Abstract
Introduction
To evaluate treatment response of uterine fibroids after ultrasound guided high-intensity focused ultrasound (USgHIFU) with a special focus on fibroid size and characterization based on Funaki classification scheme, as well as clinical response to treatment of leading fibroid-associated symptoms.
Materials and methods
Uterine fibroids treated by USgHIFU were assigned to Funaki type 1–3 based on T2-w-MRI. Differences in size, non-perfused volume ratio (NPVR) and volume reduction over time were determined using T1-/T2-w MRI sequences and contrast-enhanced sonography. Treatment effects on three leading fibroid-associated symptoms were also evaluated. Measurements were compared by mixed model, Bland–Altman’s plot and Spearman’s correlation.
Results
In this prospective single-center study, 35 patients with 44 symptomatic uterine fibroids were treated by USgHIFU (n = 22, n = 12 and n = 10 assigned to Funaki type 1, 2 and 3, respectively). NPVRs of Funaki type 1 and 2 fibroids were significantly higher compared to type 3 (p = .0023). A significant fibroid shrinkage was observed independent of Funaki type compared to baseline: 38.8 ± 26.9%, 46.7 ± 30.3% and 54.5 ± 29.3% at 3, 6 and 12 months, respectively (each p < .05). Moreover, patients experienced a significant improvement of fibroid-associated hypermenorrhea (3.9 ± 1.3 vs. 2.3 ± 1.3), pressure in the pelvic area (3.5 ± 1.3 vs. 2.1 ± 0.9) and frequent urination (2.8 ± 1.5 vs. 1.9 ± 0.8) one year post-procedure (each p < .05), regardless of fibroid Funaki type.
Conclusion
Following USgHIFU, a significant shrinkage of uterine fibroids and improvement of leading fibroid-associated symptoms were demonstrated regardless of the Funaki type.
Introduction
In recent years, high-intensity focused ultrasound (HIFU) has been regarded with increased interest while expanding its application for treatment of various noninvasive solid tumors via tissue ablation. These also include gynecological diseases such as uterine fibroids, adenomyosis, breast fibroadenoma and even breast cancer in some exploratory studies [Citation1–4]. The clinical value of HIFU has been evaluated for nearly two decades proving to be a safe and effective treatment option offering a large clinical benefit with only minimal and very short-lasting impairment on patients’ well-being [Citation5–7].
The noninvasive nature of HIFU makes it a unique treatment option as it does not require the insertion of needles, probes or electrodes into the human body to unfold its function. In contrast to diagnostic ultrasound, energy levels of the therapeutic beam are several orders of magnitude greater, although the utilized frequencies do partially overlap (0.8–3.5 MHz for ultrasound therapy, 1–5 MHz for transabdominal ultrasound diagnostics) [Citation2]. During ablation, thermal tissue damage in the target region is facilitated without any ionizing radiation; rather, external ultrasonic energy is transmitted into a lesion causing coagulation necrosis at its focus, whereas tissue outside the focus remains undamaged. Hence, lesions are destroyed in situ, leaving the skin intact [Citation2]. In consequence of HIFU, follow-up examinations of treated lesions present a gradual shrinkage of the ablated volume over time.
At present, HIFU treatment is either guided by ultrasound (USgHIFU) or magnetic resonance imaging (MRgHIFU). USgHIFU devices use diagnostic ultrasound for locating the target region and observing the treatment response in real-time as grey-scale changes caused by cavitation indicate successful tissue ablation following each exposure [Citation8,Citation9]. MRI-guided devices use MRI to locate target regions to be treated by therapeutic ultrasound [Citation8,Citation9] and indirect MRI thermometry serves to confirm target exposure and measure the response.
Over the last two decades, USgHIFU has been widely used in Asia to treat uterine fibroids. Many studies from this region with a total of more than 10,000 treated patients as well as one recent European study [Citation10] have demonstrated that US-guided HIFU is a safe, effective and cost-effective alternative for treatment of uterine fibroids [Citation5,Citation7,Citation11]. However, the vast majority of these studies lack a sufficient standardization, which would have been necessary for evidence-based recommendations. Against this backdrop, the presented study investigated the following hypotheses:
USgHIFU treatment of uterine fibroids (1) significantly reduces fibroid size over time (6-week, 3, 6 and 9 months, 1 year post-HIFU), regardless of fibroid type according to the Funaki classification scheme; (2) significantly mitigates the leading myoma-associated symptoms (hypermenorrhea, tension/pressure in the pelvic area, and urination urge/frequent urination) at above mentioned follow-ups.
Furthermore, comparison of both utilized MRI sequences (T2-weighted (w) and contrast-enhanced (CE) T1-w) shows no significant differences between both methods of measurement at baseline and any time point of follow-up (3).
