Abstract
Background
An intrathoracic goiter (ITG) is defined as a thyroid extension below the sternal notch. Compared to cervical goiters, surgery for ITG is more challenging, with a higher risk of an extracervical approach. Ultrasound (US)-guided radiofrequency ablation (RFA) is a minimally invasive treatment modality. The purpose of this study was to prospectively evaluate the safety and efficacy of RFA in patients with ITG.
Methods
From a total of 324 patients who underwent thyroid RFA at a single medical center, 15 patients (mean age 52.2 years; 73.3% female) with 16 ITGs were included and classified into three grades and three types using the cross-section imaging CT system. Clinical features and demographics, degree of extension, RFA details, goiter volume, and complications were analyzed.
Results
Mean pre- and post-RFA goiter volumes as measured by US were 106.62 ± 61.82 and 25.09 ± 14.22 mL respectively, with a volume reduction rate (VRR) of 75.5% (p < 0.001) at 6 months. The VRR as measured by CT/MRI was 57.0 ± 10.0% (p < 0.001) at 6 months. The intrathoracic length reduction rate at 6 months was 44.9 ± 39.2% (p = 0.001). In addition, 4 (25%) ITGs had total regression of the intrathoracic extension, with a downgrade from grade 1 to cervical goiter. Mean pre- and post-RFA symptom and cosmetic scores were 1.53 and 0.15 (p = 0.001), and 2.67 and 2.00 (p = 0.001), respectively. One patient had transient vocal cord palsy and another had perithyroidal and mediastinal hemorrhage.
Conclusion
US-guided RFA is an effective treatment for ITG in terms of both cervical and intrathoracic reductions with an acceptable complication rate.
Introduction
Multinodular goiter (MNG) is a common cause of thyroid enlargement, which requires surgery. As the goiter grows, it may grow inferiorly, extending to the mediastinum. Intrathoracic goiter (ITG) is defined clinically and/or radiologically as the presence of thyroid tissue below the sternal notch or thoracic inlet with the patient in the supine position [Citation1,Citation2]. The prevalence of ITG among patients having undergone thyroid surgery has been reported at between 4.8% and 30.4% [Citation1,Citation3–5]. Surgical resection is the most effective treatment for ITG. The most common indication in 65% of surgeries for ITG are compressive symptoms, cosmetic concerns, suspicion of malignancy, and prevention of progressive enlargement or intrathoracic extension [Citation1,Citation6,Citation7]. Of note, the choice of surgical modality is individual in nature, and dependent on a variety of factors.
Although in a majority of ITG cases the goiter can be approached by cervical incision, some cases may still require an extracervical approach, such as a manubriotomy, sternotomy, or thoracotomy [Citation5,Citation8,Citation9]. Patients with high-grade ITG have a greater risk for extracervical approach and postoperative dysphonia. Furthermore, the complication rate and postoperation hospital stay for ITG patients increase with the extracervical approach [Citation9–11]. Though surgery is a safe and effective treatment for ITG, the risks of complications, recurrence, and possibility of an extracervical approach remain concerns of patients suffering from ITG.
With regards to the medicinal management of ITG, thyroid hormone suppressive therapy has a limited role and risks of side effects associated with iatrogenic thyrotoxicosis [Citation8,Citation12]. While radioactive iodine therapy may result in a 40–60% reduction in volume, it is however associated with risks of radiation thyroiditis, subsequent hypo- or hyperthyroidism, and radiation-induced malignancies [Citation13,Citation14]. The effectiveness of these conservative treatments are limited; thus, radiofrequency ablation (RFA) offers a promising alternative treatment for patients with ITG.
RFA is a well-established, effective, and minimally invasive treatment for patients with benign thyroid nodules. RFA can result in significant volume reduction with cosmetic and/or symptomatic improvement, and presents a low recurrence rate [Citation15,Citation16]. The advantages of RFA include a minimally invasive procedure under local anesthesia, outpatient surgery, and lower complication rate compared with surgery [Citation17]. Of note, the complications may include voice changes, nerve injury, tumor rupture, hematoma, skin burn, and hypothyroidism [Citation18]. Nonetheless, RFA provides an alternative treatment modality to surgery in patients with lTG who refuse surgery, or cannot undergo a surgical procedure, or as a down-staging procedure prior to surgery to avoid an extracervical approach.
Although RFA is an effective and safe treatment for benign thyroid nodules, the volume reduction rate (VRR) and clinical efficacy of RFA for ITG remain unclear. Thus, the purpose of this study is to investigate the efficacy and safety of RFA for the treatment of ITG, and to descriptively illustrate the technique behind US-guided RFA for ITG.
