Abstract
Background
The survival of children with recurrent hepatoblastoma (RHB) is still unsatisfactory and the treatment for relapsed patients is challenging.
Purpose
To compare short-term results between open liver resection (OLR) and percutaneous thermal ablation (TA) in the treatment of RHB and provide evidence to support the treatment options for such patients.
Methods
A retrospective data of 21 patients with RHB in two Chinese centers were analyzed from January 2013 to May 2019. The baseline indicators and clinical effect of the two groups of children were compared.
Results
There was no statistical difference in baseline indicators between the two groups of children, and complete remission (CR) was achieved after comprehensive treatment. The median follow-up time was 30 months (IQR 38.5 months) in the TA group, and 23 months (IQR 21.7 months) in OLR group (p = .57). The 2-year OS rates were 92.3% in the percutaneous TA group and 87.5% in the OLR group (p = .68, HR = 1.6, 95% confidence interval [CI]: 0.2–12.4). The 2-year EFS rates were 66.7%, in the TA group and 50.0% in the OLR group (p = .51, HR = 0.6, 95% CI: 0.2–2.6). Compared with the OLR group, TA group had shorter operation time (3.5 ± 1.8 vs. 0.5 ± 0.1, p < .001) and postoperative hospitalization time (11.8 ± 3.0 vs. 9.5 ± 6.8 d, p = .045). No major complications occurred in both groups.
Conclusions
Ultrasound-guided percutaneous TA for RHB is a safe and effect treatment option for children. It has comparable effect with surgery within 2 years after treatment. Particularly, due to its minimally invasive advantage, it needs shorter operation and hospitalization time. Percutaneous ablation may be an alternative minimally invasive treatment for RHB children.
Introduction
Hepatoblastoma (HB) mostly occurs in children younger than three years of age and is the most common primary pediatric liver malignancy [Citation1,Citation2]. In recent years, with the use of cisplatin-based chemotherapy, the prognosis of patients with HB has been significantly improved, and the five-year overall survival (OS) rate has increased from 30% to 80% [Citation3]. Although this is an inspiring result, the prognosis of HB patients who relapse is still unsatisfactory. According to the International Childhood Liver Tumors Strategy Group (SIOPEL), the three-year event-free survival (EFS) and OS rates of patients with recurrent HB are only 34% and 43%, respectively [Citation4]. Therefore, the identification of a suitable and effective treatment to improve the prognosis of relapsed patients is an urgent need.
Open liver resection (OLR) in combination with chemotherapy remains a key intervention for relapsed HB [Citation5,Citation6], but due to the limited remaining liver volume and the proximity of the tumor to the main vascular structure, success with the second surgical resection and complete remission (CR) after disease recurrence are not assured. For example, in the SIOPEL series, only 52% of the included relapsed patients achieved a second CR [Citation7]. Disease progression becomes an important reason for the low survival rate among relapsed patients. In addition, although liver transplantation can provide the best chance of curing unresectable HB patients after chemotherapy, the five-year OS rate following rescue liver transplantation is much lower (30%) than that reported following primary liver transplantation for unresectable tumors (80%) [Citation8,Citation9]. Therefore, it is necessary to explore other methods to deal with the current dilemma of recurrent HB.
Thermal ablation (TA) has achieved great progress in the treatment of solid tumors. It has a similar treatment effect to that of open surgery in small liver cancers with the advantage of minimal invasion. At present, some studies have preliminarily discussed the efficacy of TA in the treatment of HB, having observed favorable treatment effects [Citation10–14]. This study sought to determine the differences in treatment effect and safety profiles between TA and OLR in the treatment of recurrent HB and provide evidence of other feasible treatments for children with HB.
