Abstract
Introduction
Hepatocellular carcinoma (HCC) patients with microvascular invasion (MVI) have worse survival. Whether the presence of MVI indicates the necessity of more aggressive locoregional treatments for recurrences remains to be elucidated.
Methods
We reviewed patients who underwent curative hepatectomy for primary HCC in our institution, and 379 patients with recurrent HCC up to three nodules smaller than 3 cm were enrolled. The Kaplan–Meier method was adopted to compare the secondary recurrence-free survival (sRFS) and post-recurrence survival (PRS) among patients undergoing hepatectomy, RFA and transarterial chemoembolization plus RFA (TACE-RFA). Cox regression analyses were performed to identify independent prognostic factors.
Results
Both the sRFS and PRS of the MVI (−) group were significantly longer than those of the MVI (+) group (p = 0.001 and 0.011). For patients with MVI (−), no significant difference was found in sRFS or PRS among recurrent HCC patients receiving hepatectomy, RFA or TACE-RFA (p = 0.149 and 0.821). A similar trend was found in patients with MVI (+) (p = 0.851 and 0.960). Further analysis found that TACE-RFA provided better sRFS than hepatectomy or RFA alone in patients with MVI (+) and early recurrence within two years (p = 0.036 and 0.044).
Conclusion
For HCC patients with MVI (+) and early small recurrence, TACE-RFA could achieve better prognosis than hepatectomy or RFA alone, while RFA alone provided comparable survival benefits compared with hepatectomy or TACE-RFA in other HCC patients with small recurrence.
Introduction
Hepatocellular carcinoma (HCC), one of the most prevalent malignancies, ranks as the third leading cause of cancer-related mortality worldwide [Citation1]. With improvements in surveillance for high-risk population and advances in surgical techniques, the number of HCC patients suitable for hepatectomy has been increasing [Citation2]. But approximately 70% of HCC patients experience recurrence within five years after curative resection [Citation3,Citation4]. Appropriate radical treatments against recurrences can improve the long-term survival of HCC patients. Due to improvements in imaging techniques and postoperative follow-up, increasing numbers of HCC patients are detected with early-stage recurrences.
For patients with recurrent HCC after hepatectomy, reresection can be unsuitable considering the potential hepatic dysfunction and insufficient remnant liver [Citation5,Citation6]. Radiofrequency ablation (RFA), a minimally invasive and cost-effective modality, is as effective as hepatectomy in HCCs up to 3 cm [Citation7]. Incomplete ablation is an unfavorable prognostic factor in HCC patients receiving RFA. As reported, the combination of transarterial chemoembolization (TACE) and RFA (TACE-RFA) can increase the success rate of RFA by decreasing blood flow and minimizing heat loss [Citation8,Citation9]. Even for medium-sized HCC, TACE-RFA can make similar survival benefits similar to those of hepatectomy [Citation10]. Nonetheless, studies focusing on TACE-RFA for recurrent HCCs are lacking. In addition, the consistency of patients is poor in studies that have compared different treatments for recurrent HCCs, and many studies have compared curative treatments with TACE alone for early-stage recurrences, which might result in biases in patient selection [Citation11,Citation12].
Microvascular invasion (MVI) usually indicates a high risk of recurrence and poor survival [Citation13,Citation14]. Recent studies mainly focus on improving postoperative survival by adopting adjuvant therapies including TACE or sorafenib in HCC patients, but whether the status of MVI can guide the treatments for recurrences remains to be elucidated [Citation15]. Considering that MVI is a poor prognostic factor, whether more aggressive treatments should be adopted for recurrent HCCs in patients with MVI has not been explored, especially for those with HCC that recurs within two years after initial curative hepatectomy, as these HCCs are considered to originate from residual microscopic lesions[Citation16].
This study aimed to compare the survival benefits of hepatectomy, RFA and TACE-RFA in patients with recurrent HCC up to three nodules smaller than 3 cm, according to MVI status confirmed by surgical specimens from the primary HCC. These results can help clarify whether treatment modalities for recurrent HCC should be selected based on MVI status, especially for those with early recurrent HCCs.