Materials and methods
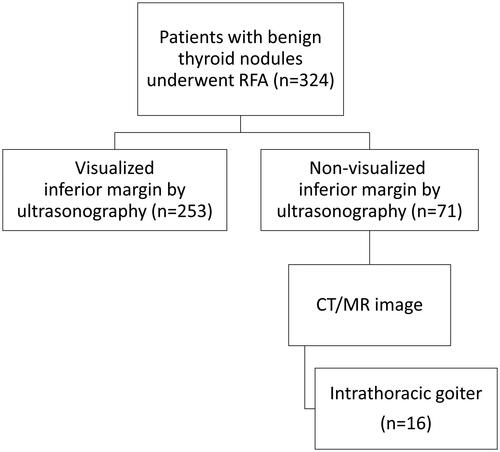
This retrospective analysis was approved by the institutional review board (201900550B0), and each participants’ private information was protected. A total of 324 patients with benign thyroid nodules underwent thyroid RFA at single medical center in Taiwan during the period of December 2016 to March 2019. In the pre-RFA evaluation, the non-visualized inferior margin of the thyroid nodule by ultrasonography (US) were noted in 71 patients (21.9%). The CT or MRI scan of the neck and upper thorax was performed to evaluate the extension of the thyroid into the thoracic inlet. After the evaluations, fifteen patients confirmed with 16 ITG were included in the study. Of the patients, one presented with a bilateral ITG (). At least two US-guided fine-needle aspirations (FNA) or core-needle biopsy (CNB) were performed prior to RFA to confirm the benign nature of the goiter. Benign thyroid nodules were confirmed in all of the 16 ITG patients. The RFA for ITG, as a treatment choice, has been discussed in multidisciplinary team meetings. All patients refused surgical treatment after a visit to the surgery clinic.
Inclusion and exclusion criteria
We included subjects who satisfied the following inclusion criteria: (1) age ≧18, (2) ITG, and (3) benign nature of the goiter proved by FNA or CNB (Bethesda ≦2). Subjects were eliminated based on the following exclusion criteria: (1) hypothyroidism or subclinical hypothyroidism, (2) patient with a pacemaker, and (3) pregnant women.
CT cross-section imaging classification system
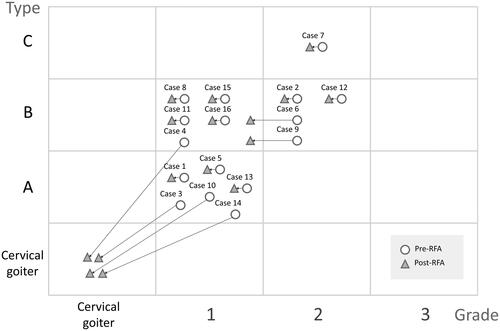
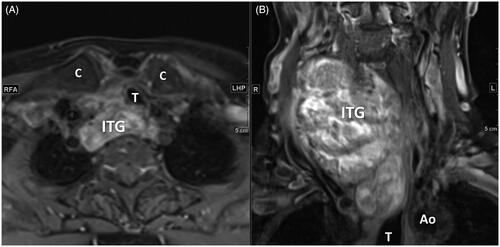
The 16 ITG were classified into three grades and three types using the CT cross-sectional imaging (CSI) classification system [Citation10]. The classification of an ITG based on a CT scan considers three dimensions of space: the cranio-caudal (sagittal), anteroposterior (axial), and latero-lateral (coronal) planes. Classification into the cranio-caudal plane is based on the lower margin of the thyroid, as follows: grade 1 (the inferior margin is between the thoracic inlet and the aortic arch convexity, ); grade 2 (the inferior margin is between the aortic arch convexity and concavity, ); and grade 3 (the inferior margin is below the aortic arch concavity). Classification in the anteroposterior plane is based on the relation of the main mass of the thyroid gland and the aortic arch or its branches and the trachea, as follows: type A (prevascular, ); type B (retrovascular-paratracheal, ); and type C (retrotracheal, ). The latero-lateral extension is classified as monolateral (only one lobe extends into the thoracic inlet), and bilateral (both lobes extend into the thoracic inlet).
