shows a single peripheral lesion in the left lung (arrow); Upper right: CT findings during MWA treatment; puncture needle guide electrode reaches the lesion site for MWA; Lower left: CT image 3 months after MWA treatment shows ground-glass changes in the treatment area and decreased density in the central area of the lesion; Lower right: CT image 6 months after MWA treatment shows a reduced ablation area with surrounding signs of inflammation.
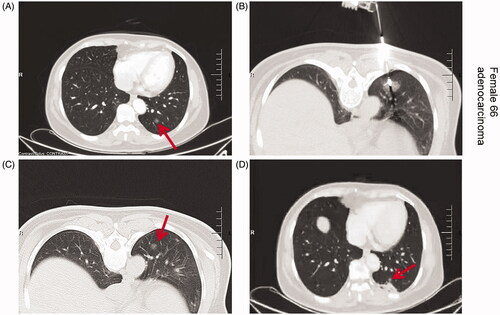
Table 3. Comparison of short-term efficacy between the two groups [n (%)].
Comparison of tumor markers
Before treatment, there was no statistical difference in tumor markers between the two groups. After treatment, the concentration level of serum CEA, NSE, CYFRA21-1 and SCCA of the two groups were decreased, and there was no statistical difference between the two groups (p > 0.05), as shown in . Therefore, it could be concluded that RFA and MWA both could effectively reduce the serum level of tumor markers and inhibit tumor growth, and the therapeutic effects of both were similar.
Table 4. Comparison of serum tumor markers between the two groups (μg·L−1, X ± s).
Local tumor control rate
Local tumor control was achieved in 90.6% (29/32) of patients in MWA () group and 78.1% (25/32) of patients in RFA () group without tumor recurrence or residue. The recurrence rate of lesions in MWA group (6.9%; 7 out of 101 lesions) was significantly lower than that in RFA group (20.4%; 11 out of 54 lesions). The difference between the two groups was statistically significant (p < 0.05), which might be related to the difference in operation between the two ablation techniques.
Postoperative complications
As to RFA group, there were 2 cases with skin burn and 2 cases with wound infection, and the complication rate was 12.5% (4/32). As to MWA group, there were 4 cases with wound exudation and 2 cases with sub-capsular hematoma of liver, and the complication rate was 18.8% (6/32). There was no statistical difference in the complication rate between the two groups (p > 0.05). The results showed that there was no difference in safety between systemic chemotherapy combined with RFA and systemic chemotherapy combined with MWA in the treatment of lung cancer, and both could be used as clinical treatment means of lung cancer.
Survival analysis
The median follow-up time for all patients was 16.8 months. The median PFS time of RFA group was 9.2 months, and that of MWA group was 10.4 months. There was a certain difference in PFS between the two groups (p < 0.05) (). The median OS was 14.6 months in RFA group and 15.2 months in MWA group. There was no significant difference between the two groups (p > 0.05) ().
Discussion
Lung cancer is the first cause of death of human beings. It features high incidence, poor prognosis and high recurrence, which seriously threatens human life and safety [Citation15]. At present, when patients are diagnosed with lung cancer clinically, the cancer has developed to middle and late stages, and the patients then miss the best time of surgical resection. Meanwhile, considering the influence of patient’s constitution, age, and other affected diseases, patients cannot be cured through surgical operation [Citation16]. Chemotherapy and radiotherapy are commonly used in the treatment of malignant tumors, but the prognosis of patients treated with such methods is poor and the survival time is limited [Citation17]. However, with the development of medical technology, thermal ablation technology has been studied and widely used in the treatment of malignant tumors, and it has been an option of relatively high safety so far.
As an alternative to radical surgery, thermal ablation has been used to treat patients with early-stage cancers in the past decade. At present, thermal ablation technique combined with other methods including surgery, chemoradiotherapy and molecular targeted drugs has made great progress in the treatment of tumors. Research on the combination of ablation and chemotherapy for progressive NSCLC is increasing gradually, while such method can improve the local tumor control rate and prolong the survival time of patients to a certain extent [Citation18], and it may become a new treatment mode for progressive NSCLC. A previous study showed that MWA combined with chemotherapy could improve PFS in advanced NSCLC in comparison with chemotherapy alone [Citation19]. Similarly, another study reported that RFA combined with chemotherapy could significantly improve the 2-year survival rate of patients with Stage IIIb and Stage IV NSCLC [Citation20]. In a phase III randomized controlled trial, MWA and RFA had similar results in patients with hepatocellular carcinoma, but MWA had the advantages of short treatment course, simple operation, short operative time and low cost [Citation21]. Additionally, the effectiveness of RFA and MWA in lung tumor ablation was evaluated in a LUMIRA trial. The results showed that there were no significant differences in survival time and complications between the two methods. However, MWA produced less intraprocedural pain and significantly reduced tumor mass compared with RFA treatment [Citation22]. In our study, it was found that MWA significantly prolonged PFS compared with RFA. However, there was no significant difference in OS between the two groups. Besides, the two methods both had good safety.
