Abstract
Objective
This retrospective study aimed to investigate the efficacy and safety of partial ablation (PA) for benign thyroid nodules (BTNs) using microwave ablation (MWA) in a long-term follow-up.
Materials and methods
Between February 2015 and April 2019, 236 patients with 236 BTNs (maximum diameter ≥2 cm) treated with ultrasound-guided MWA were enrolled. Contrast-enhanced ultrasound (CEUS) was performed within 3 d after ablation to determine whether there was residual tissue according to which the patients were assigned PA or complete ablation (CA). The volume reduction ratio (VRR) and complications were evaluated during follow-up.
Results
Eighty-two patients were enrolled in the PA group, and 154 were enrolled in the CA group. Both groups achieved continuous reductions in nodule volume and increases in VRR within 2 years after ablation. Although the VRR of the PA group at 4 years was lower than that of the CA group (65.54 vs. 95.08%; p<.05), PA still achieved ‘technical efficacy’ with a volume reduction of more than 50%. The complication and side effect rates between the two groups showed no significant difference (p>.05).
Conclusions
Both PA and CA were safe and effective in reducing the volumes of BTNs in the long-term follow-up. For nodules with a large initial volume and dangerous location, PA with a small amount of residual tissue may be acceptable.
Introduction
Thyroid nodules have become a common clinical problem, with a high incidence of up to 19–68% in some populations owing to the widespread use of high-resolution ultrasonography [Citation1]. Although most nodules are benign [Citation2], many of them may require treatment because they gradually grow and cause pressure symptoms or cosmetic problems [Citation3,Citation4]. Nonsurgical, minimally invasive treatment modalities, including radiofrequency ablation (RFA), microwave ablation (MWA) and laser ablation (LA), have been proven to be effective and safe [Citation5–8]. Thus, thermal ablation techniques have been recommended by several guidelines as alternative methods to surgery for benign thyroid nodules (BTNs) [Citation4,Citation9,Citation10].
However, as distinct therapies from surgical resection that thoroughly remove the nodule, thermal ablation methods usually aim to alleviate pressure symptoms or cosmetic problems without the intention of achieving complete ablation (CA), and a more than 50% volume reduction of the initial nodule volume over a 1-year period can be defined as technical efficacy [Citation10–12]. On the other hand, many nodules that meet the indications are extremely large [Citation13]; thus, it is usually difficult to achieve CA, and these large nodules require more treatment sessions than smaller nodules [Citation14,Citation15]. In addition, to limit adverse events, operators are usually inclined to reduce the output power and action time for nodules adjacent to vital structures, such as the laryngeal recurrent nerve and common carotid artery (CCA), which may lead to incomplete treatment of the nodules [Citation16]. As the overall efficacy of thermal ablation for BTNs varies [Citation5,Citation17], it is important to investigate whether partial ablation (PA) will weaken the efficacy of thermal ablation methods.
Few studies on RFA for BTNs have reported that marginal regrowth might occur in incompletely treated nodules during follow-up [Citation14,Citation18]. However, although the regrowth rate was as high as 5.6%, RFA still showed excellent efficacy in shrinking nodules, with a mean volume reduction rate of 93.4 ± 11.7% at the 4-year follow-up [Citation14]. MWA, as an increasingly used thermal ablation method for BTNs, has been proven to be as effective and safe as RFA [Citation5,Citation19]. However, most of these studies have not reported the initial ablation rate (IAR) and performed a relatively short-term follow-up. Thus, it is not clear whether PA through MWA for BTNs would be effective in maintaining the long-term efficacy for debulking.
This study aimed to investigate the efficacy and safety of PA for BTNs using MWA. Pretreatment and post-treatment contrast-enhanced ultrasound (CEUS) was performed to determine whether there was residual tissue, and then the residual volume was measured. Patients in this cohort were defined as the PA group, while the contemporaneous CA group was used as a reference. Subsequently, both groups received regular follow-up, and the volume reduction ratio (VRR) at each follow-up visit and side effects were evaluated.