Materials and methods
Patient selection
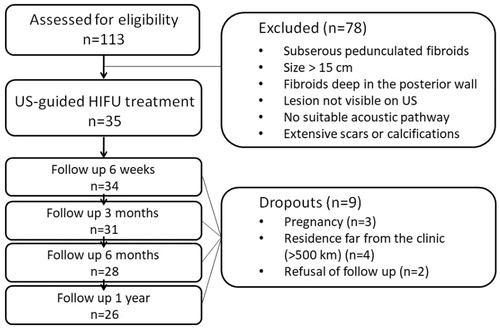
Selection criteria are summarized in . Eligibility for HIFU was confirmed via an interdisciplinary board consisting of gynecologists and radiologists (). Written informed consent was obtained from each patient. The study was approved by the local Ethics Committee at the Medical Faculty of the Rheinische Friedrich-Wilhelms-Universität Bonn and performed in accordance with the Declaration of Helsinki.
Table 1. Selection criteria for HIFU treatment of uterine fibroids.
Patients’ and fibroids’ characteristics are summarized in . Treated patients exhibited clinical fibroid-related symptoms: the three most frequent symptoms were hyper-/dysmenorrhea (n = 32, 91.4%), pelvic pain or pressure (n = 31, 88.6%) and urination urge/frequent urination (n = 26, 74.3%). The number of ablated uterine fibroids in affected patients varied from 1 (n = 9), 2 (n = 6), to more than 2 (n = 20) lesions. Three patients had been previously treated by oral contraceptives, nine patients by selective progesterone receptor modulators (ulipristal acetate); five patients had undergone myomectomy (n = 5) and three patients Caesarean section (n = 3). Fibroids were classified into three Funaki types according to their signal intensity in T2-w sequences on pretreatment MRI compared to T2-intensity of the myometrium and skeletal muscle: type 1 with very low T2-intensity comparable to that of skeletal muscle, low vascularization; type 2 with T2-intensity between that of skeletal muscle and myometrium; type 3 with heterogeneous T2-intensity equal to or higher than that of the myometrium [Citation12].
Table 2. Demographic and clinical characteristics of HIFU-treated patients with uterine fibroids.
Therapeutic procedure
USgHIFU treatment was performed in a multidisciplinary approach with a medical team of two radiologists, one anesthesiologist, one radiological and one anesthesiological nurse. On the day prior to the treatment, patients were asked to follow a bowel preparation consisting of a 12 h fasting and ingestion of prescribed laxatives. The skin in the acoustic pathway was prepared by shaving, degreasing and degassing the anterior abdominal wall to prevent skin burning. During procedure, a balloon filled with degassed water was placed between the patient’s anterior abdominal wall and transducer to optimize the acoustic window (). HIFU ablation was performed in prone position under intravenous conscious sedation. To avoid potential nerve damage, patients were asked to continuously give feedback concerning pain affecting the legs.
Figure 2. US-guided HIFU system: schematic drawing of the US-guided HIFU unit JC Tumor Therapeutic System (HAIFU Medical Technology, Chongqing, China). Patients with uterine fibroids are positioned prone on the treatment table where there is an opening (1) with a water basin (2). Patient’s skin of the lower abdominal wall over the uterus is at the level of this opening, thus the skin is in contact with cooled water. The parabolic therapeutic transducer (3) with the convex diagnostic ultrasound probe in the center is located in this water basin. The therapeutic ultrasound beam (4) is transmitted by a 20 cm diameter ceramic transducer with a focal length of 15 cm, operated at a frequency of 0.8 MHz. To compress the subcutaneous tissue and dislocate the bowel away from the acoustic path, a balloon filled with degassed water (5) is positioned between the transducer and the skin resp. anterior abdominal wall. During HIFU procedure, the extracorporeal therapeutic transducer (3) generates an ultrasound beam (4) forming an oval-shaped focus sized 1–3 mm in width and 8–15 mm in length. This way a lesion of coagulation necrosis can be induced in the target area. In case of visible grey-scale changes in the target area suggesting effective ablation, the transducer is moved to the next focus. Multiple juxtaposed lesions generate linear and discoidal necrosis areas and finally allow the ablation of the whole tumor area.
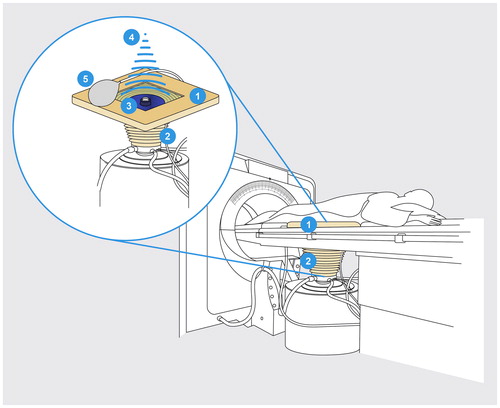
For US-guided ablation, the Focused Ultrasound Tumor Therapeutic System (JC Chongqing HAIFU Technology, Chongqing, China) equipped with a 1–8 MHz ultrasound imaging device (MyLab 70, Esaote, Genoa, Italy) was utilized. Therapeutic ultrasound was generated by a ceramic transducer (diameter 20 cm, focal length 15 cm, frequency 0.8 MHz). B-mode images visualized grey-scale changes during treatment allowing for monitoring of fibroid tissue response. A sagittal scanning mode was used for treatment planning and ablation.