Methods
Patients
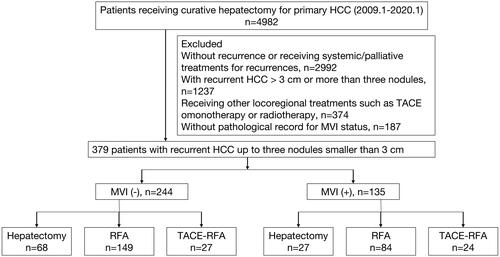
We reviewed the clinical data of HCC patients consecutively at our institution from January 2009 to January 2020. During this period, 4982 patients received curative hepatectomy for primary HCC. The inclusion criteria were as follows: (1) age at diagnosis ≥18 years; (2) pathologically diagnosed as HCC and (3) receiving R0 hepatic resection for primary HCC. The exclusion criteria were as follows: (1) no recurrence at the last follow-up; (2) receiving systemic or palliative treatments for recurrent HCC; (3) with recurrent HCC larger than 3 cm or more than three nodules; (4) receiving other locoregional treatments such as TACE monotherapy or radiotherapy; and (5) no pathological record of MVI status. Eventually, we enrolled 379 HCC patients with recurrences up to three nodules smaller than 3 cm. The patient enrollment flowchart is presented in . Among these patients, 64.4% (244/379) were negative for MVI and 35.6% (135/379) were positive for MVI. The diagnosis of recurrent HCC was based on the criteria of the European Association for the Study of the Liver [Citation2]. Hepatectomy, RFA, and TACE-RFA were described in our previous studies [Citation10,Citation17]. In the TACE-RAF group, conventional TACE was performed in all the patients, and then RFA was performed within 2–4 weeks after TACE. To achieve complete ablation, we ensured a safety margin ≥5 mm during RFA procedure, which was previously described in detail [Citation18]. This study was approved by the Ethical Committee of Sun Yat-Sen University Cancer Center.
MVI diagnosis
The diagnosis of MVI was based on the pathology reports of the surgical specimens, which were independently reviewed by two experienced pathologists. All specimens were cut along the maximal cross section of HCC with sectioning every 1 cm. Tissues at the transition region between the HCC and HCC-adjacent tissues were sampled at the 12, 3, 6, and 9 o’ clock positions separately. MVI was diagnosed as positive when tumor cells were present within a vascular space lined by the endothelium of the surrounding hepatic tissues by microscopy [Citation19]. The MVI grade was assessed according to the pathological diagnosis of HCC of the Liver Cancer Pathology Group of China [Citation20].
Statistical analysis
The Chi-square test was adopted to assess the demographic and clinicopathological characteristics among groups undergoing hepatectomy, RFA or TACE-RFA for recurrent HCC. Fisher exact test was performed when the expected frequency was less than five in one or more table cells. Univariate and multivariate Cox regression analyses were used to identify independent prognostic factors for secondary recurrence-free survival (sRFS) and post-recurrence survival (PRS), including sex, age at diagnosis, hepatitis B virus (HBV) infection, albumin–bilirubin (ALBI) grade, serum alpha-fetoprotein (AFP) level, MVI status, number and size of recurrent HCC, BCLC stage of primary HCC, and time to recurrence after initial hepatectomy (TTR). Only statistically significant variables in the univariate analyses were included in the multivariate analyses. The Kaplan–Meier method with the log-rank test was adopted to compare the sRFS and PRS among subgroups with different treatment modalities for recurrences in the whole cohort and subgroups classified by MVI status and TTR. The sRFS was defined as the time interval from the date of treatments for recurrent HCCs to the date of first detection of recurrences on imaging or death. The PRS was defined as the period from the date of treatments for recurrences to the date of death or the last follow-up. A two-tailed p value less than 0.05 was considered statistically significant. All the statistical analyses were performed with R software version 3.6.1 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients
The baseline clinical characteristics of recurrent HCC patients are listed in . Patients were further classified into three groups according to the treatment modalities for recurrent HCC. Ninety-five patients underwent hepatectomy, 233 patients received RFA and 51 patients underwent TACE-RFA. The median follow-up time after treatments for recurrences was 30.61 months. No significant difference was found among the three groups in terms of sex (p = 0.169). Patients receiving hepatectomy were younger than those receiving RFA or TACE-RFA. Most patients in each group were infected with HBV. The RFA and TACE-RFA groups had higher proportion of patients with ALBI grade II compared with the hepatectomy group (p < 0.001). The serum AFP level was similar among groups with different treatment modalities (p = 0.705). In terms of MVI, the TACE-RFA group had the highest proportion of positive MVI in primary HCC while the proportion of positive MVI was lowest in the hepatectomy group. The status of MVI did not differ significantly among the three groups (p = 0.081). As for characteristics of recurrent HCCs, the tumor size was similar among the hepatectomy, RFA and TACE-RFA groups (p = 0.638), while the TACE-RFA group had higher proportion of multiple HCCs. For the BCLC stage of primary HCC, most patients had primary HCC at BCLC 0-A stage in the hepatectomy group and the TACE-RFA groups had the highest proportion of patients with primary HCC at BCLC B stage (p = 0.018). Higher proportions of patients in the hepatectomy and RFA group had TTR longer than 2 years. In contrast, more than half of patients had TTR less than 2 years in the TACE-RFA group. A significant difference was found among different treatment groups for TTR (p < 0.001). Compared with patients with MVI (−), more patients had TTR ≤2 years in the MVI (+) group (p = 0.010).