Materials and methods
Patients
This study was retrospective and was approved by the Ethics Committee of our hospital. Written informed consent was obtained from each patient before treatment. The patients were selected based on the following inclusion criteria: (1) all nodules confirmed as pathologically benign through at least two US-guided fine-needle aspiration (FNA) or core needle biopsy (CNB) procedures; (2) maximum nodule diameter ≥2 cm and no US imaging findings of malignant or suspicious characteristics; (3) presence of nodule-related symptoms, such as compressive symptoms, neck discomfort or pain, and foreign body sensations or presence of cosmetic problems; and (4) willingness to undergo treatment but inability to tolerate surgery or refusal to undergo surgery. The exclusion criteria were as follows: (1) coagulation dysfunction or severe bleeding tendency; and (2) cystic nodules (<10% solid components).
From February 2015 to April 2019, 389 patients with BTNs larger than 2 cm underwent ablation by MWA. After excluding 87 patients who had not received CEUS within 3 d after ablation, 23 were excluded because their images could not be retrieved from the DICOM workstation, and 43 were lost to follow-up. Finally, 236 patients (191 females and 45 males; mean age: 46.30 ± 12.948; range: 18–76 years) with 236 nodules were enrolled in the study ().
Pre-ablation assessment
Before treatment, all patients underwent US, CEUS, US-guided FNA or CNB and laboratory tests. A GE LOGIQ E9 scanner (GE Healthcare, Milwaukee, WI, USA) with high-resolution linear probes (6.0–12.0 MHz) was used in this study. Transverse and longitudinal US images were obtained for each target nodule, and three orthogonal diameters were measured. The volume of the nodule was calculated based on the following equation: V = π a × b × c/6 (where V represents the volume, a is the largest diameter, and b and c are the other two perpendicular diameters). All of the US imaging were interpreted independently by two radiologists with more than 5 years of experience, and a consensus was reached through negotiation or referral to another radiologist with more than 10 years of experience if their initial interpretations were different. The composition of the nodules was subjectively evaluated by the ultrasound examiner and was classified as mainly solid (solid portion >90%), predominately solid (50% < solid portion <90%) or predominantly cystic (10% < solid portion < 50%). Vascularity was graded using a four-point scale (grade 0, no vascularity; grade 1, perinodular vascularity only; grade 2, intramodular vascularity; and grade 3, peri- and intra-modular vascularity). According to the distance (<5 mm) between the target nodules and surrounding vital structures, the locations of the nodules were divided into several categories: adjacent to the trachea or not, adjacent to the carotid artery or not, and adjacent to the danger triangle area or not.
The laboratory tests included triiodothyronine, free thyroxine, thyroid-stimulating hormone, complete blood count and blood coagulation tests. Before treatment, the patients were asked to rate their symptoms on a 10-point visual analog scale, while the cosmetic score was assessed by the physician [Citation20].
Procedure
All MWAs were performed by six experienced radiologists. The microwave unit (KY-2000, Kangyou Medical, Nanjing, China) consists of a microwave generator (producing 1–100 W of power at 2450 MHz), a flexible low-loss coaxial cable, and a 16 G antenna (1.6 mm in diameter and 10 cm in length with a 3-mm active tip) coated with polytetrafluoroethylene to prevent adhesion. The antenna was cooled continuously by distilled water circulating through dual channels inside the antenna shaft to prevent shaft overheating. Each patient was placed in the supine position with a hyperextended neck. Then, local anesthesia was administered using 1–2% lidocaine around the thyroid gland. The hydro-dissection technique was applied in all patients using 0.9% physiological saline-infused outside of the thyroid capsule to isolate the vital structures of the neck (carotid artery, trachea, danger triangle area, etc.). The trans-isthmic approach and moving shot technique were generally used [Citation21]. We first inserted a metal trocar into the anterior capsule and then placed the needle through the channel into the nodule so that we could conveniently move the needle by moving the trocar without the need for repeated punctures. During MWA, a power output of 30–50 W was usually used. During the whole procedure, we continuously monitored the changes on the ultrasonogram and intermittently talked with the patients to assess the phonation status. The treatment was not terminated until the whole nodule appeared hyperechoic. For predominantly cystic nodules, we routinely performed MWA after the aspiration of internal fluid. After MWA, each patient was asked to press on their neck for 15–20 min and was observed for 1–4 h. Complications and side effects during or after the procedure were identified according to the criterion outlined in the quality improvement guidelines of the Society of Interventional Radiology [Citation22] and a multicenter study of complications for thyroid ablation [Citation23].