Evaluation of fibroid size and leading fibroid-associated symptoms
The evaluation of fibroids following USgHIFU included radiological and clinical evaluation. Contrast-enhanced MRI (1.5-Ingenia MRI, Philips, The Netherlands) and CE ultrasound (CEUS) (Logiq E9, GE, Tampa, FL) were performed for all patients before and after HIFU treatment. Contrast-enhanced sonography was used to assess perfusion of fibroids at baseline and post-procedure. Exact lesion diameters in three planes (anterior-posterior, cranio-caudal, right-left) were measured on MR images using T2-w and CE-T1-w sequences. In order to estimate fibroid volumes, volume formula of an elongated ellipsoid (V = a*b*c*(π/6)) was used. The objective primary lesion response was assessed by determination of non-perfused volume ratio (NPVR, %) on CE T1-w imaging and was defined as the ratio of non-perfused volume in first post-HIFU MRI compared to whole fibroid volume. Long-term follow-up was accomplished after 6 weeks, 3, 6 and 9 months and 1 year. Lesion volume reduction or fibroid shrinkage rate (%) over time was calculated as ratio between fibroid volume at each timepoint (6 weeks, 3, 6, 9 months, 1 year after HIFU) and corresponding initial fibroid volume. CEUS was subjectively evaluated by two experienced investigators for differences in image quality and assessability compared to CEMRI. HIFU-associated changes in the three leading and most common fibroid-associated symptoms in our patient collective ((1) hypermenorrhea; (2) feeling of tension or pressure in the pelvic area; (3) urination urge and frequent urination) were evaluated comparing levels at baseline and at each timepoint of follow-up (symptom scale 1–5: 1 ‘not at all’, 5 ‘very heavy’). Adverse events and complications were recorded.
Statistical analysis
Data of this prospective observational longitudinal study were analyzed using Stata Version 14 (StataCorp, StataCorp LP, College Station, TX) and SPSS (Version 25, SPSS Inc., Chicago, IL). Assessing agreement between T1-/T2-w MRI measurements was analyzed by the Bland–Altman plot [Citation11] and Spearman’s correlation. Changes of fibroid volumes and leading symptoms following HIFU were evaluated by mixed model considering values at baseline and each follow-up as dependent variables. The influence of various interventional parameters (total energy, sonication time, power) and assignment to corresponding Funaki types on non-perfused volumes and volume reduction over time was analyzed using logistic regression and mixed model, respectively. A p value of < .05 was considered statistically significant.
Results
Thirty-five female patients (aged 28–53 years, median 43 years) with symptomatic uterine fibroids (n = 44, largest diameter between 2 and 12 cm) were successfully treated by USgHIFU at our center. A single HIFU session was performed in most patients (n = 33/35, 94%). Due to recurring disease-associated symptoms, HIFU ablation was repeated in 2/35 patients (once at 16 and 7 months after 1st session, respectively). Intervention parameters are shown in . Treated fibroids were allocated to Funaki type (type 1: n = 22, 50%; type 2: n = 12, 27%; type 3: n = 10, 23%). Estimated NPVR averaged 60.1 ± 31.8% (median 69.4%) and significantly correlated to Funaki types (p = .0023). NPVR on first post-interventional imaging ranged from 73.2 ± 24.2%, 59.7 ± 31.9% and 30.1 ± 30.9% for Funaki type 1, 2 and 3, respectively (). Other than slight lesion swelling in some cases (n = 16, 36%), no significant difference in fibroid volumes was observed between baseline and first post-interventional imaging (1-week follow-up) (). Comparing initial volumes to fibroid volumes over time, a significant volume reduction was shown for all fibroids and each Funaki type at each further follow-up (6 weeks, 3, 6, 9 months, 1 year, ) confirming hypothesis 1. For lesion shrinkage, NPVR was the decisive factor (p = .006); some non-significant effect of total energy was also observed as a potentially relevant factor toward volume reduction. Values of fibroid volumes and volume reduction are shown in . Comparison between results of both utilized MRI sequences (T2-w and CE T1-w) by Bland–Altman’s test revealed no significant differences between both methods of measurement at baseline and any time point of follow-up (). Thus, hypothesis 3 was confirmed. In the early follow-up after HIFU, CEUS could not be performed in 6/35 patients due to edema in the acoustic pathway.
Figure 3. Non-perfused volume rate (NPVR in %) of treated uterine fibroids according to different Funaki types. NPVR values were measured using T1-w MRI sequences in first post-interventional MRI (within 1st week following HIFU procedure). Significant differences in NPVR were observed between Funaki groups.