Table 1. Baseline characteristics of recurrent HCC patients receiving different treatments.
Survival outcomes
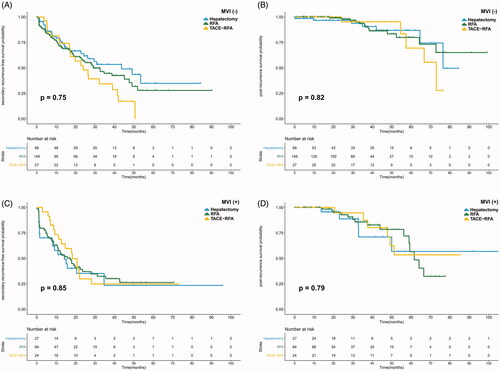
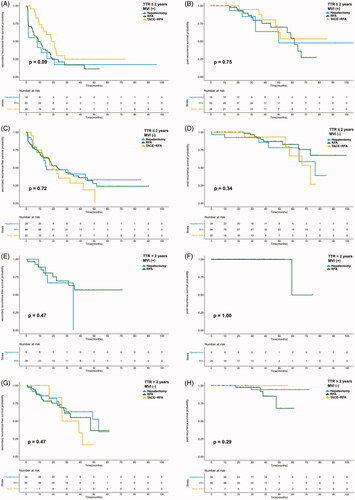
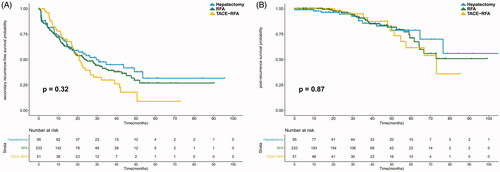
The median sRFS and PRS were 23.60 and 76.45 months, respectively. The median sRFS was 30.87 and 15.77 months in the MVI (−) and MVI (+) groups (p = 0.001). The PRS of the MVI (−) group was significantly longer than that of the MVI (+) group (p = 0.011). Both the sRFS and PRS were similar among the hepatectomy, RFA, and TACE-RFA groups (p = 0.323 and 0.868) (shown in ). For patients with MVI (−), no significant difference was found in sRFS or PRS among the three treatment groups (p = 0.149 and 0.821). Similarly, the sRFS and PRS were comparable among the different treatment groups in patients with MVI (+) (p = 0.851 and 0.960). The survival curves are plotted in . This study further compared the sRFS and PRS of recurrent patients receiving hepatectomy, RFA or TACE-RFA in subgroups classified by TTR and MVI status considering that early recurrent HCCs mainly originate from residual primary HCCs. For patients with MVI (+) and TTR ≤ 2 years, TACE-RFA provided better sRFS than hepatectomy or RFA alone (p = 0.036 and 0.044). No significant difference was found in the other subgroups among HCC patients receiving different treatment modalities. Detailed survival curves are presented in .
Figure 2. The survival curves of recurrent HCC patients receiving hepatectomy, RFA or TACE-RFA. No significant difference was found in sRFS (A) or PRS (B) among the different treatment groups.