Materials and methods
Patients
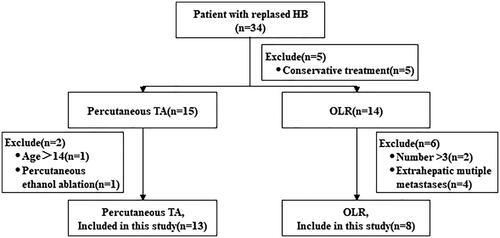
The electronic medical records of consecutive recurrent HB patients who visited the Chinese PLA General Hospital and the First Affiliated Hospital of Sun Yat-sen University from January 2013 to May 2019 were retrospectively reviewed. The study inclusion criteria were as follows: (1) pathologically confirmed HB recurrence, (2) age of less than 14 years old at initial diagnosis and having received radical treatment (OLR combined with chemotherapy) and (3) the number of recurrent tumors was less than three, and each recurrent tumor was less than 5 cm in size. Patients with severe liver damage (Child–Pugh C) or other organ failure were excluded from this investigation. Because the patients in this study were minors, their parents signed informed consent forms before treatment was initiated, and this study was approved by the institutional review committee of the two hospitals. Patient demographics and oncological information (pretext stage, size, number and alpha-fetoprotein (AFP) level) were identified.
Treatments
In the absence of standardized treatment strategies for recurrent HB, the surgical plan was determined by surgeons with more than five years of treatment experience based on the patients’ previous treatment(s) and current condition. OLR was performed as previously described [Citation15]. Percutaneous TA was performed using ultrasound guidance with either radiofrequency ablation (RFA) or microwave ablation as previously described [Citation14,Citation16]. If the ablation was incomplete, an additional session of TA was conducted, with contrast-enhanced ultrasound (CEUS) and magnetic resonance imaging (MRI) performed the next day after OLR/TA to confirm the treatment effect.
Follow-up and outcomes
Complications and side effects were classified using the Clavien–Dindo system. Intraoperative and postoperative data were collected and included the estimated AFP level, operating time, and postoperative hospitalization time. The follow-up protocol consisted of contrast-enhanced MRI/computed tomography or CEUS and AFP level measurement at one month after treatment and every three months thereafter. The definition of CR used was as described in the SIOPEL series [Citation4,Citation17]. The definition of local tumor progression was defined as previously described [Citation18]. The OS and EFS rates were evaluated from the beginning of treatment.
Statistical analysis
Statistical analysis was performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), run in R Studio version 1.2.1335 (RStudio Inc., Boston, MA). Data were presented as mean ± standard deviation values or median values with quartiles in parentheses for continuous variables. Categorical variables were expressed as frequencies with percentages. The t-test or Wilcoxon rank-sum test was used for continuous variables, and the chi-squared test or Fisher’s exact test was used for categorical variables. OS and EFS rates were estimated with the Kaplan–Meier method and compared by the log-rank test. All tests were two-sided, with p > .05 considered to indicate a statistically significant difference.
Results
Patients
A total of 21 recurrent HB patients were included in this study, eight of whom underwent OLR and 13 of whom underwent ultrasound-guided percutaneous TA (). The patients’ baseline characteristics are shown in ; 90.4% of patients were in PRETEXT stage III/IV at first diagnosis, and the mean interval time from initial disease onset to recurrence in the two procedure groups was 13.9 ± 7.3 months (16.9 ± 9.0 vs. 12.0 ± 5.5 months; p = .134). There was no statistical difference between the two groups in terms of the number or size of relapsed tumors ().
Table 1. Baseline participant characteristics.
Intraoperative and postoperative outcomes
All patients received adjuvant chemotherapy after OLR/TA. Thirteen patients in the TA group were checked with CEUS/MRI one day after treatment, and the results showed that the treatment was successful. AFP levels were high in all patients before OLR/TA () but dropped significantly after treatment (). There was no significant statistical difference between the two procedure groups at the end of treatment (8.9 vs. 16.6 ng/mL; p = .972) (). The operation time of the OLR group was longer than that of the TA group (3.5 ± 1.8 vs. 0.5 ± 0.1 h; p < .001). As compared with the OLR group, the TA group experienced a shorter postoperative hospitalization time (11.8 ± 3.0 vs. 9.5 ± 6.8 d; p = .045). The comparison of treatment cost between the two groups showed no statistically significant difference ($6751.7 ± $3061.8 vs. $5980 ± $5022; p = .238) ().
Table 2. Comparison of intraoperative and postoperative outcomes between the OLR and TA.
