Abstract
Purpose
Ultrasound-guided thermal ablation (including microwave ablation [MWA] and radiofrequency ablation [RFA]) has emerged as a remarkable technology for the treatment of benign and malignant diseases. The objective of this multicenter study was to assess the efficacy and safety of thermal ablation in a large cohort of patients with papillary thyroid microcarcinoma (PTMC).
Materials and methods
Retrospective study of 725 patients who underwent MWA/RFA at 11 centers between March 2015 and March 2020. The mean age of patients was 46 ± 11 years (range, 22–81); the mean follow-up time was 21 ± 13 months (range, 6–60). Changes in size of tumor, the rates of tumor disappearance, disease progression, and complications were assessed.
Results
From 6 months post-ablation, the size of tumors was significantly reduced compared with those recorded pre-ablation (p < 0.001 for all). Five hundred and fifteen (71.0%) PTMCs had completely disappeared as assessed by ultrasound examination. Six (0.8%) patients developed disease progression post-ablation; of these, 5 (0.7%) patients developed new PTMCs, while one (0.1%) patient developed cervical lymph node metastasis. Nineteen (2.6%) patients developed complications post-ablation; of these 14 (1.9%) patients developed voice hoarseness, 4 (0.6%) developed hematoma, and one (0.1%) patient developed cough.
Conclusions
Ultrasound-guided thermal ablation represents an effective and safe treatment for patients with PTMC besides active surveillance and surgery.
Introduction
The incidence of papillary thyroid microcarcinoma (PTMC), defined as papillary thyroid carcinoma with a maximum diameter ≤1 cm, has rapidly increased across the world since the early 1980s [Citation1]. In some countries, active surveillance (AS) is currently suggested as an alternative to immediate surgical resection of incidental PTMC to reduce surgery-related morbidity [Citation2–4]. Indeed, AS can be considered as a valid option for management of appropriately selected PTMC patients. However, some PTMC patients may not accept AS due to anxiety caused by increase in tumor size and cervical lymph node metastasis [Citation5]. Therefore, there is a dilemma among clinicians about whether to treat the disease aggressively or conservatively.
Ultrasound (US)-guided thermal ablation, a moderate approach between AS and surgery, may be a potential option for selected patients with PTMC. US-guided thermal ablation is a novel, minimally invasive, and potentially acceptable candidate technique for the management of thyroid lesions. Some ablation guidelines and consensus statements indicate that thermal ablation can be used in benign and selected malignant thyroid nodules [Citation6–10]. For instance, microwave ablation (MWA), radiofrequency ablation (RFA), or laser ablation were introduced to treat low-risk T1a papillary thyroid carcinoma [Citation11,Citation12], and even T1b [Citation13]. However, most of these studies were single-center studies with relatively small number of patients, and the results were sometimes inconsistent [Citation14]. Thus, the value of thermal ablation in the management of PTC remains debatable. In this multicenter study, we investigated the role of US-guided thermal ablation (MWA/RFA) in a large number of patients with PTMC with the goal of establishing its efficacy and safety.
Methods
Study design and patients
This study was conducted at 11 medical centers in China. The retrospective multicenter study protocol was approved by the institutional review boards (IRB) of the medical centers. The requirement for informed consent of patients was waived off because the personal details were kept confidential.
Between March 2015 and March 2020, a total of 725 patients (573 women and 152 men) were finally included in this study. Included patients met the following criteria: (1) PTMC confirmed by US-guided core needle biopsy or fine needle aspiration; (2) solitary PTMCs; (3) no extrathyroidal extension, no lymph node metastasis (LNM), or distant metastasis on imaging examinations; and (5) patients who refused AS or surgery. The exclusion criteria were: (1) multiple PTMCs; (2) follow-up period of <6 months; (3) incomplete follow-up data.
Pre-ablation assessment
High-resolution US examination of the neck was performed for each patient to evaluate the tumor size (including three meridians: the largest diameter and two other perpendicular diameters), location, and to detect LNM. Tumor volume (V) was reported in mm3 using the ellipsoid volume formula: V = 0.524abc, where a is the largest diameter, and b and c are the two perpendicular diameters. CT of the neck and lung was performed in all patients to exclude metastasis. US-guided fine needle aspiration or core needle biopsy was performed in all patients to obtain pathology specimens. Specimens were sent for cytological or histological pathology and BRAF V600E mutation tests. Blood examinations, including measurements of thyroid function (serum triiodothyronine, serum free thyroxine, and serum thyrotropin), platelet count, and blood coagulation tests were performed.