Figure 2. A 37-year-old female had left grade 1 and type A ITG. (A) Axial enhanced CT shows the main mass of ITG was in the prevascular region with right deviation and backward displacement of the trachea. (B) Coronal enhanced CT shows the inferior margin of ITG between the thoracic inlet and the aortic arch convexity. (C, D) Axial and coronal enhanced CT shows the regression change of ITG with decreased volume and enhancement of the main mass after RFA. (ITG: intrathoracic goiter; Ao: aorta; SCV: subclavian vein; SCA: subclavian artery; CCA: common carotid artery; T: trachea).
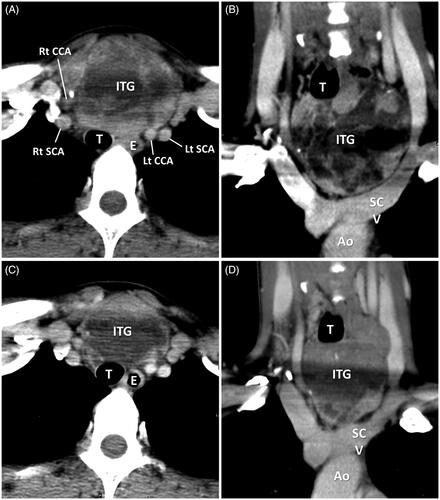
Figure 3. A 58-year-old female with left grade 2 and type B ITG suffered from progressive chest ecchymosis with pain and mild hoarseness after RFA. (A–C) Axial enhanced CT and (D) coronal enhanced CT in day 6 post-RFA shows the main mass of ITG was in the paratracheal region with right deviation of the trachea. The inferior margin of ITG between the inferior margin of ITG between the aortic arch convexity and concavity. Some poor enhanced and hypodense area within the ITG represents the post-RFA change. Subcutaneous, perithyroidal, and focal mediastinal hematomas were also noted. (ITG: intrathoracic goiter; Ao: aorta; SVC: superior vena cava; T: trachea; H: hematoma).
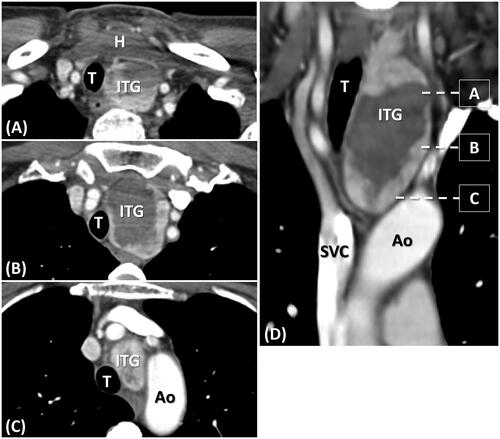
Figure 4. A 73-year-old female had right grade 2 and type C ITG (A) Axial T1 enhanced MRI shows the posterior extension of ITG to retrotracheal region with left deviation and compression of the trachea. (B) Coronal T1 enhanced MRI shows the inferior margin of ITG below the aortic arch convexity. (ITG: intrathoracic goiter; Ao: aorta; T: trachea; C: clavicle).
Pre- and post-RFA assessment
The clinical evaluations of symptoms and cosmetic issues were performed as part of the pre- and post-RFA assessments. The symptom score was obtained by patients filling out a questionnaire concerning five clinical symptoms: compression, cough, difficulty swallowing, voice change, and pain. We allocated one point for each positive symptom (ranged from 0 to 5). The cosmetic score was obtained using the following scale: 0, no palpable mass; 1, no cosmetic problem but palpable mass; 2, a cosmetic problem during neck extension and/or during swallowing; 3; readily detected cosmetic problem [Citation19]. The 10-point visual analog scale for pain (VAS Pain) [Citation20] was used to assess immediate post-RFA pain.
The US was evaluated in all patients both before, and at 1, 3, 6, and 12 months after RFA procedure. Serum thyroid function levels, and CT or MRI scan were evaluated before and at 6 months after RFA. The goiter length (cm) was separately measured as cervical and intrathoracic parts by CT or MRI scan. The tracheal lumen was measured as the narrowest diameter of trachea compressed by the goiter, while the tracheal deviation was defined as the deviation distance between the center of the tracheal lumen and the midline of the sternum (mm). The goiter volume was calculated using the ATA thyroid nodule volume calculator (https://www.thyroid.org/professionals/calculators/thyroid-with-nodules/) by entering the length, width, and depth as measured by US, CT, or MRI scan.
Laryngoscopy was evaluated in all patients both before and immediately after the RFA procedure to assess possible vocal cord paralysis. Major and minor complications were assessed according to the standard terminology and rating system of the Society of Interventional Radiology (SIR). Minor complications include SIR classifications A–B, while major complications include SIR classifications C–F [Citation21].