CEUS evaluation pre and post treatment
CEUS was performed before starting the ablation procedure and within 10–20 min after MWA to determine whether supplemental ablation during the operation was necessary and 1–3 d after treatment to evaluate the initial ablation efficacy. For CEUS, a low mechanical index and linear transducer (range: 6–12 MHz) were used. The SonoVue contrast agent (SonoVue, Bracco, Milan, Italy) was injected into an antecubital vein as an intravenous bolus of 2.4 ml via a 20 gauge cannula, followed by a 5–10 ml normal saline flush. The thyroid nodule was observed continuously for 1 min and then intermittently until the microbubbles cleared from circulation. All the CEUS images and videos were digitally recorded for further analysis. The target nodule was defined as completely ablated when nonenhancement was observed in both the arterial phase and venous phases. In contrast, PA was defined as persistence of enhancement within or in the surrounding nodule after treatment, which was a sign of residual viable tissue. IAR = (initial volume − residual volume) × 100/initial volume (%).
Post-procedural follow-up
Post-procedural follow-up was carried out at 1, 3, 6 and 12 months after treatment and every 6–12 months thereafter (median 12 months; range, 1–58 months). At each follow-up, grayscale and color Doppler and laboratory tests were performed. CEUS was performed only if it was difficult to identify the residual portion of the nodules. The three orthogonal diameters and thyroid nodule volumes were evaluated in the same manner before and after ablation. The VRR of the treated nodule was calculated as follows: VRR=(initial volume − final volume) × 100/initial volume (%). Technique efficacy was defined as a volumetric reduction ≥50% of the initial nodule volume over the 12 months after ablation [Citation10–12]. Any delayed complications that occurred during the follow-up period were also recorded.
Statistical analysis
SPSS version 23 (IBM, Armonk, NY and MedCalc, Ostend, Belgium) was used to analyze the data. The primary outcome was the volume reduction rate at different follow-up timepoints. Continuous variables are reported as the mean ± SD. Data with a skewed distribution were described as the median and range. Qualitative variables were analyzed by χ2 test or Fisher’s exact test. Normally distributed quantitative data were compared by one-way analysis of variance. Data with a skewed distribution, such as the largest diameter and volume before or after MWA, were analyzed using the Mann–Whitney U test. A p value less than .05 was considered statistically significant.
Results
The demographic characteristics of all enrolled patients before ablation are summarized in . Compared with patients in the CA group, those in the PA group had larger diameters and initial volumes and higher cosmetic and symptom scores. In addition, more nodules in the PA group were adjacent to the carotid artery [76.8% (63/82) vs. 63.0% (97/154)] and danger triangle area [59.8% (49/82) vs. 39.0% (60/154)] than in the CA group, and those in the PA group showed more abundant vascularity on color Doppler US (p<.05); in contrast, the echogenicity, composition, calcifications and number of nodules adjacent to the trachea did not show significant differences between groups (all p>.05). In addition, there was no significant difference in pathological diagnosis between the two groups (Supplementary appendix ).
Table 1. Patients’ demographic data and information of nodules.