Univariate and multivariate analyses
The results of the univariate and multivariate analyses for sRFS and PRS are shown in . For sRFS, MVI status, tumor number, BCLC stage of primary HCC, and TTR showed significant correlation with secondary recurrence after curative treatments in the univariate analyses (p = 0.001, <0.001, 0.001, and <0.001). The multivariate analyses showed that MVI status, tumor number, and TTR were independent prognostic factors for sRFS (p = 0.009, <0.001, and <0.001). In terms of PRS, only MVI status and TTR showed significant impact on survival in the univariate analyses (p = 0.013 and 0.024), which remained statistically significant in the multivariate analyses. To explore whether prognostic factors were different in subgroups with different MVI statuses, this study also identified independent prognostic factors in the MVI (−) and MVI (+) groups. In MVI (−) group, both tumor number and TTR showed significant association with sRFS (p < 0.001 and =0.003). For PRS, no independent factor was found in this study. In the MVI (+) group, BCLC stage of primary HCC and TTR correlated to sRFS significantly in the univariate analyses (p = 0.045 and <0.001), while no significant factor was identified for PRS. Detailed data are shown in Supplementary Table 1.
Table 2. Univariate and multivariate analyses of prognostic factors for post-recurrence survival (PRS) and secondary recurrence-free survival (sRFS).
Treatment complications
The major complications after each treatment are presented in Supplementary Table 2. No treatment-related death occurred in this study. Although the hepatectomy group had higher incidences of complications, no significant difference was found among the hepatectomy, RFA and TACE-RFA groups.
Discussion
In this study, we found that TACE-RFA could achieve better prognosis than hepatectomy or RFA alone for recurrent HCCs up to three nodules smaller than 3 cm in patients with MVI (+) and TTR ≤ 2 years. For other patients with early recurrent small HCCs, RFA alone provided comparable survival benefits to hepatectomy or TACE-RFA. These results can assist in formulating the optimal treatments for recurrent HCCs, which can simultaneously ensure the treatment efficiency and reduce the damage to liver function.
MVI, which is reported to be a poor prognostic factor for HCC, indicates poorer differentiation, higher rates of recurrence and worse survival [Citation13,Citation14]. The guiding value of MVI in the treatment selection for recurrent HCC, however, had rarely been discussed, especially for recurrent HCCs within 2 years after initial curative hepatectomy, which are considered to originate from residual microscopic lesions and have characteristics similar to those of primary HCCs [Citation16]. By comparing the prognosis of recurrent HCC patients receiving different treatment modalities in groups with negative or positive MVI, we found that patients with positive MVI receiving TACE-RFA had longer sRFS than those receiving hepatectomy or RFA alone in the early recurrent group, while HCC patients did not benefit from more aggressive treatments in terms of sRFS or PRS in the late recurrent or MVI (−) groups. As an unfavorable survival factor, whether the presence of MVI affects the prognosis of patients with small HCC remains further clarified. Shindoh et al. found that MVI has no significant impact on the survival of patients with solitary HCC up to 2 cm, while significant survival difference was found in early-stage HCC patients with and without MVI in another study [Citation21,Citation22]. For patients whose primary HCC was positive for MVI, postoperative adjuvant therapy with sorafenib could not improve the RFS of HCC patients [Citation23]. In the current study, we found that there was no significant difference in the prognosis between recurrent HCC patients receiving RFA alone and those receiving more aggressive treatments including hepatectomy or TACE-RFA in HCC patients with MVI (−) or MVI (+) but TTR > 2 years. These results indicated RFA alone might be sufficient for recurrent HCC ≤3 cm, even with the presence of MVI.
In the current data, for patients with recurrent HCC up to three nodules smaller than 3 cm, RFA could provide similar survival benefits compared to hepatectomy. For primary HCC, patients receiving ablation had similar prognosis with those receiving partial hepatectomy [Citation17]. Xia et al. have found that there is no statistically significant difference in long-term survival between recurrent HCC patients treated with repeat hepatectomy and those treated with RFA, who suffered from recurrence within Milan Criteria after curative resection for primary HCC, and they also found hepatectomy provided longer survival benefits than RFA for recurrent patients with HCC >3 cm or serum AFP level >200 ng/ml [Citation24]. In addition, for HCC patients with recurrence after liver transplantation, RFA has high technical success rates and local control [Citation25,Citation26]. Although different cutoff values of tumor size are reported in which RFA can achieve comparable efficiency to hepatectomy, the most acknowledged threshold is 3 cm in the consideration of heat sink [Citation9,Citation10].