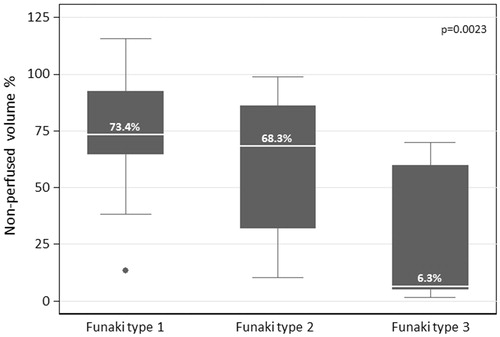
Figure 4. Significant fibroid volume reduction was observed 6 weeks after HIFU compared to baseline (95%CI: –52%, –74%, p<.05). Lesion shrinkage improved steadily over the observational period at 3-, 6-, 9-month and 1-year follow-up (each p<.001, compared to baseline, mixed model). Significant decrease in fibroid volume was also shown for each time point (3-, 6-, 9-month and 1-year follow-up) compared to the lesion volumes within first week after HIFU (each p<.001).
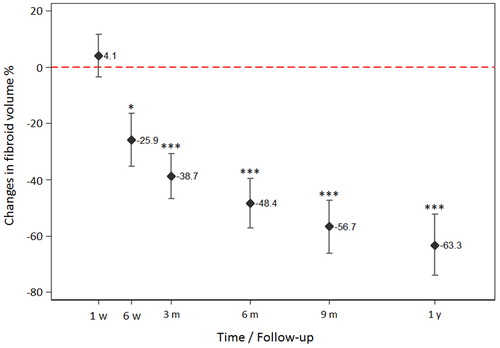
Figure 5. Comparison of results using two MRI sequences (contrast-enhanced T1- and T2-w) by Bland–Altman’s plot showed no significant differences between both methods of measurement at any time point. Most values lie within the predictive interval (limits of agreement). The Spearman test did not reveal a significant trend in the differences. For higher true values, T2-weighted imaging revealed a tendency toward discretely higher measurements. For lower true values, T1-weighted imaging showed a trend toward discreetly lower measurements. No significance was found for both trends.
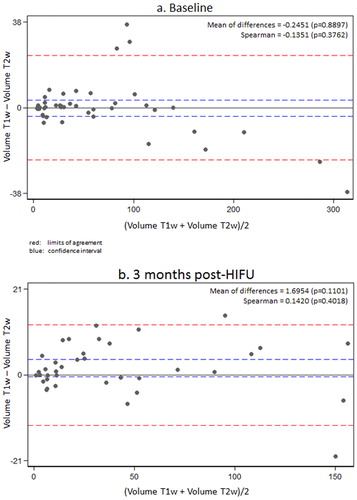
Table 3. Therapeutic parameters of US-guided HIFU (n = 35) in patients with uterine fibroids.
Table 4. Lesion volumes of HIFU-treated uterine fibroids at baseline and follow-up (1 and 6 weeks, 3, 6 and 9 months, 1 year after HIFU); corresponding volume reduction in % compared to initial lesion volumes.
Uterine fibroids with different T2-w signal behavior showed a varying therapeutic response regarding both NPVR and lesion shrinkage over time. Uterine fibroids of Funaki type 1 and 2 showed a significantly higher NPVR (0.0023) compared to type 3 (). Although all fibroids showed a significant shrinkage over time compared to baseline and first postinterventional imaging, lesion shrinkage was highest for Funaki type 1 and 2 fibroids with best treatment response (). The three leading clinical symptoms were significantly improved (p < .05 at 6-month and 1-year follow-up) after USgHIFU confirming hypothesis 2: (1) hypermenorrhea from 3.9 ± 1.3 at baseline to 3.0 ± 1.5, 2.6 ± 1.3, 2.3 ± 1.3 at 3-, 6-month and 1-year follow-up, respectively; (2) feeling of tension or pressure in the pelvic area from 3.5 ± 1.3 at baseline to 2.7 ± 1.2, 2.5 ± 1.0 and 2.1 ± 0.9 at 3-, 6-month and 1-year follow-up, respectively; (3) urinary urgency and frequent urination from 2.8 ± 1.5 at baseline to 2.4 ± 1.3, 2.2 ± 1.1 and 1.9 ± 0.8 at 3-, 6-month and 1-year follow-up, respectively.
Figure 6. Fibroid volume reduction over time compared to initial volumes for different Funaki types. All fibroids showed a significant shrinkage over time compared to baseline volumes and volumes in the first postinterventional imaging (for both p<.05 at 6-week follow-up; p<.001 at 3-, 6-, 9-month and 1-year follow-up). One year after HIFU treatment, the decrease in volume was more pronounced for Funaki type 1 and 2 fibroids. Volumes of ablated Funaki 3 fibroids seem to stay stable between 6 months until 1 year after HIFU which may be explained by the significantly lower NPVR for this group.
