Abstract
Background
Hyperthermia has been reported to cause cancer stage regression, thus providing surgical opportunities in patients with unresectable tumors and improving the quality of life of patients by preserving certain organs.
Methods
A prospective open-label phase II trial was conducted to evaluate the efficacy of hyperthermia combined with induction chemotherapy in patients with locally advanced resectable oral squamous cell carcinoma (OSCC). Patients received hyperthermia combined with two cycles of 5-fluorouracil, cisplatin, and docetaxel (TPF) induction chemotherapy regimens or TPF induction chemotherapy alone, followed by radical surgery with postoperative radiotherapy. The primary endpoint was the clinical response rate of the induction chemotherapy. The secondary endpoints were overall survival (OS), disease-free survival (DFS), and toxicity.
Results
A total of 120 patients were enrolled, and 115 patients were included in the clinical response analysis. The clinical response rate was significantly higher in the experimental arm than in the control arm (65.45% vs. 40.00%, p = 0.0088). There were no unexpected toxicities, and hyperthermia and induction chemotherapy did not increase the perioperative morbidity rate. Moreover, there was a significant improvement in DFS, but no significant difference in OS between the two arms. In the subgroup analysis, increased OS and DFS rates were associated with patients with favorable clinical response after induction chemotherapy in the total population, experimental arm, and control arm.
Conclusions
Our study demonstrates that hyperthermia combined with induction chemotherapy is associated with a high response rate and provides a new treatment option for patients with resectable stage III or IVA OSCC.
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the maxillofacial and oral regions and has the highest prevalence in developing countries [Citation1]. Although substantial progress has been achieved in recent decades, the prognosis of OSCC patients remains poor, with approximately a 50% 5-year survival rate, and even poorer clinical outcomes in patients with locally/regionally advanced lesions [Citation2].
Induction chemotherapy is considered a possible strategy for shrinking or downstaging locally advanced head and neck cancers (including OSCC), enhancing organ preservation rates, and/or lowering the risks of distant and/or local recurrence, and eventually improving treatment outcomes [Citation3]. Induction chemotherapy combined with 5-fluorouracil, cisplatin, and docetaxel (TPF) has been suggested as the standard combination chemotherapeutic regimen for palliative treatment or the induction of patients with advanced OSCC [Citation4]. Nevertheless, there was no statistical evidence of improvement in recurrence-free survival, distant failure-free survival, or three-year overall survival in the PARADIGM and DeCIDE trials [Citation5,Citation6]. Compared with upfront surgery in patients with resectable stage III or IVA OSCC, there was also no demonstrable improvement in overall survival (OS) or disease-free survival (DFS) [Citation7]. Since these studies failed to show significant improvements in OS or DFS among patients receiving TPF induction chemotherapy, the efficacy of TPF induction chemotherapy has been questioned.
Hyperthermia is used clinically as a local therapeutic strategy in OSCC patients [Citation8]. Hyperthermia may enhance the treatment efficacy of chemotherapy and radiation therapy in various solid tumors by increasing tumor penetration of anticancer drugs and by synergistically enhancing cytotoxicity [Citation9]. Our previous study demonstrated that an ultrasound hyperthermia heating temperature of 39 °C to 45 °C and a heating time of 30–45 min yielded the best efficacy for OSCC [Citation10]. However, the OS and DFS after hyperthermia therapy have never been studied in OSCC patients, and the long-term curative effect remains unknown.
We hypothesized that hyperthermia combined with TPF administered preoperatively would improve the clinical response rate among patients with resectable locally advanced OSCC. To confirm this hypothesis, we conducted a randomized phase II trial by combining TPF induction chemotherapy with subsequent surgery and postoperative radiotherapy, and compared these with traditional induction chemotherapy with TPF followed by postoperative surgery and radiotherapy in the patient population.
Materials and methods
Study design
The present prospective multicenter open random phase II trial (ChiCTR1800014391) was conducted at three hospitals in China. The study protocol was approved by the institutional ethics committee of the Ninth People’s Hospital affiliated with the Shanghai Jiao Tong University School of Medicine and the regional ethics committees of all participating hospitals. Written informed consent was obtained from all participants and was approved by the institutional review board of each center.