RFA devices and technique
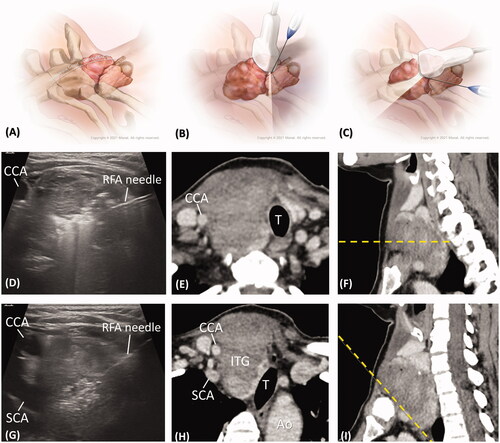
All RFA procedures were performed under perithyroidal local anesthesia. Hydrodissection with 5% dextrose water was injected perineurally to prevent thermal damage to the nerves. The RFA was administered using an internally cooled 18 G electrode, 7 cm in length, and with a 7 or 10 mm active tip size, and powered by the RF generator (VIVA, STARmed, or M2004, RF Medical). The choice of active tip length (7 or 10 mm) was determined according to goiter size, contour, and the relative position of perithyroidal structures. For the cervical part of the ITG, RFA was performed using a free-hand, trans-isthmic approached, ‘moving-shot’ technique under US-guidance as standard procedure. For the intrathoracic part of the ITG, the transducer was placed cranially to the clavicle with the axis parallel to the clavicular long axis. With the caudal tilted transducer, the intrathoracic part of the goiter could be partially seen. Intrathoracic RFA was performed via medial-to-lateral or lateral-to-medial direction, using a ‘fixed needle’ rather than ‘moving-shot’ technique to avoid extrathyroidal damage in the nonvisible field ().
Figure 5. (A–C) The medical illustrations show the anatomy of an ITG and the echo planes of RFA from cervical level to intrathoracic level. To see the ITG, the transducer was placed cranially to the clavicle with the caudal tilting angle. (D) The intra-procedural echo image shows the RFA for the cervical goiter with an axial view, as in the technique in . The post-RFA enhanced CT images with (E) an axial view and (F) a dotted line in the sagittal image displaying the same cervical section as in . (G) The intra-procedural echo image shows RFA for ITG in an oblique view with a tilting transducer, as in the technique in . The post-RFA enhanced CT images with (H) an oblique axial view and (I) a dotted line in the oblique sagittal view displaying the same intrathoracic section as . (CCA: common carotid artery; SCA: subclavian artery; T: trachea; Ao: aorta, ITG: intrathoracic goiter).
Statistical analysis
Clinical features and demographics, degree of extension, RFA details, goiter volume, and complications were analyzed by SPSS (Version 23.0; IBM, Armonk, New York, USA) statistical software. Data measurements are expressed as mean, range and SD. Comparison of the pre- and post-RFA data was analyzed with ANOVA. The threshold for statistically significant differences was defined as p < 0.05.
Results
Clinical characteristics and imaging subgroups
A total of 15 patients with 16 ITGs, including 11 grade 1 and five grade 2 ITGs were included in the study. Patient demographic data and clinical characteristics are presented in and . The overall mean age of the patients with ITG was 52.2 years (range 37–73; SD 11.6), with 11 female patients (73.3%) and four male patients (26.7%). The mean goiter volume as measured by US was 106.62 ml (range 29.2–252; SD 61.82), and as measured by CT or MRI was 97.74 ml (range 22.8–229.4; SD 56.47). The mean cervical and intrathoracic length of goiters as measured by CT or MRI was 7.18 cm (range 4.13–9.6; SD 1.48) and 1.55 cm (range 0.38–2.85; SD 0.76), respectively. The mean percentile of intrathoracic extension was 18.01% (range 5.44–40.83; SD 9.96).
Table 1. Demographic data.
Table 2. Clinical characteristics and efficacy at 6 months post-RFA.
The mean RFA procedure time was 74.13 min (range 49–103; SD 17.12), and the mean energy was 19.23 kcal (range 7.62–45.5; SD 12.32). Either 7 mm or 10 mm active tips were used in these procedures. The mean pain score (VAS) during and after RFA procedure were 4.00 (range 0–8; SD 2.42) and 2.53 (range 0–7; SD 2.00, p = 0.028).