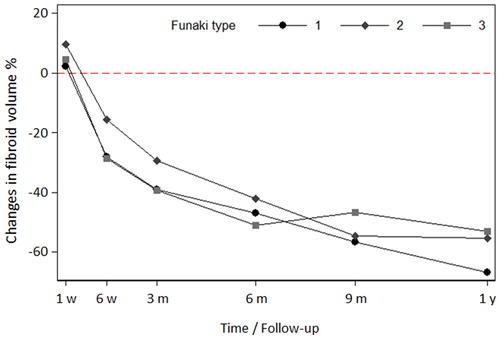
Figure 7. Representative MRI images (T2-w, sagittal plane) of large uterine fibroids show different signal intensities of the fibroids according to the Funaki classification scheme: Funaki type 1 (a) with very low T2-w intensity comparable to that of skeletal muscle, low vascularization; Funaki type 2 (b) with T2-w intensity between that of skeletal muscle and myometrium; Funaki type 3 (c, d) with heterogeneous T2-w intensity equal to or higher than that of the myometrium.
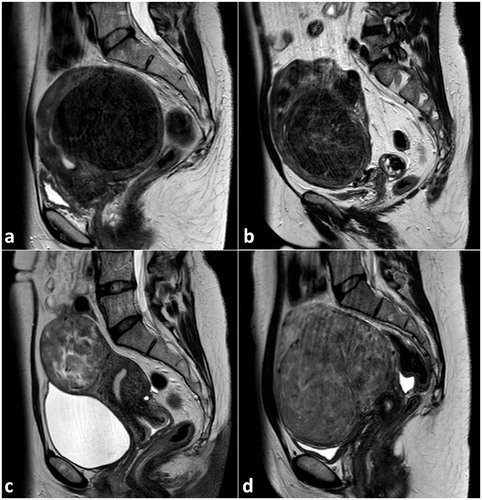
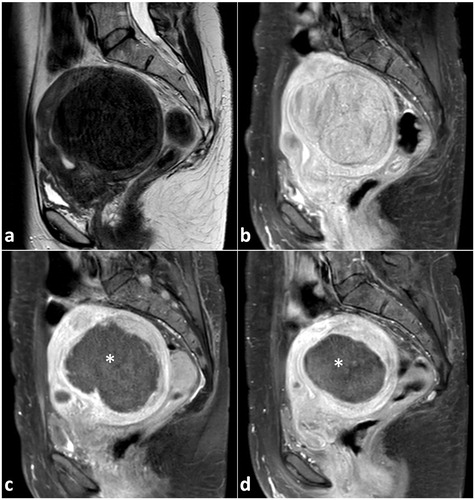
Figure 8. A 47-year old woman with a large uterine fibroid of Funaki type 1 (largest diameter 9.4 cm) and fibroid-associated symptoms was treated by US-guided HIFU at our institution. Representative MRI images (a T2-w, b–d contrast-enhanced T1-w, sagittal plane) of the large uterine fibroid pre- and post-procedure are shown. At baseline, the fibroid volume was about 300 ml in T2-w (a) and 275 ml in T1-w MRI (b). Three hours after HIFU procedure, the treated fibroid was unchanged in size and volume, but largely nonvascularized (c: black region, marked with a white star): a non-perfused volume ratio of 73% was achieved indicating an effective ablation and technical success. Three months after US-guided HIFU, a considerable volume reduction of about 50% was observed (fibroid volume in T1-w MRI of 153 ml, d; largest diameter 7.4 cm).
No major complications or permanent damage was observed in any HIFU treated patients. Peri-interventional short-lasting side effects included vaginal bleeding (6%), transient short-lasting abdominal pain (20%), increased vaginal discharge (10%) and subcutaneous edema of the lower abdominal wall (32%). These side effects were self-limiting or treated symptomatically by over the counter medication (NSAI, paracetamol, hyoscinbutylbromid).
Discussion
To our knowledge, this prospective single-center study is the first documented clinical experience of USgHIFU for treating symptomatic uterine fibroids in German-speaking countries. It demonstrates a significant shrinkage of uterine fibroids and clinical improvement of leading fibroid-associated symptoms following USgHIFU regardless of the Funaki type. The results of this study are in concordance to the findings made so far in Asia [Citation5,Citation7,Citation11,Citation13], including the large, recently published, non-randomized, observational study with 2411 patients treated by USgHIFU (n = 1353), hysterectomy (n = 472) and myomectomy (n = 586) across 20 Chinese hospitals [Citation5]. Despite the very large patient numbers, the vast majority of published results lack a sufficient standardization with regard to timepoints of follow-up with imaging modalities and questionnaires, which are necessary for evidence-based recommendations. In Europe, there is only one other European study reporting on USgHIFU for symptomatic uterine fibroids [Citation10]. This observational study with 10 female patients showed considerable relief in fibroid-related clinical symptoms and size reduction of the dominant fibroid in 9/10 patients.
Regarding the reliable and valid assessment of fibroid volume at baseline and at any time point of follow-up, there was no statistically significant difference between the two most commonly used MRI sequences. Therefore, intravenous contrast agent is only necessary at baseline and in the first control imaging after USgHIFU; further follow-up studies may be performed by only using T2w-sequences and additional CEUS to evaluate lesion perfusion.