Eligibility criteria
Patients aged between 18–75 years with histologically confirmed OSCC were eligible. According to the surgeon’s judgment, patients needed to have resectable stage III and IVA disease (T1-3N1M0 or T1-3N2M0 or T4aN0-2M0 based on the Union for International Cancer Control system, 2002). The other inclusion criteria were: serum creatinine and bilirubin levels <1.5× the normal upper limit, aspartate aminotransferase and alanine aminotransferase levels <2.5× the normal upper limit, platelet count >80,000/µL, hemoglobin concentration >8 g/L, white blood cell count >3,000/µL, Karnofsky performance status >60%, Eastern Cooperative Oncology Group performance status score of 0–2, no prior surgery, and radiation therapy or chemotherapy for OSCC. Patients were excluded if they had other cancers or distant metastases if they had undergone surgery involving lymph nodes or primary tumors (except biopsy for diagnosis) if they had received prior chemotherapy or radiotherapy, if they had a creatinine clearance less than 30 ml/min, or if they had other malignant tumors within 5 years.
Treatment
On the basis of a computer-generated number list, eligible participants were randomly assigned to the center of central data collection before the treatment was initiated. According to the results of randomization, the participants were (1:1) allocated to receive therapy with neoadjuvant therapy and chemotherapy with TPF and hyperthermia or TPF neoadjuvant chemotherapy alone, followed by surgery and postoperative radiotherapy. Chemotherapy comprised 75 mg/m2 cisplatin and 75 mg/m2 docetaxel administered intravenously on the first day and 750 mg/m2 fluorouracil every day as a 120-hintravenous, continuous infusion from day 1 to day 5. Induction chemotherapy was performed every 3 wks for 2 cycles unless unacceptable toxicity or disease progression occurred. Surgery was performed 2 wks after the induction chemotherapy was completed by the patients. The safety margins of the primary lesions were 1.5 cm away from the palpable margins. Adequate margins were confirmed by performing frozen sections intraoperatively.
Postoperative radiotherapy was started 4–6 wks after surgery. Standardized intensity-modulated radiotherapy or conformal radiotherapy was allowed at a dose of 1.8–2 Gy per day, 5 days per week for 6 wks (54–60 Gy in total). For patients with high-risk characteristics, including vascular embolism, extracapsular nodal spread, or positive surgical margins, a total radiation dose of 66 Gy was administered. Concurrent chemotherapy was administered to patients with positive margins and/or extracapsular extension. Cisplatin was administered every 3 wks at a dose of 75 mg/m2 upon creatinine clearance. Nevertheless, the doses would be delayed if there was evidence of ototoxicity, neurotoxicity, renal toxicity, or dehydration. Radiation therapy was delayed in patients with grade 2–4 dysphagia or mucositis until they recovered to toxicity grades less than 2. The patients could be withdrawn from the study treatment and evaluation at any stage of the study for the following reasons: voluntary withdrawal, death, loss of follow-up, any safety reasons (adverse events [AEs]) considered by the researchers, and poor compliance with the study protocol.
Hyperthermia
Patients were treated in the supine position using an ultrasound thermotherapy system developed by Shanghai Med-X Engineering Center for Medical Equipment and Technology affiliated to Shanghai Jiao Tong University (). Local hyperthermia was administered on days 1, 3, 5, 7, and 9, concurrent with the TPF induction chemotherapy regimens. Hyperthermia administration was conducted with cis-platinum infusion on day 1, after docetaxel infusion, and prior to 5-fluorouracil, and was performed with 5-fluorouracil on day 3 and day 5. For a proper heat-up process, the ultrasound applicator should be in close contact with the region to be heated. The thermotherapy system was adopted to treat locally advanced primary tumors, whereas disseminated lymph nodes were excluded from the hyperthermia treatment.