The treatment characteristics of the nodules are summarized in . Although the total energy in the PA group was higher than that in the CA group (25504.27 ± 16750.07 vs. 15921.23 ± 10632.35 J), the energy deposited per milliliter nodule volume was lower in the PA group (1354.72 ± 833.35 vs. 1852.64 ± 1173.47 J/ml) (p<.001). The number of ablation sessions was not significantly different between the two groups. The IAR for the PA group was 97.71% ± 2.79% (range: 84.48–99.92%).
Table 2. Treatment characteristics of nodules.
The volume of the nodules in the PA group was larger than that of the nodules in the CA group before treatment () and at each follow-up () (p<.05). In the short term, the nodule volumes of both groups decreased gradually; however, the volumes in the PA group showed a tendency to increase over 3 years ( and ). There were no differences in VRR at 1, 3, 6 and 12 months, 2 years or 3 years after ablation between the two groups. Owing to internal bleeding in one nodule, the volume at 3 years in the PA group increased, while the VRR was drastically reduced. Although the VRR of the PA group at 4 years was lower than that of the CA group (65.54 vs. 95.08%; p<.05), both approaches achieved ‘technique efficacy’ and more than a 50% volume reduction (). Six patients in the PA group received additional treatment due to incomplete alleviation of the symptoms or tumor regrowth during the follow-up (). Although the symptom score and cosmetic scores were higher in the PA group than in the CA group, both the scores before ablation and at the last follow-up were significantly decreased in both groups (p<.001) ().
Figure 2. Changes of thyroid nodule volume (a) and volume reduction ratios (VRRs) (b) before microwave ablation and at each follow-up period.
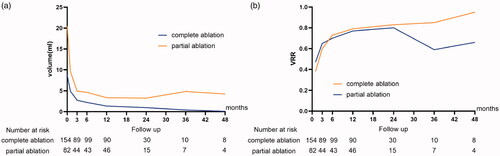
Figure 3. A 49-year-old man received MWA for a solid benign nodule in the left lobe of his thyroid gland, who refused to surgery for having suffering from HCC and undergone RFA and TACE 8 years ago. (a) Longitudinal image before MWA showed a isoechoic solid nodule with peripheral halo (the nodules were measured as 3.5 cm × 1.9 cm × 2.0 cm). (b) Transverse images before treatment showed the nodules adjacent trachea (Tr), common carotid artery (CCA), danger triangle area (circle). (c) Color Doppler shows abundant peri- and intra-nodular vascularity. (d) CEUS before treatment displays rim-like enhancement around the lesion. (e) Microwave antenna (thick arrow) was positioned in the nodule, ‘moving-shot’ technology was used until the nodule was covered by the hyperechoic microbubbles. (f) CEUS at 1 d after treatment shows residual tumor enhancement in the surrounding of the nodule (small arrow). (g–i) 1, 3 and 4 years after treatment, the residual volume increased gradually (small arrow). HCC: hepatocellular carcinoma; RFA: radiofrequency ablation; TACE: transcatheter arterial chemoembolization; MWA: microwave ablation.
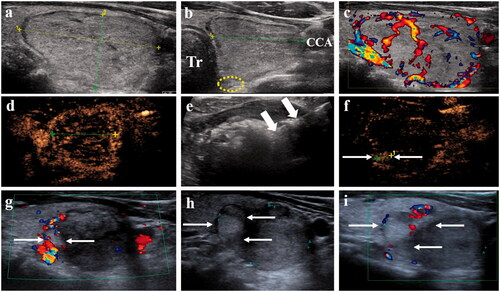
Table 3. The outcomes at each follow-up period after treatment.
Table 4. Information of six patients in partial ablation group received additional treatment.