Compared with RFA, MWA has been seldom involved, but MWA has advantages in theory as it provides larger ablation area, shorter procedural time, less heat sink effect, and higher tumor control rate [Citation23], which is consistent with our result that MWA is superior to RFA in local tumor control rate. Although MWA and RFA both induce tumor necrosis by heating tissue, there is a great difference between the two methods in heat generation, which may be the reason for the different therapeutic effects in the treatment of lung cancer. As to RFA, needle electrodes are used to deliver current, which increases the temperature around the electrodes from 60 °C to 100 °C. However, direct and active heating is limited to the small area around the electrodes, while the large part of the final ablation area can only be ablated with passive heat conduction. This method is mainly for small tumors and not suitable for multiple or large tumors. In addition, the tissue adjacent to the RFA electrodes can be carbonized to act as an electrical insulator, which will reduce the effectiveness of RFA [Citation24]. In contrast, MWA is more effective in inducing tumor necrosis because of its heat generation mechanism, that is, the displacement of molecules (water) of rotating dipole ions and ions mediated by microwave transmission does not depend on electrical conduction and is not limited to tissue carbonization, which makes MWA play a great role in multiple or large tumors as well [Citation25]. So far, the clinical efficacy of RFA and MWA in the treatment of lung cancer is still controversial. Whether MWA is superior to RFA needs more research to confirm.
There are some limitations in this study. Firstly, this study was conducted in a retrospective manner and is inevitably with potential selection and indication bias. Secondly, this study is mainly for Chinese population of a relatively small size, thus the guiding significance for other countries still needs to be further explored.
In conclusion, our study showed that both RFA and MWA are safe and effective treatment methods, while MWA shows advantages in terms of local tumor control and PFS. Our research results may provide a reference for thermal ablation in lung cancer treatment.
Author contributions
XF and SJ contributed to the study design. XF, SJ and ZZ conducted the literature search. HY acquired the data. WJ wrote the article. WJ performed data analysis and drafted. YY and ZZ revised the article. ZZ gave the final approval of the version to be submitted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
Additional information
Funding
Reference
- Dane B, Grechushkin V, Plank A, et al. PET/CT vs. non-contrast CT alone for surveillance 1-year post lobectomy for stage I non-small-cell lung cancer. Am J Nucl Med Mol Imaging. 2013;3:408–416.
- She K, Yan H, Huang J, et al. miR-193b availability is antagonized by LncRNA-SNHG7 for FAIM2-induced tumour progression in non-small cell lung cancer. Cell Prolif. 2018;51(1):12406.
- Palussiere J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 13(1):91. 2018;13:91.
- Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18:63–70.
- de Baere T, Tselikas L, Catena V, et al. Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging. 2016;97:1019–1024.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–260.
- Donington JS. Radiofrequency ablation in high-risk stage I non-small cell lung cancer. Cancer. 2015;121:3393–3394.
- Murphy KP, Maher MM, O'Connor OJ. Abdominal ablation techniques. AJR Am J Roentgenol. 2015;204:W495–502.
- An C, Li WZ, Huang ZM, et al. Small single perivascular hepatocellular carcinoma: comparisons of radiofrequency ablation and microwave ablation by using propensity score analysis. Eur Radiol. 2021. DOI:https://doi.org/10.1007/s00330-020-07571-5
- Wei Z, Yang X, Ye X, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. Eur Radiol. 2020;30(5):2692–2702.
- Palussiere J, Catena V, Lagarde P, et al. Primary tumors of the lung: should we consider thermal ablation as a valid therapeutic option? Int J Hyperthermia. 2019;36:46–52.
- Sone K, Oguri T, Ito K, et al. Predictive role of CYFRA21-1 and CEA for subsequent docetaxel in non-small cell lung cancer patients. Anticancer Res. 2017;37:5125–5131.
- Ye X, Fan W, Wang H, et al. Chinese expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer. 2015;6:112–121.
- Cheng M, Fay M, Steinke K. Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: a retrospective study. Int J Hyperthermia. 2016;32(3):316–323.
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543.
- Yang X, Ye X, Zheng A, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763.
- Wei Z, Ye X, Yang X, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142.
- Wei Z, Ye X, Yang X, et al. Advanced non small cell lung cancer: response to microwave ablation and EGFR Status. Eur Radiol. 2017;27:1685–1694.
- Wei Z, Ye X, Yang X, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464.
- Du S, Qin D, Pang R, et al. Long-term efficacy of radiofrequency ablation combined with chemotherapy in the treatment of patients with advanced non-small cell lung cancer–a retrospective study. Zhongguo Fei Ai Za Zhi. 2017;20:675–682.
- Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172–1173.
- Macchi M, Belfiore MP, Floridi C, et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol. 2017;34(5):96.
- Planche O, Teriitehau C, Boudabous S, et al. In vivo evaluation of lung microwave ablation in a porcine tumor mimic model. Cardiovasc Intervent Radiol. 2013;36:221–228.
- Lucchina N, Tsetis D, Ierardi AM, et al. Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol. 2016;29:460–465.
- Yao W, Lu M, Fan W, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int J Hyperthermia. 2018;34:1329–1336.