Figure 1. (A) Ultrasonic thermal therapy system; (B) Representative CT scan of OSCC patients pre- and post-hyperthermia therapy; (C) Representative section of hematoxylin and eosin staining post-hyperthermia therapy in OSCC patients.
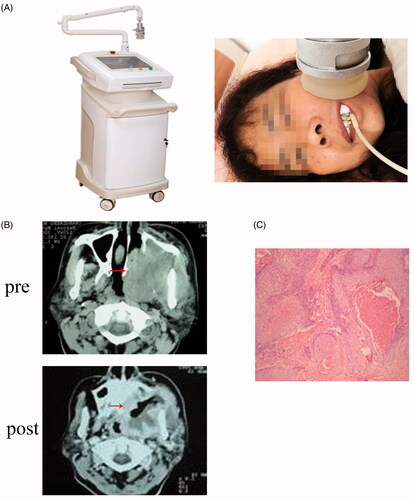
The ultrasounds were produced from four square transducers (PZT-5) with dimensions of 32 × 32 mm. There were two resonant frequencies, 1 MHz and 3.5 MHz, on the system for different objects to be treated. The amplified signal that feeds the transducer was generated by a frequency generator and amplified by a syntonic LC circuit (composed of a capacitor C and an inductor L).
The output signal of the amplifier was matched to the transducer by a matching network. A needle sensor implanted in the tumor was utilized to test the true temperature of the treatment target so that the power of the ultrasound transducers could be correctly controlled.
Movement of the treatment in the x, y, or z directions was executed by a three-dimensional (3 D) movement mechanical system. A plastic membrane covered the treatment head such that the liquid could be infused into the head. An ultrasound hyperthermia system, including the target location, ultrasound dose, frequency, and temperature control, could be implemented by the investigators and engineers from Shanghai Med-X Engineering Center for Medical Equipment and Technology using the supporting software.
In this study, the output frequency and power of the system were set to 1 MHz and 25 W, respectively. The output voltage of the controllable switching power supply was 33 V. The voltage control range of the switching power supply was 0–45 V. The actual output voltage of the switching power supply was controlled through communication with the switching power supply. The output voltage of the controllable switching power supply acted on the source end of the metal-oxide-semiconductor field-effect transistor (MOSFET), which generated a sine wave through the half-bridge resonant circuit to drive the transducer. In the participating centers, the treatment temperature was set at 41 ± 1 °C and maintained for 30 min. The upper limit of the temperature was set at 44 °C to prevent overheating.
Patient assessment
A complete medical history was obtained from each participant, and a baseline assessment of the tumor was performed. Imaging studies and clinical assessments were utilized to evaluate the response of the clinical tumor (executed at the baseline and 2 wks after two cycles of induction chemotherapy). The responses were characterized according to the Response Evaluation Criteria in Solid Tumors, version 1.0 [Citation11]. Toxicity was assessed weekly during and after the completion of radiotherapy and induction chemotherapy in accordance with the Common Terminology Criteria for AEs (version 3.0).
Statistical considerations
The size of the sample was calculated to test the differences in the rates of remission among the groups, assuming a clinical response rate of 23.33% for the chemotherapy-only group and 51.85% for the hyperthermia + chemotherapy group based on the results of previous studies [Citation12]. Fisher’s exact test with a 0.05 two-sided significance level would have 80% power if the sample size in each group was 46, with type I and II error rates set to 0.05 and 0.2, respectively. Allowing for a 20% dropout rate, the number of enrolled patients was determined to be 60 patients for each group.
The key endpoint was the response to induction chemotherapy. Toxicity, DFS, and OS were included as the secondary endpoints. Categorical data are shown as percentages and numbers for the descriptive analysis, and the analysis of survival was performed using the log-rank test and the Kaplan–Meier method. Hazard ratios (HRs) were determined using Cox proportional hazards analysis. The rank correlation coefficient of Spearman’s test was used to compare the relationship between clinical and pathological responses and baseline factors. Efficacy was analyzed using the intention-to-treat principle. All patients who underwent treatment were included in the studies of AEs. The significance level of all hypothesis-generating tests was set at <0.05.