Efficacy
Mean post-RFA goiter volume as measured by US was 25.09 ± 14.22 ml (VRR 75.5 ± 9.1% at 6 months, p < 0.001), and as measured by CT or MRI was 41.91 ± 29.01 ml (VRR 57.0 ± 10.0% at 6 months, p < 0.001). The intrathoracic length reduction rate at 6 months was 44.9 ± 39.2% (p = 0.001).
Shrinkage of goiter was accompanied by improvements in symptom and cosmetic scores. Prior to RFA, 75% of patients complained of compressive symptoms, with a mean symptom score of 1.53 ± 1.30; meanwhile, 93.8% of patients complained of cosmetic issues, with a mean score of 2.67 ± 0.82. At 6 months post-RFA procedure, the percentage of patients with compressive and cosmetic problems decreased to 12.5 and 81.3%, respectively. Both the symptom and the cosmetic score differences between baseline and 6 months were also significant (p = 0.001) ().
Subgroups
A higher intrathoracic length reduction rate was noted in the grade 1 ITG subgroup as compared to grade 2 (54.4 vs. 24.0%), but without statistical significance (p = 0.074). Otherwise, no significant differences of VRR or clinical improvement were noted among the grades and types. In addition, four of the 16 (25%) ITGs had total regression of the intrathoracic extension, with a downgrade from grade 1 prior to RFA to cervical goiter after the RFA procedure. Two ITGs had a downgrade from grade 2 prior to RFA to grade 1 after the RFA procedure ().
Complications
Post-RFA complications were absent in 14 out of 16 RFA cases. Of the complications reported, one patient had hoarseness during the RFA procedure, and gradually recovered within 3 months (minor complication, SIR classification A). Another patient presented with progressive chest ecchymosis with pain and mild hoarseness after the RFA procedure. She was admitted for closer observation at post-RFA day 6. Subcutaneous, perithyroidal, and focal mediastinal hematomas () were confirmed by CT scan. The symptoms failed to progress during hospitalization, and the patient ultimately recovered without further surgical treatment (major complication, SIR classification C). In addition, no cases of hypothyroidism or long-term vocal cord paralysis were noted at 6 months post-RFA procedure.
Discussion
This study demonstrates the effectiveness and relatively low complication rate of the RFA procedure for treatment of patients with ITG. Although we only performed RFA in the cervical part of the ITG, the RFA treatment at 6 months achieved significant reductions of 75.5% VRR measured by US, 57% VRR measured by CT or MR, and 44.9% reduction of intrathoracic length. Of note, the differences of the VRR as measured by US or CT/MR is understandable. More specifically, the US can only measure the cervical goiter, which is not obscured by the sternum; meanwhile, the CT/MR scans can provide more complete images of the features of an ITG, including the total volume of the goiter, the grade of extension, and the impact on the trachea [Citation22]. It is important to note that no long-term complications were reported in this study.
To our knowledge, this is the first study reporting on the efficacy of the RFA procedure for treatment of ITG. For patients with large thyroid nodules, a single-session RFA may achieve a satisfactory clinical result, but will rarely achieve complete ablation [Citation23]. The VRR of large benign thyroid nodules according to previous reports is in the range of 45–83% [Citation24–26]. In our previous retrospective study of single-session RFA for benign thyroid nodules, the VRR of large thyroid nodules (defined as volume >30 ml) was 75.0% at the 6-month follow-up [Citation19], similar to the VRR results reported herein as measured by US. However, there are indeed several differences between the RFA procedure for cervical goiter and for ITG. The inability of US to scan the intrathoracic part of the ITG, due to obscuration by sternum, is a limitation. Even though the ablation area is limited in terms of cervical sections under US-guided RFA, the reduction of the intrathoracic extension at 6 months post-RFA remains significant. This may be attributable to the shrinkage of the cervical goiter causing an upward migration of the intrathoracic section. Another possibility is that some intrathoracic sections may still be ablated by tilting the transducer. Although the volume reduction in the intrathoracic sections is less extensive than that of the cervical goiter due to the US limitations, RFA nonetheless remains an effective treatment for ITG.