We also found no significant difference in prognosis between recurrent patients receiving hepatectomy and TACE-RFA in patients with MVI (−) or MVI (+) but TTR > 2 years, which was similar to the results reported previously [Citation27]. Studies have found that for primary or recurrent HCC within Milan criteria, TACE-RFA provided comparable prognosis to hepatectomy [Citation27,Citation28]. In this study, TACE-RFA achieved better sRFS than hepatectomy in patients with MVI (+) and recurrent HCC within 2 years. Similarly, previous study observed that for patients with HCCs beyond the Milan criteria but within up-to-seven criteria, TACE-RFA prolonged the survival significantly better than hepatectomy [Citation10]. TACE can provide synergistic effects to RFA due to the reduction of heat loss by decreasing blood flow, which assist ablation to treat larger lesions curatively [Citation8,Citation9]. In addition, TACE can also diminish the dissemination of HCC [Citation29]. The combination of RFA and ethanol injection can also improve the efficiency of RFA and achieve comparable prognosis to hepatectomy for patients with small recurrent HCC [Citation30]. Both MVI and early recurrence are adverse prognostic factors for HCC [Citation16]. For patients with MVI (+) and early recurrent HCCs, their recurrences may be more malignant and microlesions can exist in the liver except for the small recurrent lesions detected by imaging. These potential lesions can be treated by additional TACE.
Although TACE-RFA can achieve larger ablation region than RFA alone, they provided similar survival benefits in patients with MVI (−) or MVI (+) but TTR > 2 years when treating recurrent HCCs up to three nodules smaller than 3 cm. Previous studies also demonstrated similar findings. Shibata et al. prospectively enrolled patients with HCC up to 3 cm and randomly assigned them to receive TACE-RFA or RFA alone, and in their study, both the overall survival and local progression-free survival were similar between HCC patients receiving TACE-RFA and those receiving RFA alone, which indicates the combination therapy may be not necessary [Citation31]. But TACE-RFA achieved longer sRFS in recurrent HCC patients with MVI (+) and TTR ≤2 years, which may be due to the efficiency of additional TACE for potential undetectable HCCs. These findings indicated that only patients with more malignant small recurrent HCCs needed TACE-RFA, while RFA alone could be enough for the rest of patients with small recurrences. HCC patients treated with TACE-RFA reports more frequent discomfort and complications and longer hospitalization [Citation32]. Either RFA or TACE can impair the liver function such as infarction, abscess or failure, and the combination of these two treatments increase the risk of damages to liver [Citation32,Citation33]. Thus, identifying HCC patients needing TACE-RFA can both ensure the treatment efficiency and avoid unnecessary treatments.
Although studies have explored the survival benefits of different treatment modalities in recurrent HCC, various inclusion criteria were adopted for these researches. Among these studies, the maximum HCC diameter ranged from 3 to 8 cm, the stage of recurrent HCC varied from early to advanced stage and the tumor number ranged from single to multiple [Citation11,Citation12,Citation27,Citation34]. Diverse inclusion criteria may increase the potential bias and reduce the comparability of results among studies. In this study, we only enrolled recurrent HCCs up to three nodules smaller than 3 cm and compared the survival benefits of hepatectomy, RFA and TACE-RFA for these patients, which ensured the homogeneity of patients.
This study has several limitations. First, it is a single-center retrospective study, so there might exist potential biases among different treatment groups. To avoid biases as much as possible, the patients were consecutively reviewed for enrollment, which can reduce selection bias in the retrospective study. Although the TTR were not well balanced among groups, we compared the survival benefits of hepatectomy, RFA and TACE-RFA in subgroups classified by MVI status and TTR. Second, this study was based on the single institution with oncologists experienced in RFA, so these results might depend on the experience of doctors. Third, the sample size was limited in this study, especially for the TACE-RFA group. Fourth, the MVI status can be different between primary and recurrent HCCs. Although the MVI status of primary HCC is not always the same as that of the recurrent ones, HCC patients with MVI have poorer prognosis, which raises the doubt on the necessity of more aggressive treatments for their recurrences. This study indicated that TACE-RFA can provide better prognosis for patients with MVI in early recurrent HCC, while RFA alone is efficient enough for the rest of HCC patients with small recurrence. Multicenter prospective clinical trials are needed to further validate these results.