Results
Patients
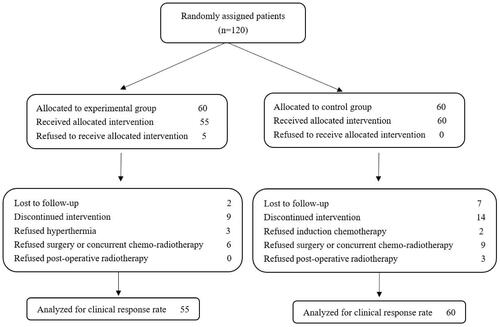
From February 2015 to December 2017, 120 eligible patients were randomly assigned to two study arms (60 patients in each arm). shows the baseline demographic and clinical characteristics of the patients. After random assignment, five patients withdrew, and 46 patients did not complete the full treatment protocol (). Eight patients withdrew during the follow-up stage. The median follow-up time was 35 months.
Table 1. Baseline patient demographic and clinical characteristics.
Treatment characteristics
The target thermal dose was to reach a temperature between 40 °C and 42 °C for approximately 30 min. Thermometry was conducted every time hyperthermia was administered; ten hyperthermic fields per patient and a total of 600 hyperthermic fields were applied. Of these fields, six had a treatment temperature of less than 40.0 °C, 13 had a treatment temperature between 42 °C and 44 °C, and the other 581 had a treatment temperature between 40.0 °C and 42 °C. The minimum hyperthermia temperature was 38.0 °C, and the maximum was 44 °C. The average duration of hyperthermia was 28.71 min. In the experimental arm, chemotherapy dose reductions were implemented in seven patients. In the control arm, chemotherapy dose reductions were implemented in four patients. lists the pathological stage and tumor characteristics.
Response to induction chemotherapy
A total of 115 patients were evaluated for their clinical response after two treatment cycles. A significant improvement in the clinical response rate was observed in the experimental arm compared to the control arm (65.45% vs. 40.00%, χ2 = 7.45, p = 0.0088), and the primary endpoint was reached. In the hyperthermia arm, eight (14.5%) patients achieved a complete response (CR), and 28 (50.9%) patients achieved a partial response (PR). In the control arm, no (0%) patients achieved CR, and 24 (40%) patients achieved PR. No patients died as a result of nontreatment or non-cancer-related causes during induction chemotherapy ().
Table 2. Clinical response of hyperthermia.
OS and DFS
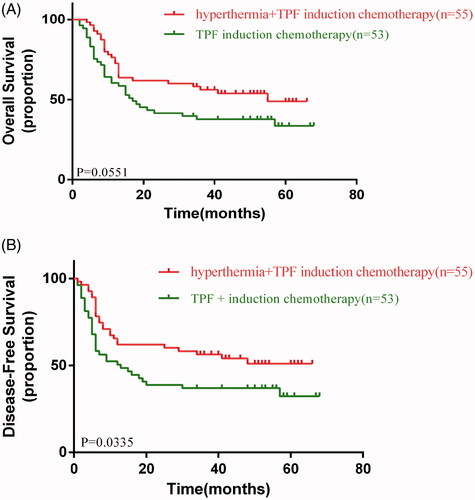
In the data analysis, 48 patients died (34 and 26 in the control and experimental arms, respectively). There were no significant differences in OS between the two arms (HR, 0.6022; 95% confidence interval [CI], 0.3587–1.011; p = 0.0551) (). Nevertheless, there was a significant improvement in DFS between the two arms (HR, 0.5671; 95% CI, 0.3362–0.9565; p = 0.0335) ().