Ablation procedure
All procedures were performed by radiologists with at least 3 years of experience in thyroid ablation. For the ablation, RFA or MWA was performed based on the preference of the physician. MWA was performed with an 16 G or 17 G cooled MWA antenna and an active tip of a 0.3 or 0.5 cm tip, with a 10 or 15 cm shaft length, (KY-2000, Nanjing, China; ECO Microwave System, Nanjing, China). RFA was performed with an 17 G RF generator and an active tip of 0.5, or 0.7 cm, with a 15 cm shaft length (Cooltip Radiofrequency Ablation System, Shanghai, China). All operations were performed under US guidance. Prior to ablation, contrast-enhanced US examination was performed to evaluate tumor vascularity. Skin sterilization was performed, and topical anesthesia with 1% lidocaine was administered at the subcutaneous puncture site and the thyroid anterior capsule. Hydrodissection techniques were performed before the insertion of the ablation electrode [Citation15]. Hydrodissection techniques were used to obtain a separation zone of at least 5 mm and to prevent thermal injury to critical structures, such as the esophagus, trachea, RLN, carotid artery, vagus nerve, and the cervical sympathetic ganglion. To achieve an adequate safety margin by hydrodissection, slow and continuous injection of fluid was maintained during the procedure in some of the patients. A trans-isthmic approach or direct nodule puncture was used for the ablation procedure according to the location of the tumor. The power output ranged from 20 W to 60 W according to the electrode tip size. The ablation was performed using moving-shot or fixed-applicator technique. The ablation procedure was terminated when the tumor was completely covered with hyperechoic zone in three dimensional space on US. Intraoperative US was used intermittently to assess the patient's vocal cord movement to detect potential recurrent laryngeal nerve (RLN) injury [Citation16]. Post-ablation, contrast-enhanced US was performed to evaluate whether the ablation was complete, and that the range was enough. If the unenhanced zone on the contrast-enhanced US covers the entire tumor, the ablation is considered complete ( and Supplementary Material 1). If the unenhanced zone extends beyond the margins of the tumor for at least 2 mm, the range is considered adequate. If the marginal ablation of the tumor is inadequate, a complementary ablation is performed immediately (). After ablation, all patients were monitored for potential complications associated with the procedure.
Figure 1. A 35-year-old woman with papillary thyroid microcarcinoma in the right lobe was treated with microwave ablation. (A) Pre-ablation, B-mode ultrasound shows hypoechoic target tumor (arrowheads); (B) Pre-ablation, contrast-enhanced ultrasound shows hypo-enhancement pattern (arrowheads); (C) Hydrodissection technique (arrows) was used to protect the vagus nerve and carotid artery surrounding the tumor (arrowheads); (D) Hyperechoic pattern in the tumor (arrowheads) during ablation (arrows); (E) Post-ablation, the CEUS shows no enhancement (arrowheads) in the tumor; (F) One day post-ablation, US shows the hypoechoic ablation zone (arrowheads).
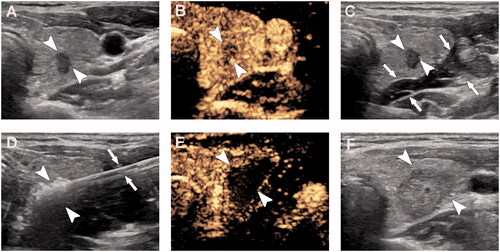
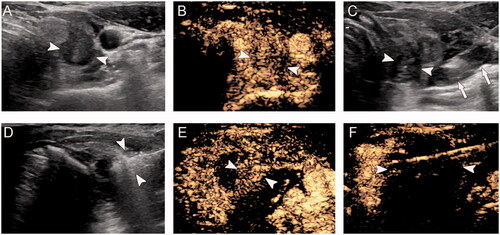
Figure 2. A 40-year-old woman with papillary thyroid microcarcinoma in the right lobe was treated with microwave ablation. Contrast-enhanced ultrasound showed partial ablation of the tumor. Complementary ablation was performed in this patient. (A) Pre-ablation, B-mode ultrasound shows hypoechoic target tumor (arrowheads); (B) Pre-ablation, contrast-enhanced ultrasound shows hypo-enhancement pattern (arrowheads); (C) Hydrodissection technique (arrows) was used to protect the vagus nerve and carotid artery surrounding the tumor (arrowheads); (D) Hyperechoic pattern in the tumor (arrowheads) during ablation; (E) Post-ablation, the CEUS shows partial ablation of the tumor (arrowheads); (F) After complementary ablation, the CEUS shows no enhancement (arrowheads) in the tumor.