The complications and side effects are summarized in , and there were no significant differences between the two groups (p>.05). The overall complication rate was 5.8% (9/154) in the CA group and 8.5% (7/82) in the PA group. Major and minor complication rates were 3.9% (6/154) and 1.9% (3/154) in the CA group and 6.1% (5/82) and 2.4% (2/82) in the PA group, respectively. The side-effect rate was 4.5% (7/154) in the CA group and 7.3% (6/82) in the PA group. All eight patients with voice changes that occurred during or just after ablation recovered completely within 1 week − 3 months after the procedure. Three nodule ruptures were detected within 30 d after ablation, which appeared on US images as disruption of the anterior thyroid capsule and the formation of a mixed echo mass in the anterior neck. Two of these patients showed abscess formation, and one showed internal bleeding comorbid with an inflammatory reaction. All of these patients recovered within 3 months after treatment with antibiotics and debridement. All of the patients with minor complications and side effects recovered without treatment within 1–3 d after ablation, except for one with a skin burn who recovered 1 month after the procedure and was left with an inconspicuous scar. No patients experienced life-threatening complications during the follow-up.
Table 5. Complications and side effects.
Discussion
In this study, we retrospectively analyzed the outcomes of 82 patients who underwent PA through MWA for BTNs and 154 contemporaneous patients who underwent CA as the reference. The results showed that both PA and CA were safe and effective in reducing the volumes of the nodules in short-term and long-term follow-up.
In terms of efficacy, both groups achieved satisfactory continuous reductions in nodule volume and increases in VRRs within 2 years after ablation. Although the VRR of the PA group at 4 years was lower than that of the CA group (65.54 vs. 95.08%; p < .05), PA still achieved a volume reduction of more than 50%, which was considered to be ‘technical efficacy’ [Citation11], and only six patients (2.5%) in the PA group received additional treatment during follow-up because of incomplete alleviation of symptoms or tumor regrowth. The reason for the satisfactory efficacy in this study is that we had long noted that potentially incomplete treatment might cause recurrence [Citation16], so, we ablated the lesions of each patient as completely as possible. To do so, we carefully designed the treatment strategy, applied moving shot and hydro-dissection techniques and more importantly, routinely performed CEUS to determine if supplemental therapy was necessary during the session. Therefore, the initial ablation ratio was extremely high (97.71% ± 2.79%; range 84.48–99.92%) in the PA group. As Sim et al. revealed in their research, the IAR is highly correlated with the VRR. They found that if the IAR exceeded 70%, a 50% VRR could be achieved in most cases [Citation24]. Their results were consistent with those of our study, in which PA with a small amount of residual tissue did not necessarily mean significant regrowth or the need for additional treatment.
Several studies, with both RFA and LA, have revealed many risk factors correlated with in CA, which would ultimately lead to regrowth, but the risk factors were inconsistent in different studies [Citation18,Citation19,Citation25,Citation26]. The results showed that more nodules in the PA group had a larger diameter and initial volume, were adjacent to the carotid artery and danger triangle area, and showed abundant vascularity on color Doppler US (p<.05). As other researches had reported, it would be difficult for MWA to cover all of the nodule tissue in a three-dimensional space if the nodule is large [Citation13,Citation16]. Furthermore, larger nodules would receive less energy per ml of volume than smaller nodules [Citation16], which was also proven by our study. In addition, nodule locations adjacent to surrounding vital structures might hinder complete treatment because of safety concerns. In addition, nodules with abundant vascularity may require more energy per ml to achieve complete necrosis than nodules with poor vascularity [Citation16,Citation27]. However, as many innovative techniques and strategies, including hydro-dissection, moving shot and vascular ablation techniques, are routinely used [Citation21,Citation28], these factors do not seem to prevent satisfactory efficacy, even in long-term follow-up. However, this study should not be interpreted as advocating for incomplete treatment, which would definitely result in the regrowth of residual hyperplastic tissue over time. As demonstrated in this research, one nodule with residual tissue developed internal bleeding during follow-up, and six patients received additional treatment due to incomplete alleviation of their symptoms or tumor regrowth. Although all of these patients ultimately achieved satisfactory outcomes, they had to suffer from additional physical and financial burdens.