Adverse events
Our results revealed that there were no significant differences in the incidence of AEs between the two arms. The most frequent AEs were hematologic toxicity (41.67% in hyperthermia arm vs. 61.67% in control arm), diarrhea (23.33% in hyperthermia arm vs. 26.67% in control arm), abnormal liver enzyme levels (21.67% in hyperthermia arm vs. 18.33% in control arm), febrile neutropenia (5% in hyperthermia arm vs. 3.33% in control arm), and nausea and/or vomiting (13.33% in hyperthermia arm vs. 10% in control arm). The most frequent AEs with grade 3–4 hematologic toxicity were hematologic toxicity (8.33% in hyperthermia arm vs. 3.33% in control arm), oral mucositis (10% in hyperthermia arm vs. 6.67% in control arm), and dysphagia (8.33% in hyperthermia arm vs. 11.67% in control arm). Scalded skin was the only complication specific to the hyperthermia group and exerted a rare incidence without III-IV adverse events (n = 2, 3.33%). Induction chemotherapy did not improve the postoperative morbidity rate. Moreover, no radiotherapy-, surgery-, or chemotherapy-related deaths occurred ().
Table 3. Toxicity.
Subgroup analysis
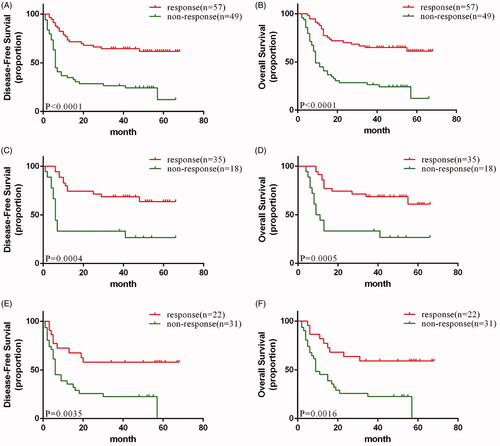
In the subgroup analysis, enrolled patients with a favorable clinical response (PR + CR) after induction chemotherapy showed a significant association with both increased OS (HR, 0.2473; 95% CI, 0.1423–0.4297; p < 0.0001) () and DFS (HR, 0.2593; 95% CI, 0.1493–0.4502; p < 0.0001) () in the total population. We also observed that a favorable clinical response was positively correlated with increased OS () and DFS () in both the experimental and control arms.
Figure 4. (A) disease-free and (B) Overall survival of response and non-response patients in total population; (C) disease-free and (D) Overall survival of response and non-response patients in experimental arm; (E) disease-free and (F) Overall survival of response and non-response patients in control arm.
Discussion
Combinations of chemotherapy and hyperthermia has been evaluated in two phase III trials. Issels et al. have added regional hyperthermia to neoadjuvant chemotherapy (ifosfamide, doxorubicin, etoposide) of high-risk soft tissue sarcomas [Citation18,Citation19]. Another ongoing phase III trial, regional hyperthermia is combined with adjuvant chemotherapy (gemcitabine and cisplatin versus gemcitabine, cisplatin and regional hyperthermia) in patients with resectable pancreatic cancers following R0 or R1 resection [Citation25]. To date, no randomized study has focused on hyperthermia combined with chemotherapy in OSCC patients. The present study was conducted to investigate the efficacy of local hyperthermia concurrent with traditional chemotherapy as a phase II randomized trial in OSCC patients.
Our study is the first prospective, randomized phase II study of hyperthermia plus TPF induction chemotherapy followed by surgery with postoperative radiotherapy or concurrent chemoradiotherapy compared to conventional induction chemotherapy with TPF followed by surgery with postoperative radiotherapy or concurrent chemoradiotherapy in patients with resectable OSCC. A significantly elevated clinical response rate was observed in the hyperthermia arm than in the control arm, and the primary endpoint was reached.
Since the biological justification of hyperthermia has been demonstrated, it has been applied in clinics. Similar to other forms of anticancer therapies (such as chemotherapy and radiotherapy), acute toxicity of hyperthermia is rare and late toxicity is unknown, in contrast with radiotherapy and chemotherapy, both of which have well-known and non-negligible toxicity spectra [Citation13]. It is one of the most potent radiosensitizers and displays thermal synergism with some chemotherapy agents [Citation14]. According to our data, the most frequent complication caused by hyperthermia was scalded skin; however, the incidence was rather low (3.33%), and no increase of grade II–IV AEs was observed. There were also no unexpected toxicities, and hyperthermia plus TPF induction chemotherapy did not increase the perioperative morbidity rate. Thus, hyperthermia is a well-tolerated therapeutic modality for OSCC.