Safety
The incidence of postoperative complications showed no statistically significant difference in the comparison between the OLR and TA groups (75.0% vs. 30.8%; p = .128); however, patients in the OLR group were more likely to experience high fever (75.0% vs. 23.1%; p = .032) and other complications (50% vs. 7.7%; p = .470). Four patients (50%) in the OLR group developed intra-abdominal infection, and one patient (12.5%) required blood transfusions. In the TA group, one patient experienced intra-abdominal infection. All complications were Dindo–Clavien grades I/II and were cured by symptomatic treatment (Supplementary Table 1).
Recurrence and survival
The median follow-up times were 30 months (range: 16.5–55 months) in the TA group and 23 months (range: 14.8–36.5 months) in the OLR group (p = 0.57). During the follow-up period, no local tumor progression was observed in any patient. However, four patients each in the OLR and TA groups experienced recurrence, with three cases of intrahepatic metastasis and one case of extrahepatic metastasis in each group. There was no significant difference in the recurrence interval time between the two groups (5.6 ± 3.3 vs. 5.4 ± 2.0 months; p = .886) (). Four patients, including one patient (12.5%) in the OLR group and three patients (23.1%) in the TA group, chose to undergo surgery after the second relapse because the lesions were located outside the liver or too large and were not suitable for treatment by TA. Three patients – two in the OLR group and one in the TA group – chose conservative treatment. Finally, one patient in the OLR group chose TA. Three HB-related deaths (n = 6 deaths total) occurred in each group. The one- and two-year OS rates were, respectively, 92.3% and 82.5% in the percutaneous TA group and 87.5% in the OLR group (hazard ratio [HR]: 1.6, 95% confidence interval [CI]: 0.2–12.4) (). The one- and two-year EFS rates were both 66.7% in the TA group and both 50.0% in the OLR group (HR: 0.6, 95% CI: 0.2–2.6) ().
Figure 2. Graphs show Kaplan–Meier survival estimates for survival between patients who underwent percutaneous thermal ablation (TA) or open liver resection (OLR). A. Graph shows cumulative overall survival (OS). The 1- and 2-OS rates, respectively, were 92.3% and 82.5% in the percutaneous TA group and both 87.5% in the OLR group (HR = 1.6, 95% CI: 0.2–12.4). There was no significant difference in cumulative OS between the percutaneous TA and OLR groups (p = .51). B. Graph shows cumulative event-free survival (EFS). The 1- and 2-year EFS rates, respectively, were both 66.7%, in the TA group and both 50.0% in the OLR group (HR = 0.6, 95% CI: 0.2–2.6).
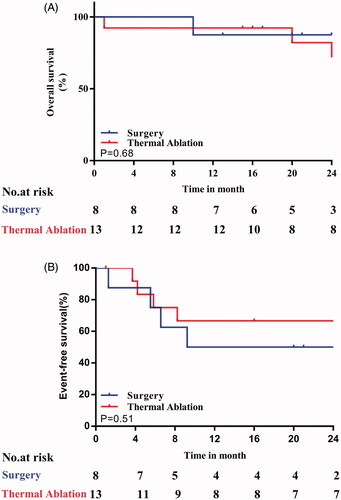
Table 3. Oncologic outcomes and recurrence.
Discussion
HB is the most common pediatric liver malignancy, accounting for over 90% of primary hepatic malignancies found in children younger than five years old [Citation19], with a rising incidence noted in recent years. At present, the PRETEXT system and multiple platinum chemotherapy regimens developed by the SIOPEL have greatly improved the prognosis of patients with primary HB [Citation20,Citation21]. Although the recurrence rate of HB is only 12% in patients who achieve CR [Citation4], the treatment of recurrent HB when required remains a difficult undertaking.