Post-ablation assessment and follow-up
Post-ablation, patients were followed up every 3–6 months for the first year and every 6–12 months thereafter. US and blood examination were performed at each follow-up visit, while CT examination was performed every 12 months. Contrast-enhanced US and fine needle aspiration were carried out in patients with suspected tumor recurrence or LNM.
Study definitions
The changes in size of tumor (ablation zone), rate of tumor disappearance, and disease progression reflected the treatment efficacy. Disease progression included local recurrence, new tumor, local LNM, distant metastasis, and mortality from PTMC. The complication rate reflected the treatment safety. Complications were recorded using the standard of “Image-guided Thyroid Ablation: Proposal for Standardization of Terminology and Reporting Criteria” [Citation17].
Statistical analysis
All data were analyzed using the version 26.0 SPSS software. p Values < 0.05 indicated a statistically significant difference. Continuous data are presented as mean ± standard deviation or median, and categorical data are presented as frequency (percentage). Changes in tumor, tumor disappearance, disease progression, and complications were evaluated. Paired t test was used to compare pre- and post-ablation changes in tumor size and volume.
Results
Clinical characteristics of participants
The baseline clinical characteristics of participants disaggregated by the 11 participating centers are listed in . A total of 725 patients (573 women and 152 men) were included in the study. The mean age of patients was 46 ± 11 years (range, 22–81). The mean follow-up time was 21 ± 13 months (range, 6–60). One hundred and ninety-six patients were followed up for 6 months, 291 for 12 months, 126 for 24 months, 87 for 36 months, 24 for 48 months, and one for 60 months. Six hundred and seventy-three (92.8%) patients were treated with single ablation, while 52 (7.2%) patients required complementary ablation owing to insufficient margins after the first ablation.
Table 1. Clinical characteristics of study population disaggregated by the 11 participating centers.
Five hundred and forty-two (74.8%) patients were treated with MWA while 183 (25.2%) patients were treated with RFA. There were no significant differences in age (p = 0.83) or sex (p = 0.12) between the two groups. Moreover, there were also no significant differences in tumor disappearance (p = 0.15), disease progression (p = 0.96), or complication rates (p = 0.96) between the two groups.
Tumor changes
The mean pre-ablation maximum diameter of tumors was 6.4 ± 1.8 mm (range, 2–10). The mean tumor volume was 110.7 ± 90.6 mm3 (range, 4–472). Changes in size of the tumors at pre- and post-ablation are listed in . The maximum diameter and volume of the tumors significantly increased at 1-month and 3-month post-ablation compare to those recorded pre-ablations due to the included safety margin (p < 0.001 for both), and then gradually decreased. The maximum diameter and volume of the tumors at 6-, 12-, 18-, 24-, 36-, and 48-months post-ablation significantly decreased compare to those recorded pre-ablation (p < 0.001 for all).
Table 2. Changes in size of the tumors at pre- and post-ablation at each follow-up time-point.
Tumor disappearance
At the most recent follow-up, 515 (71.0%) patients showed complete disappearance of the PTMC on US examination. Two PTMCs (0.3%) had disappeared in 3 months, 116 (16.0%) in 6 months, 159 (21.9%) in 9 months, 172 (23.7%) in 12 months, 56 (7.7%) in 18 months, and 10 (1.3%) in 24 months. Two hundred and ten (29.0%) PTMCs had not completely disappeared. None of the tumors showed regrowth of the residual ablated lesion during the follow-up period. The disappearance of tumors was related to the follow-up time. In this study, most patients in whom the tumors did not disappear had a relatively short follow-up time. Among these, 11 patients were followed up for less than 6 months, 84 for less than 9 months, 32 for less than 12 months, and 43 for less than 18 months.