The presented study also investigated factors affecting technical success rates and achieved lesion volume reduction over time. In 2007, Funaki et al. proposed dividing fibroids into three groups depending on the MR-tomographic appearance. In previous studies, fibroids’ Funaki type played a clear role in the technical results of MRgHIFU as represented by the NPVR: Funaki type 1, 2 and 3 showed an NPVR of 53%, 52% and 33%, respectively [Citation14]. Our results utilizing USgHIFU demonstrated a similar influence of Funaki type on NPVR as reported for MRgHIFU (73%, 60% and 30% for Funaki type 1, 2 and 3, respectively), so that NPVR of Funaki type 1 and 2 was significantly higher than that of Funaki type 3 (0.0023). This may be due to the fact that Funaki type 1 fibroids, consist to a large degree of smooth muscle, numerous nuclear areas and low collagen fiber content [Citation15] and thereby require less energy deposition at the focal point than other fibroid types [Citation16–18]. Only one study from South Africa showed no significant difference in shrinkage rate between hypointense, isointense and hyperintense fibroids in women [Citation19].
Our results also demonstrated that all treated fibroids, independent of their Funaki type, showed significant lesion shrinkage over time, which was highest for Funaki type 1 and 2 fibroids with best treatment response and lowest for Funaki type 3 fibroids. Taking into account, the observed fibroid volume reduction over time, MR-tomographic classification of Funaki type 2 or even type 3 fibroid according to T2-w sequences by itself is not an exclusion criterion for HIFU treatment, as postulated in some studies [Citation20]. Previous findings [Citation20] suggest that approximately three times as much energy is probably required to achieve a sufficient NPVR in Funaki type 3 fibroids than in type 1 or 2 fibroids. This in turn increases the risk of thermal injury due to increased energy needed to achieve ablation as more energy could be potentially deposited in the abdominal wall and adjacent structures along the acoustic pathway.
Ablation results also depend on enhancement as shown in T1-w CE images. Fibroids with only mild enhancement [Citation21] or lower blood flow and volume on dynamic contrast enhanced MRI [Citation22] showed a better ablation effect than those with a moderate/significant enhancement. In a similar way, using CEUS, fibroids with lower perfusion time parameters (arrival time, peak time and enhancement time) reflecting the blood flow velocity, and with higher intensity parameters (enhancement intensity and enhancement rate) reflecting the perfusion volume of a lesion were resistant to HIFU and had poor ablation efficacy [Citation23]. This fact again can be explained by energy deposition being less effective in cases where heat is distributed or taken away by the blood flow, a phenomenon known as a ‘cooling effect’. Therefore, T1-w contrast enhancement can reflect the blood perfusion status of uterine fibroids in predicting the effect of ablation [Citation24]. As a practical consequence, studies have shown that for MRgHIFU [Citation25] as well as USgHIFU [Citation26], NPVR was larger after additional intravenous oxytocin administration, which causes contraction of the fibroid leading to diminished vascularization.
Fibroid location within the uterus also seemed to be especially important for the ablation result. Compared to anterior, lateral and fundus fibroids, fibroids in the posterior part of uterus had the lowest NPVR [Citation27]. This was probably mostly due to the fact that the attenuation of the ultrasound beam was less and the deposited energy higher when the ultrasonic beam traveled through the shorter acoustic pathway. Technical success was also worse in transmural fibroids compared to intramural, submucosal or subserosal ones, with no significant difference in the latter group [Citation27]. Therefore, fibroids with a long distance from their ventral sides to the skin, retroverted uterine position, significant enhancement on T1-w imaging, hyperintensity on T2-w imaging, transmural type and posterior location are harder to ablate [Citation21,Citation28].
Theoretically, an influence of ethnical factors is also conceivable regarding ablation results. For example, fibroids in black women may have a poorer blood supply as findings from a previous study from South Africa suggest [Citation19]; thus, a better therapeutic effect was shown for these patients using MRgHIFU [Citation12,Citation19,Citation29,Citation30]. One black patient treated at our center also showed an excellent therapeutic response to USgHIFU: the ablated fibroid with an initial largest diameter of about 3 cm nearly disappeared one year after HIFU (volume reduction of about 95%).
It can be postulated that the major factor influencing technical success of HIFU is the deposited total energy or energy efficiency factor. As already mentioned, this is dependent on the fibroid composition, blood supply/enhancement type, location, distance from the fibroid ventral side to the skin, and possibly body habitus (abdominal wall thickness, BMI, presence of abdominal wall scars). For example, in patients with a thicker abdominal wall, more energy is absorbed in fatty tissue and consequently less energy can be deposited inside the fibroid [Citation31]. Previous studies showed that US energy is converted into thermal energy, with the highest efficiency at the fat/muscle interface and that fatty tissue easily absorbs thermal energy [Citation32]. However, one pilot study using a portable device (n = 36 patients) found no correlation between the NPVR and the abdominal wall thickness (rectus muscle thickness and subcutaneous fat thickness), original tumor volume or treatment time [Citation33]. Our study was in line with these findings.