The clinical outcomes of OSCC remain poor, and even with a great variety of chemotherapy and radiotherapy schedules, targeted patient and agent outlooks continue to be poor [Citation22,Citation23]. Previous studies failed to prove that induction chemotherapy with TPF could enhance survival compared to upfront surgery in patients with resectable stage III or IVA OSCC [Citation7]. Nevertheless, hyperthermia is a distinctive therapeutic modality and has been shown to enhance therapeutic outcomes in a wider range of malignancies, including locally advanced cervical cancer, regionally recurrent breast cancer, melanoma, and soft tissue sarcoma among others [Citation15,Citation16]. Moreover, hyperthermia appears to be a potential immunomodulating agent when utilized with radiotherapy [Citation17]. Issels et al. showed that patients with localized, high-risk soft tissue sarcoma assigned to neoadjuvant chemotherapy plus hyperthermia had increased survival than did those treated with neoadjuvant chemotherapy alone [Citation18,Citation19], indicating that hyperthermia had promising applications in neoadjuvant chemotherapy. Datta et al. confirmed that hyperthermia plus radiotherapy increased clinical response rate in HNSCC compared to radiotherapy alone, demonstrating that hyperthermia was an available therapeutic option in HNSCC patients. The researchers also analyzed several HT techniques that had been applied for HNSCC, such as capacitive heating techniques, microwaves, and radiofrequency techniques, with an enhanced clinical response rate, varying in degrees [Citation20]. The ultrasound technique could penetrate deeper inside the tissue and achieve more accurate temperature control than the above techniques [Citation21]. Here, we applied ultrasound technology to hyperthermia therapy. Our data revealed that the DFS was improved by ultrasound hyperthermia plus TPF induction chemotherapy, whereas the OS showed no significant difference when compared to induction chemotherapy, indicating that ultrasound hyperthermia plus TPF induction chemotherapy might also have an encouraging long-term effect. Therefore, OSCC is an ideal disease in which ultrasound hyperthermia can be combined with induction chemotherapy.
Most patients diagnosed with OSCC present with locally advanced disease (accompanied by synchronous lymph node metastasis) [Citation24]. Hyperthermia has been confirmed to shrink the tumor size, degrade the tumor stage, and create conditions for surgical resection. In the present study, we also showed that hyperthermia combined with TPF induction chemotherapy yielded a higher clinical response rate than TPF induction chemotherapy alone. This presents a possible utility of ultrasound hyperthermia in unresectable OSCC patients to control the tumor size and prolong survival time.
A previous study reported that stage III or IVA resectable OSCC patients who experienced better clinical response to TPF induction chemotherapy had good distant, locoregional, and OS control [Citation7]. Consistent with the previous study, we observed that a favorable clinical response after induction chemotherapy was significantly associated with increased OS and DFS in the total population, experimental arm and control arm, suggesting that a favorable clinical response was a therapeutic predictor of hyperthermia or induction chemotherapy.
In the present phase II randomized study, hyperthermia combined with TPF induction chemotherapy provided a potentially useful therapeutic option for OSCC patients. However, the evidence is not strong enough because of the limited sample size and insufficient follow-up time. Furthermore, since ultrasound hyperthermia treatment was performed with short heating-times of 30 min on days 1, 3, 5, 7, and 9, with moderate adjusted temperatures around 41 °C and assured strong synergism only for day 1, non-thermal effects may also play a role here. The local effect can only be anticipated with the combination of chemotherapy and hyperthermia. Based on the thermobiological basis and potentiation of hyperthermia with induction chemotherapy, the outcomes are expected to favor a combination of hyperthermia and induction chemotherapy. This could pave the way for a future phase III clinical trial of hyperthermia to validate the long-term effect of induction therapy among OSCC patients.