Disease progression
Six (0.8%) patients exhibited disease progression at 21 ± 13 months of follow-up period post-ablation. The clinical characteristics of patients who exhibited disease progression are listed in . Five (0.7%) patients exhibited new PTMCs, of which four were in the contralateral thyroid and one was in the ipsilateral thyroid. The mean maximum diameter of new tumors was 4.8 mm (range, 3–6). The mean volume of new tumors was 42 mm3 (range, 9–63). The mean time elapsed from the first ablation to the detection of new tumor was 21 months (range, 11–36). Five patients were subjected to second ablation treatment. One (0.1%) patient exhibited new LNM in level VI of the ipsilateral neck at 12 months post-ablation. This patient was subjected to hemithyroidectomy with neck dissection. At the most recent follow-up, none of the six patients exhibited any signs of disease progression post second treatment. None of the other patients exhibited local tumor recurrence, distant metastasis, or mortality from PTMC during the follow-up period. None of the other patients underwent delayed surgery due to anxiety.
Table 3. Clinical characteristics of patients who exhibited disease progression.
Complications
Nineteen (2.6%) patients exhibited major or minor complications during the follow-up period post-ablation. The major complication was voice hoarseness, and the minor complications were hematoma formation and cough. Fourteen (1.9%) patients developed voice hoarseness, 4 (0.6%) patients developed hematoma, and one (0.1%) patient developed choking cough. The clinical characteristics of patients who exhibited hoarseness post-ablation are listed in . All 14 patients with hoarseness had PTMC located near the posterior capsule or the middle capsule of the thyroid, which was closer to the RLN. Two patients recovered their voice completely within 1 month post-ablation, 4 within 2 months, 4 within 3 months, 1 within 4 months, and 3 within 6 months. All hematomas completely resolved within 2 weeks. The patient who developed cough showed complete recovery within 1 day. None of those complications were life threatening, and all patients recovered from major or minor complications without sequelae. One patient died of myocardial infarction at 6 months post-ablation.
Table 4. Characteristics of patients who developed hoarseness post-ablation.
Discussion
In this multicenter study, we found a high rate of complete tumor loss (71.0%), a low rate of disease progression (0.8%), and a low rate of complications (2.6%) in PTMC patients treated with thermal ablation. We also observed that thermal ablation of PTMC helped alleviate anxiety and reduced the rate of delayed surgery. These results indicate that US-guided thermal ablation is a feasible, effective, and safe therapy for PTMC besides AS and surgery.
AS has been recently proposed as an alternative to immediate surgical resection for PTMC [Citation18,Citation19]. However, delayed surgery is major problem during AS. In previous studies, 13–15.7% patients underwent delayed thyroid surgery due to anxiety, increased tumor size, or cervical LNM during AS period [Citation5,Citation20]. In a meta-analysis of nine studies (n = 4,156), the rate of cervical LNM was 1.0% during AS [Citation21]. Furthermore, previous studies found that younger and male patients may not be good candidates for AS because these patients are more likely to develop tumor volume enlargement and LNM [Citation22]. In our study, no patients delayed surgery due to anxiety, and only 1 patient (0.1%) had delayed surgery due to LNM during post-ablation follow-up. Compared with AS, thermal ablation can eliminate the anxiety, reduce the rate of LNM, and reduce the rate of delayed surgery. Therefore, our results indicated that US-guided thermal ablation was a feasible therapy for PTMC.
Tumor disappearance and disease progression (including LNM and recurrence) reflect the efficacy of thermal ablation in patients with PTMC. According to a meta-analysis of thermal ablation for PTMC, the rate of tumor disappearance ranges from 57.3% to 76.2% post-ablation [Citation12]. In our study, the tumor disappearance rate was 70%, which was similar to previous studies. Further analysis revealed that out of the 210 tumors that did not disappear, 127 were followed up for less than 12 months. The disappearance of tumor was closely related to the follow-up time. Therefore, we speculate that the rate of tumor disappearance will continue to increase with the extension of follow-up time. A meta-analysis revealed that the rate of LNM varies from 0.6% to 2.0% post-ablation, and the local recurrence rate varies from 0.01 to 1.87% [Citation12]. In our study, only one (0.1%) patient developed LNM during follow-up and none of the patients developed local recurrence. Therefore, our results indicated that US-guided thermal ablation was an effective therapy for PTMC.