In our study, for lesion shrinkage, some effect of the total energy was observed, even though not-significant, so that this parameter was considered as a potentially relevant factor toward volume reduction. Although the HIFU machine specifies intervention parameters, such as the duration and type of energy delivery, it is currently technically not possible to directly measure the energy applied in the focal zone. In addition, the acoustic environment of the surrounding focusing tissue can be dynamically affected by the expansion of the necrotic region [Citation34], thus making the ultrasonic energy more or less easily deposited. Therefore, in contrast to MRgHIFU where it is possible to monitor temperature elevation, any indication of the energy delivered or the energy efficiency factor with USgHIFU is currently only an estimate. Nevertheless, HIFU-devices with US-guidance are more powerful than those with MRI-guidance delivering shorter treatments times and reaching deeper situated fibroids given the myoma size.
Our study confirmed that HIFU is a safe and low-risk procedure. While the method is per se noninvasive and no serious complications or permanent damage were observed, the procedure itself is associated with peri-procedural pain in up to 80% of patients [Citation35]. This pain almost immediately disappeared after treatment returning to pretreatment levels within a few hours post-procedure [Citation33,Citation36]. This may be due to energy absorption and swelling of the tissue (skin, fat, abdominal wall) in the acoustic access path, which is usually clinically inapparent, but could be detected in about every sixth patient using MRI [Citation32]. In contrast to interventions, e.g., in pancreatic carcinoma, which is usually performed under general anesthesia, HIFU treatment for fibroids is usually carried out in conscious sedation. As a result, patients are responsive and can provide feedback for treatment adjustment if the energy application causes irritation of the lumbosacral plexus. However, in our study, there were no such adverse effects lending further evidence to the safety of the procedure. Care may be required in HIFU treatment of uterine fibroids located on the posterior uterine wall or in retroverted uteri, close to the surface of the sacral bone adjacent to lumbosacral nerves, to prevent sciatic nerve damage or sacral injury [Citation37,Citation38]. Occurring in approximately 4–14% of patients, such transient irritation is one of the most frequently reported side effects, along with vaginal discharge (6%) [Citation19,Citation27,Citation37,Citation38], which was reported in 10% of our patients. Serious side effects like skin burns (up to 2%) or even small bowel perforations (0.1%) are possible but have been only rarely reported [Citation7,Citation39]. Nonetheless, compared to other treatment options, such as myomectomy or hysterectomy HIFU treatment is the procedure with the lowest short-term complication rate [Citation5].
This study has several limitations. A number of 35 patients with 44 HIFU-treated fibroids are relatively small regarding different fibroid locations in the uterus and different fibroid types (Funaki type 1–3). The sample size does not allow for the detection of small effects of HIFU treatment on fibroid-associated symptoms. Furthermore, this is a single-center study so that recruitment of patients at only one site may limit validity of findings.
In conclusion, this prospective single-center study demonstrated a significant lesion size reduction after USgHIFU of symptomatic intramural uterine fibroids in a European collective independent of the MR-sequence used for evaluation. Results from CEUS correlated well with results obtained from CEMRI, so that CEUS may be used as an additional tool or even alternative for follow-up studies after HIFU, particularly in patients in whom CEMRI is not feasible due to claustrophobia or hypersensitivity to MRI contrast media. The volume reduction depended on the Funaki type with best treatment responses for type 1 and 2 fibroids; however, even Funaki type 3 fibroids demonstrated the possibility of a significant size decrease. The clinical response to treatment was independent of Funaki type of fibroids and showed a significant improvement of the most common cardinal symptoms in the majority of patients. Fibroid size is associated with patient’s symptoms, comfort and well-being, thus changes in these volumes over time represent an important outcome for the effect of treatment in patients with symptomatic uterine fibroids.
Acknowledgements
The authors acknowledge Guido Luechters for his continuous support in statistical analysis at any time as well as Olga Ramig, Ingrid Manderla-Franke, Kathrin Bird and Irene Zender for their support in clinical management of HIFU-treated patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jenne JW, Preusser T, Gunther M. High-intensity focused ultrasound: principles, therapy guidance, simulations and applications. Z Med Phys. 2012;22:311–322.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327.
- Orsi F, Arnone P, Chen W, et al. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Can Res Ther. 2010;6(4):414–420.
- Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: review of ten years of clinical experience. Front Med China. 2010;4(3):294–302.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364.
- Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269.
- Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45:3214–3221.
- Kohrmann KU, Michel MS, Steidler A, et al. Technical characterization of an ultrasound source for noninvasive thermoablation by high-intensity focused ultrasound. BJU Int. 2002;90:248–252.
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–154.