Author contributions
Study concept and design: WG and JM. Acquisition and interpretation of the data: GXR, HYJ, YTW, HS, XHM, YH, YZC, WLQ MHG AND QWZ. Drafting of the manuscript: HYJ. Statistical analysis: GXR. Critical revision of the manuscript for important intellectual content: YZC and WLQ. Obtained funding: WG and JM.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China), and informed consent was obtained from all patients (ChiCTR1800014391). The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Consent for publication was obtained from all authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Sun Y, Zhang Y, Liu L, et al. Genetic polymorphisms and HPV infection in oral squamous cell carcinomas. Curr Opin Virol. 2015;14:1–6.
- Wiegand S, Wichmann G, Dietz A. Perspectives of induction with chemo and/or immune check point inhibition in head and neck organ preservation treatment. Front Oncol. 2019;9:191.
- Ghi MG, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol. 2017;28:2206–2212.
- Cohen EE, Karrison T, Kocherginsky M, et al. DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2012(15_suppl)30:5500–5500.
- Haddad RI, Rabinowits G, Tishler RB, et al. The RARADIGM trial: a phase III study comparing sequential therapy (ST) to concurrent chemoradiotherapy (CRT) in locally advanced head and neck cancer (LAHNC). J Clin Oncol. 2012;30(15_suppl):5501–5501.
- Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31(6):744–751.
- Moniruzzaman R, Rehman MU, Zhao QL, et al. Combination of 5-aminosalicylic acid and hyperthermia synergistically enhances apoptotic cell death in HSC-3 cells due to intracellular nitric oxide/peroxynitrite generation. Cancer Lett. 2019;451:58–67.
- Sanz B, Calatayud MP, Torres TE, et al. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials. 2017;114:62–70.
- Ju H, Mao L, Zhang L, et al. Ultrasound hyperthermia enhances chemo-sensitivity in oral squamous cell carcinoma by TRIF-mediated pathway. J Oral Pathol Med. 2018;47(10):964–971.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000(3)92:205–216.
- Ren GX, Shen GF, Chen YZ, et al. A phase I/II study of ultrasound hyperthermia combined with chemotherapy in advanced oral and maxillofacial-head and neck carcinoma. Chin Clin Oncol. 2011;16(03):244–248. (In Chinese)
- Datta NR, Pestalozzi B, Clavien PA, et al. "HEATPAC" – a phase II randomized study of concurrent thermochemoradiotherapy versus chemoradiotherapy alone in locally advanced pancreatic cancer. Radiat Oncol. 2017;12(1):183.
- Gresham GK, Wells GA, Gill S, et al. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer. 2014;14:471.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Datta NR, Rogers S, Klingbiel D, et al. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: a systematic review with conventional and network meta-analyses. Int J Hyperth. 2016;32(7):809–821.
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–542.
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492.
- Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–570.
- Datta NR, Rogers S, Ordonez SG, et al. Hyperthermia and radiotherapy in the management of head and neck cancers: a systematic review and meta-analysis. Int J Hyperth. 2016;32(1):31–40.
- Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clinical cancer research: an official journal of the American Association for. Clin Cancer Res. 2007;13(12):3482–3489.
- Yin J, Jung JE, Choi SI, et al. Inhibition of BMP signaling overcomes acquired resistance to cetuximab in oral squamous cell carcinomas. Cancer Lett. 2018;414:181–189.
- Ozawa H, Ranaweera RS, Izumchenko E, et al. SMAD4 loss is associated with cetuximab resistance and induction of MAPK/JNK activation in head and neck cancer cells. Clin Cancer Res. 2017;23(17):5162–5175.
- Freier K, Engel M, Lindel K, et al. Neoadjuvant concurrent radiochemotherapy followed by surgery in advanced oral squamous cell carcinoma (OSCC): a retrospective analysis of 207 patients. Oral Oncol. 2008;44(2):116–123.
- Tschoep-Lechner KE, Milani V, Berger F, et al. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29(1):8–16.