The rate of complications reflects the safety of thermal ablation for patients with PTMC. Hemithyroidectomy is the most common management option for PTMC. In a study of 340 patients who underwent thyroid surgery, the incidence of transient RLN injury post hemithyroidectomy was 3.2%, while the incidence of permanent RLN injury was 0.3% [Citation23]. In our study, the rate of transient RLN injury was low (1.9%), and none of the patients developed permanent RLN injury. In a previous study of 7123 patients who underwent thyroidectomy, the rate of temporary hypocalcemia post hemithyroidectomy was 12%, while the rate of permanent hypocalcemia was 1.4% [Citation24]. However, none of the patients in our study developed temporary or permanent hypocalcemia. In a previous study, the rate of levothyroxine replacement 6 months after hemithyroidectomy was 84.4% [Citation25]; however, none of the patients in our study required hormone replacement. Compared with hemithyroidectomy, the rate of RLN injury in this study was lower; moreover, the patients' serum calcium levels were not affected and none of the patients required hormone replacement. In previous studies, the overall complication rate post thermal ablation for malignant thyroid nodules ranged from 1% to 6% [Citation12,Citation26,Citation27]. Similar to those reports, the overall complication rate in our study was 2.6%. Therefore, our results indicated that US-guided thermal ablation was a safe therapy for PTMC.
The large number of cases (n = 725) with strict enrollment criteria, the multicenter study design, and medium-term duration of follow-up represent major strengths of this study. To the best of our knowledge, this is currently the largest study of PTMC patients treated with US-guided thermal ablation. A total of eleven representative centers were included in this study, and most centers had relatively long follow-up period, with the longest follow-up period being 60 months.
A few limitations of our study should be acknowledged. The first limitation is the potential heterogeneity among the patients and operator. Some centers had relatively short treatment experience, and some centers enrolled relatively small number of patients, as is often seen in multicenter studies. Secondly, this was a retrospective study; therefore, our results may have been affected by an element of selection bias. Lastly, it was single arm, nonrandomized study lacking a parallel control group and can only be compared with the results of past studies. Therefore, to generalize the outcomes of thermal ablation, a prospective and randomized study comparing thermal ablation and AS or surgery is warranted.
In summary, this large multicenter study demonstrates US-guided thermal ablation is an effective and safe treatment option for patients with PTMC besides AS and surgery.
Disclosure statement
There is no financial or other potential conflict of interest.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Haser GC, Tuttle RM, Su HK, et al. Active surveillance for papillary thyroid microcarcinoma: new challenges and opportunities for the health care system. Endocr Pract. 2016;22(5):602–611.
- Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44(3):307–315.
- Oh HS, Ha J, Kim HI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid. 2018;28(12):1587–1594.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015;18(4):423–430.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J. 2020;9(4):172–185.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol. 2020;11:575152.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Ma B, Wei W, Xu W, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: a retrospective case review and literature review. Thyroid. 2018;28(9):1134–1142.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Kumar A, Sinha C, Kumar A, et al. Assessment of functionality of vocal cords using ultrasound before and after thyroid surgery: an observational study. Indian J Anaesth. 2018;62(8):599–602.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Koshkina A, Fazelzad R, Sugitani I, et al. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(6):552–560.
- Jeon MJ, Lee YM, Sung TY, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: a cross-sectional study. Thyroid. 2019;29(7):956–962.
- Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center's experience in Korea. J Clin Endocrinol Metab. 2017;102(6):1917–1925.
- Saravana-Bawan B, Bajwa A, Paterson J, et al. Active surveillance of low-risk papillary thyroid cancer: a meta-analysis. Surgery. 2020;167(1):46–55.
- Jeon MJ, Kim WG, Chung KW, et al. Active surveillance of papillary thyroid microcarcinoma: where do we stand? Eur Thyroid J. 2019;8(6):298–306.
- Zakaria HM, Al Awad NA, Al Kreedes AS, et al. Recurrent laryngeal nerve injury in thyroid surgery. Oman Med J. 2011;26(1):34–38.
- Vaiman M, Nagibin A, Olevson J. Complications in primary and completed thyroidectomy. Surg Today. 2010;40(2):114–118.
- Sarfati-Lebreton M, Toqué L, Philippe JB, et al. Does hemithyroidectomy still provide any benefit? Ann Endocrinol. 2019;80(2):101–109.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26(3):420–428.
- Lim HK, Cho SJ, Baek JH, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20(12):1653–1661.