- Lyon PC, Rai V, Price N, et al. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschall Med. 2020;41(5):550–556.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19:724–732.
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–589.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Funaki K, Fukunishi H, Funaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–184.e6.
- Huang H, Ran J, Xiao Z, et al. Reasons for different therapeutic effects of high-intensity focused ultrasound ablation on excised uterine fibroids with different signal intensities on T2-weighted MRI: a study of histopathological characteristics. Int J Hyperthermia. 2019;36:477–484.
- Gong C, Setzen R, Liu Z, et al. High intensity focused ultrasound treatment of adenomyosis: the relationship between the features of magnetic resonance imaging on T2 weighted images and the therapeutic efficacy. Eur J Radiol. 2017;89:117–122.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5–13.
- Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82:e43–e49.
- He M, Jacobson H, Zhang C, et al. A retrospective study of ultrasound-guided high intensity focussed ultrasound ablation for multiple uterine fibroids in South Africa. Int J Hyperthermia. 2018;34:1304–1310.
- Andrews S, Yuan Q, Bailey A, et al. Multiparametric MRI characterization of Funaki types of uterine fibroids considered for MR-guided high-intensity focused ultrasound (MR-HIFU) therapy. Acad Radiol. 2019;26(4):e9–e17.
- Fan HJ, Cun JP, Zhao W, et al. Factors affecting effects of ultrasound guided high intensity focused ultrasound for single uterine fibroids: a retrospective analysis. Int J Hyperthermia. 2018;35:534–540.
- Wei C, Fang X, Wang CB, et al. The predictive value of quantitative DCE metrics for immediate therapeutic response of high-intensity focused ultrasound ablation (HIFU) of symptomatic uterine fibroids. Abdom Radiol. 2018;43(8):2169–2175.
- Wang YJ, Zhang PH, Zhang R, et al. Predictive value of quantitative uterine fibroid perfusion parameters from contrast-enhanced ultrasound for the therapeutic effect of high-intensity focused ultrasound ablation. J Ultrasound Med. 2019;38(6):1511–1517.
- Kim YS, Lim HK, Park MJ, et al. Screening magnetic resonance imaging-based prediction model for assessing immediate therapeutic response to magnetic resonance imaging-guided high-intensity focused ultrasound ablation of uterine fibroids. Invest Radiol. 2016;51:15–24.
- Lozinski T, Filipowska J, Krol P, et al. Oxytocin administration in high-intensity focused ultrasound treatment of myomata. Biomed Res Int. 2018;2018:7518026.
- Yu SC, Cheung EC, Leung VY, et al. Oxytocin-augmented and non-sedating high-intensity-focused ultrasound (HIFU) for uterine fibroids showed promising outcome as compared to HIFU alone or uterine artery embolization. Ultrasound Med Biol. 2019;45:3207–3213.
- Fan HJ, Zhang C, Lei HT, et al. Ultrasound-guided high-intensity focused ultrasound in the treatment of uterine fibroids. Medicine (Baltimore). 2019;98(10):e14566.
- Liu Z, Gong C, Liu Y, et al. Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2018;34:77–86.
- Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol. 2012;56:409–416.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771–777.
- Keserci B, Duc NM. Magnetic resonance imaging parameters in predicting the treatment outcome of high-intensity focused ultrasound ablation of uterine fibroids with an immediate nonperfused volume ratio of at least 90. Acad Radiol. 2018;25:1257–1269.
- Yin N, Hu L, Xiao ZB, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34(8):1298–1303.
- Lee JY, Chung HH, Kang SY, et al. Portable ultrasound-guided high-intensity focused ultrasound with functions for safe and rapid ablation: prospective clinical trial for uterine fibroids-short-term and long-term results. Eur Radiol. 2020;30:1554–1563.
- Chen L, ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol. 1997;23:921–931.
- Liao D, Xiao Z, Lv F, et al. Non-contrast enhanced MRI for assessment of uterine fibroids' early response to ultrasound-guided high-intensity focused ultrasound thermal ablation. Eur J Radiol. 2020;122:108670.
- Hou R, Wang L, Li S, et al. Pilot study: safety and effectiveness of simple ultrasound-guided high-intensity focused ultrasound ablating uterine leiomyoma with a diameter greater than 10 cm. Br J Radiol. 2018;91:20160950.
- Cun JP, Fan HJ, Zhao W, et al. Factors influencing MR changes associated with sacral injury after high-intensity focused ultrasound ablation of uterine fibroids. Int J Hyperthermia. 2019;36(1):21–28.
- Zhang YJ, Xiao ZB, Lv FR, et al. MRI evaluation of endopelvic fascial swelling and analysis of influencing factors in patients with uterine fibroids after high-intensity focused ultrasound ablation. Int J Hyperthermia. 2020;37(1):175–181.
- Zou Q, Zhang G, Liu Y. Health education using telephone and WeChat in treatment of symptomatic uterine myoma with high-intensity focused ultrasound. Med Sci Monit Basic Res. 2018;24:127–133.