Percutaneous TA is the first-line treatment for early liver cancer in adults, especially when the tumor is less than 3 cm in size, and can achieve an effect similar to that of surgery [Citation22–24]. Percutaneous TA has also been gradually applied in the treatment of pediatric liver neoplasms. Hetzer et al. [Citation11] safely deployed stereotactic RFA (11 sessions, 15 lesions) in 10 pediatric patients with liver masses and extrahepatic metastasis, and follow-up imaging studies (median: 55 months, range: 18–129 months) revealed no local or distant recurrence of disease in any patient. Steven et al. [Citation13] used RFA to treat five patients with 23 HB metastases in the lungs, liver, or bone and achieved local control in 22 of them (95.6%) at a median follow-up period of 30.1 months (range: 18.9–65.7 months) after RFA. Although some [Citation10,Citation13,Citation14,Citation16] studies have initially offered details on the safety and effectiveness of TA in the treatment of pediatric HB, these investigations were published in a case report format, and it is still unknown whether TA can achieve a similar effect as that of OLR in HB.
This study compared the effects of OLR and TA in recurrent HB, and the demographic characteristics, tumor information, intraoperative and postoperative outcomes, and medium-term follow-up of 21 patients were retrospectively analyzed. With no significant differences in baseline characteristics, the TA group experienced shorter operative and postoperative hospitalization times (p < .05). There was no statistical difference in the two-year OS (87.5% vs. 82.5%; HR: 1.6, 95% CI: 0.2–12.4) and two-year EFS (66.7% vs. 50%; HR: 0.6, 95% CI: 0.2–2.6) rates between the TA and OLR groups. Also, the incidence of postoperative complications in the OLR group was not statistically different from that in the TA group (75.0% vs. 30.8%; p = .128), and all symptoms resolved after symptomatic treatment; it is worth noting that postoperative complications, such as infection are prone to occur after the initial surgery [Citation25]. As compared with repeated surgical treatment, percutaneous TA boasted the advantages of less trauma, shorter procedure time, and faster recovery [Citation26]. More importantly, the United States Food and Drug Administration issued a warning in December 2016 that the use of anesthetics and sedatives in children under three years of age may affect brain development. Repeated or long-lasting (> 3 h) anesthesia may exacerbate this risk. In this study, 11 patients (52.4%) were younger than three years. For patients experiencing a disease relapse in poor physical condition, TA required a shorter anesthesia time, which can help to limit the incidence of anesthesia-related complications. Moreover, image-guided TA will not reduce residual liver volume; it can achieve precise treatment and maintain long-term physiological needs. In short, TA constitutes a safe option for patients with poor tolerance and high risk for resection without sacrificing the tumor-control effect in the treatment of relapsed HB.
This study has some limitations. First, although the number of patients in this study was the largest among current studies reporting on TA as a treatment for HB, its population was still small, and selection bias may have had an impact on the research results. We look forward to studies involving larger populations of patients in the future. Second, the tumor size in this study was no more than 3 cm, and whether the conclusion of this study is applicable to children with larger tumors still needs to be further demonstrated. Moreover, percutaneous TA treatment in this study included two methods: RFA and microwave ablation. However, many studies have indicated a similar efficacy and safety profile exists for these two techniques [Citation27,Citation28]. Finally, combination therapy has been a hot topic in recent years. Some studies have shown that TA can achieve good results [Citation29–32], but few were focused on multidisciplinary combination therapy. This study, too, did not explore this aspect, and we hope to conduct further research in this area in the future.
In conclusion, this cohort study indicated that percutaneous TA for recurrent small HB is a safe treatment option for children, with an effect comparable to that of surgery shown within two years after treatment. As compared with surgery, TA leads to less trauma and faster recovery. We look forward to larger, prospective studies in the future that will provide more and stronger evidence supporting the clinical application of percutaneous TA in HB.
Supplemental Material
Download PDF (83.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ng K, Mogul DB. Pediatric liver tumors. Clin Liver Dis. 2018;22(4):753–772.
- Dasgupta P, Henshaw C, Youlden DR, et al. Global trends in incidence rates of childhood liver cancers: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2020;34(5):609–617.
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122–131.
- Semeraro M, Branchereau S, Maibach R, et al. Relapses in hepatoblastoma patients: clinical characteristics and outcome-experience of the International Childhood Liver Tumour Strategy Group (SIOPEL)). Eur J Cancer. 2013;49(4):915–922.