Regarding safety, the complication and side effect rates between the two groups showed no significant differences (p>.05), which was comparable to previous studies [Citation29–31]. None of the complications were serious; most of the patients recovered without treatment, while a few patients required only routine intervention. No patients experienced life-threatening complications during the follow-up. In the PA group, even the much larger initial volume and much higher prevalence of dangerous nodule locations did not necessarily mean an increased complication rate. This may be because the operators, who knew that greater complications are possible for larger nodules, preferred to create safe zones rather than perform CA [Citation16]. In addition, continuous real-time monitoring with ultrasound during treatment and application of the moving shot and hydro-dissection techniques may be important in reducing potential side effects.
There are several limitations in our study. First, our study is a nonrandomized, retrospective study, and selection bias may affect the results; a prospective randomized study in the future is necessary. Second, considering that the residual tissue in this study was unintentional, the initial efficacy rate was extremely high (97.71% ± 2.79%; range 84.48–99.92%), and the residual tissue was relatively small, so the excellent efficacy of PA in this study should not be extrapolated to cohorts with more residual tissue, and further studies are needed.
In conclusion, both PA and CA were safe and effective for treating BTNs in the long-term follow-up. For nodules with a large initial volume and dangerous location, PA with a small amount of residual tissue may be acceptable.
Acknowledgments
The authors acknowledge Dr. Miao Liu for help with statistical analysis. This research did not receive any specific grant from any funding agency in the public, commercial or nonprofit sector.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Burman KD, Wartofsky L. Clinical practice. Thyroid nodules. N Engl J Med. 2015;373(24):2347–2356.
- Chen AY, Bernet VJ, Carty SE, et al. Surgical Affairs Committee of the American Thyroid A: American Thyroid Association statement on optimal surgical management of goiter. Thyroid. 2014;24(2):181–189.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Zheng BW, Wang JF, Ju JX, et al. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine. 2018;62(2):307–317.
- Gharib H, Hegedüs L, Pacella CM, et al. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013;98(10):3949–3957.
- Cheng Z, Che Y, Yu S, et al. US-guided percutaneous radiofrequency versus microwave ablation for benign thyroid nodules: a prospective multicenter study. Sci Rep. 2017;7(1):9554.
- Zhi X, Zhao N, Liu Y, et al. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34(5):644–652.
- Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):622–639.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Khanh HQ, Hung NQ, Vinh VH, et al. Efficacy of microwave ablation in the treatment of large (≥3 cm) benign thyroid nodules. World J Surg. 2020;44(7):2272–2279.
- Wang B, Han ZY, Yu J, et al. Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia. 2017;33(4):459–464.
- Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation for the management of thyroid nodules: a critical appraisal of the literature. Clin Endocrinol . 2017;87(6):639–648.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33(8):905–910.
- Yue WW, Wang SR, Lu F, Sun LP, et al. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine. 2017;55(2):485–495.
- Jung SL, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018;19(1):167–174.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Burke DR, Lewis CA, Cardella JF, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14(9 Pt 2):S243–S246.
- Baek JH, Lee JH, Sung JY, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Magri F, Chytiris S, Molteni M, et al. Laser photocoagulation therapy for thyroid nodules: long-term outcome and predictors of efficacy. J Endocrinol Invest. 2020;43(1):95–100.
- Negro R, Salem TM, Greco G. Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia. 2016;32(7):822–828.
- Zhao CK, Xu HX, Lu F, Sun LP, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of CEUS evaluation. Clin Hemorheol Microcirc. 2017;65(4):393–405.
- Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol. 2012;2012:919650.
- Liu YJ, Qian LX, Liu D, et al. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med. 2017;242(15):1515–1523.
- Wu W, Gong X, Zhou Q, et al. US-guided percutaneous microwave ablation for the treatment of benign thyroid nodules. Endocr J. 2017;64(11):1079–1085.
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017;27(8):3128–3137.