In conclusion, for HCC patients with MVI (+) and early recurrence, TACE-RFA could achieve better prognosis than hepatectomy or RFA alone. In patients with MVI (−) or late recurrent HCCs, RFA alone provided comparable survival benefits to hepatectomy or TACE-RFA. These findings can assist in identifying HCC patients who needs TACE-RFA for small recurrent HCCs.
Ethical approval
This study was approved by the Ethical Committee of Sun Yat-sen University Cancer Center (B2020-350-01) and carried out according to the 1964 Helsinki Declaration.
Supplemental Material
Download PDF (185.9 KB)Supplemental Material
Download PDF (97.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873.
- Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237(4):536–543.
- Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234(1):63–70.
- Shimada M, Takenaka K, Taguchi K, et al. Prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg. 1998;227(1):80–85.
- Shuto T, Kinoshita H, Hirohashi K, et al. Indications for, and effectiveness of, a second hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology. 1996;43(10):932–937.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Yamakado K, Nakatsuka A, Akeboshi M, et al. Combination therapy with radiofrequency ablation and transcatheter chemoembolization for the treatment of hepatocellular carcinoma: short-term recurrences and survival. Oncol Rep. 2004;11(1):105–109.
- Yamakado K, Nakatsuka A, Ohmori S, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13(12):1225–1232.
- Pan T, Mu LW, Wu C, et al. Comparison of combined transcatheter arterial chemoembolization and CT-guided radiofrequency ablation with surgical resection in patients with hepatocellular carcinoma within the up-to-seven criteria: a multicenter case-matched study. J Cancer. 2017;8(17):3506–3513.
- Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41(2):236–242.
- Chen SL, Xiao H, Xie ZL, et al. The presence of microvascular invasion guides treatment strategy in recurrent HBV-related HCC. Eur Radiol. 2020;30(6):3473–3485.
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207.
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24.
- Yang J, Liang H, Hu K, et al. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: a systematic review and meta-analysis. Cancer Cell Int. 2021;21(1):92.
- Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493.
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328.
- Sun X, Li L, Lyu N, et al. Follow-up schedule for initial recurrent hepatocellular carcinoma after ablation based on risk classification. Cancer Imaging. 2020;20(1):42.
- Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855.
- Sheng X, Ji Y, Ren GP, for the Liver Cancer Pathology Group of China (LCPGC), et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int. 2020;14(6):1034–1047.
- Shindoh J, Andreou A, Aloia TA, et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol. 2013;20(4):1223–1229.
- Shindoh J, Kobayashi Y, Kawamura Y, et al. Microvascular invasion and a size cutoff value of 2 cm predict long-term oncological outcome in multiple hepatocellular carcinoma: reappraisal of the American Joint Committee on cancer staging system and validation using the surveillance, epidemiology, and end-results database. Liver Cancer. 2020;9(2):156–166.
- Xu L TY, Li H, Zhou J, et al. Sorafenib as an adjuvant therapy for hepatocellular carcinoma with microvascular invasion after radical resection: a prospective multicenter nonrandomized controlled study. J Clin Oncol. 2020;38:e16685.
- Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020;6(2):255–263.
- Liu B, Huang G, Xie X, et al. Feasibility and outcomes of percutaneous radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma after liver transplantation: a single-center experience. Int J Hyperthermia. 2020;37(1):1202–1209.
- Yang W, Wu H, Zhang ZY, et al. Long-term outcome of percutaneous radiofrequency ablation in recurrent hepatocellular carcinoma after liver transplantation. Int J Hyperthermia. 2018;34(1):68–76.
- Peng Z, Wei M, Chen S, et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol. 2018;28(8):3522–3531.
- Takuma Y, Takabatake H, Morimoto Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269(3):927–937.
- Zhao M, Wang JP, Pan CC, et al. CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement. Eur J Radiol. 2012;81(10):2717–2725.
- Chen S, Peng Z, Xiao H, et al. Combined radiofrequency ablation and ethanol injection versus repeat hepatectomy for elderly patients with recurrent hepatocellular carcinoma after initial hepatic surgery. Int J Hyperthermia. 2018;34(7):1029–1037.
- Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252(3):905–913.
- Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol. 2019;44(6):2283–2292.
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7):S377–S390.
- Yang W, Chen MH, Wang MQ, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res. 2009;39(3):231–240.