- Venkatramani R, Furman WL, Fuchs J, et al. Current and future management strategies for relapsed or progressive hepatoblastoma. Paediatr Drugs. 2012;14(4):221–232.
- Hafberg E, Borinstein SC, Alexopoulos SP. Contemporary management of hepatoblastoma. Curr Opin Organ Transplant. 2019;24(2):113–117.
- Yang T, Whitlock RS, Vasudevan SA. Surgical management of hepatoblastoma and recent advances. Cancers (Basel). 2019;11(12):1944.
- Pham TA, Gallo AM, Concepcion W, et al. Effect of liver transplant on long-term disease-free survival in children with hepatoblastoma and hepatocellular cancer. JAMA Surg. 2015;150(12):1150–1158.
- Trobaugh-Lotrario AD, Meyers RL, Tiao GM, et al. Pediatric liver transplantation for hepatoblastoma. Transl Gastroenterol Hepatol. 2016;1:44.
- Hesh CA, Gill AE, Soler Rodriguez D, et al. Percutaneous image-guided microwave ablation as primary therapy for PRETEXT II hepatoblastoma. Pediatr Blood Cancer. 2020;67(10):e28641.
- Hetzer B, Vogel GF, Entenmann A, et al. Stereotactic radiofrequency ablation of a variety of liver masses in children. Int J Hyperthermia. 2020;37(1):1074–1081.
- Zhang YT, Chang J, Yao YM, et al. Novel treatment of refractory / recurrent pulmonary hepatoblastoma. Pediatr Int. 2020;62(3):324–329.
- Yevich S, Calandri M, Gravel G, et al. Reiterative radiofrequency ablation in the management of pediatric patients with hepatoblastoma metastases to the lung, liver, or bone. Cardiovasc Intervent Radiol. 2019;42(1):41–47.
- Cui R, Yu J, Gu Y, et al. Microwave ablation assisted by three-dimensional visualization system as local therapy for relapsed hepatoblastoma: a small pilot study. Abdom Radiol (NY). 2019;44(8):2909–2915.
- Sunil BJ, Palaniappan R, Venkitaraman B, et al. Surgical resection for hepatoblastoma-updated survival outcomes. J Gastrointest Cancer. 2018;49(4):493–496.
- Liu B, Zhou L, Huang G, et al. First experience of ultrasound-guided percutaneous ablation for recurrent hepatoblastoma after liver resection in children. Sci Rep. 2015;5(1):16805.
- Zsíros J, Maibach R, Shafford E, et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28(15):2584–2590.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Darbari A, Sabin KM, Shapiro CN, et al. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology. 2003;38(3):560–566.
- Towbin AJ, Meyers RL, Woodley H, et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48(4):536–554.
- Czauderna P, Haeberle B, Hiyama E, et al. The Children’s Hepatic tumors International Collaboration (CHIC): novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer. 2016;52:92–101.
- Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2015;103(1):126–135.
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307.
- Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020;6(2):255–263.
- Kingham TP, Correa-Gallego C, D'Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220(4):471–479.
- Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett. 2016;370(1):78–84.
- Facciorusso A, Abd El Aziz M, Tartaglia N, et al. Microwave ablation Versus radiofrequency ablation for treatment of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Cancers. 2020;12(12):3796.
- Glassberg M, Ghosh S, Clymer J, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438.
- Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1(6):756–765.
- Peng Z, Wei M, Chen S, et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. Eur Radiol. 2018;28(8):3522–3531.
- Huang Y, Song J, Zheng J, et al. Comparison of hepatic resection combined with intraoperative radiofrequency ablation, or hepatic resection alone, for hepatocellular carcinoma patients with multifocal tumors meeting the University of California San Francisco (UCSF) criteria: a propensity score-matched analysis. Ann Surg Oncol. 2020;27(7):2334–2345.
- Ryu T, Takami Y, Wada Y, et al. Combined hepatectomy and microwave ablation for multifocal hepatocellular carcinoma: long-term outcomes and prognostic factors. Asian J Surg. 2021;44(1):186–191.