In the present study, the RFA procedures were more effective in grade 1 than in grade 2 ITG patients, with a higher rate of cranio-caudal downgrade. This may be due to the above-mentioned reasons, and also possibly related to the narrow thoracic inlet. We propose a bottleneck hypothesis to explain the effect. This bottleneck hypothesis suggests that while the thyroid goiter extends from the cervical to thoracic level, the narrowest site is the thoracic inlet, which is also the bottleneck of the ITG. As such, the ITG might become stuck at the thoracic inlet while in the process of shrinking after RFA. For grade 1 ITG, the intrathoracic extension is limited, with a tapering contour. For higher grades of ITG, including grades 2 and 3, the intrathoracic volume becomes larger, effectively enhancing the bottleneck effect. This study indicates the tendency for a better intrathoracic reduction rate in grade 1 ITG patients. Although this study indicates that US-guided RFA may be more effective for grade 1 ITG, conclusive evidence requires further investigation.
Basically, the selection of the active tip of the ablation needle is depended on the main size and shape of the intrathoracic component, and their relative anatomy between goiter and critical structure. If the intrathoracic goiter was huge with a round shape which means it can well maintain the safety margins from major vessels, tracheal, and esophagus, the 10 mm active tip would be applied. On the other hand, if the intrathoracic part is relatively deep and narrow, the 7 mm active tip would be better to avoid complications. Compared to 7 mm, the 10 mm active tip usually needs a higher power (50–60 watt) to achieve better tissue resistance during the RFA procedure. Although the different size of the active tip has different resistance, the internal content of the goiter (solid, cyst, hemorrhage, spongiform…) would also obvious influent the resistance during the RFA procedure. We continuously adjusted the resistance by using different power until the ablating zone becoming whiteout under US.
RFA offers a safe treatment modality with a low complication rate. According to previous guidelines and studies, the overall complication rate of RFA for benign thyroid goiters is approximately 2.1–3.3%, while the major complication rate was between 1.27 and 1.35% [Citation15,Citation18]. Hoarseness is the most common major complication of RFA, with a reported incidence rate of 0.97–1.45% in benign thyroid nodules, and a rate of permanent change of 0.17% [Citation18,Citation27]. In this study, two of the 16 cases exhibited transient hoarseness immediately after RFA. The vocal cord paralysis was diagnosed by post-RFA laryngoscopic evaluation, while both patients recovered spontaneously within 3 months. Although no previous reports have discussed the complication rate regarding RFA for ITG, the complication rate may be correlated with nodular size. Our previous study reported higher rates of hoarseness and other complications in larger thyroid nodule groups [Citation19]. One explanation may involve variation or distortion of the vagus nerve close to the thyroid nodule, caused by the bulging of large thyroid nodules [Citation28,Citation29]. Another complication noted in this study was a subcutaneous, perithyroidal, and focal mediastinal hematoma, from which the patient recovered spontaneously without surgical intervention. Hematoma is a minor complication with a relatively low incidence rate, usually not requiring surgical intervention. The hematoma may be caused by mechanical or ablation injury to vessels due to the needle or electrode, and are categorized as perithyroidal, supcapsular, and intranodular hematomas [Citation18,Citation30]. It has been suggested that larger goiters, including ITGs, have higher blood supplementation and are usually hypervascular with prominent perithyroidal vessels [Citation31,Citation32], potentially contributing to the susceptibility to bleeding. This study indicates that RFA for ITG possibly exhibits higher complication rates due to larger volumes, hypervascularity, and distortion of the perithyroidal structures.
Surgical excision is one curative treatment option for ITG, with en bloc resection of the goiter. The surgical management of ITG is, however, relatively complex and may be combined with an extracervical approach. One particular study reported an extracervical approach rate for ITG of 7.2%, which increased in association with the intrathoracic extension of the goiter, up to 45.1% with the ITG reaching the aortic arch level (CSI classification grade 2) [Citation33], indicating that regression of the intrathoracic extension can decrease the extracervical approach rate for ITG. Indeed, RFA as a down-staging procedure prior to surgery may reduce the need for an extracervical approach, such as a manubriotomy, sternotomy, or thoracotomy. Of note, complications associated with surgery for ITG are slightly higher than those of standard thyroid surgeries, which include laryngeal nerve injury, hypoparathyroidism, bleeding, and hematoma. Meanwhile, although incidence of tracheomalacia and pneumothorax are rare, they may be of importance to patients with ITG [Citation6,Citation8,Citation34,Citation35]. When compared with surgical intervention for ITG, RFA exhibits relatively fewer and less severe complications, generally not requiring inpatient care. There was no post-RFA hypothyroidism or hypoparathyroidism noted in our data. Although it must be noted that a particular limitation of RFA compared with surgery lies in the lack of a gross pathology of the ITG. However, the incidence rate of cancer in ITG patients having undergone surgery was between 13 and 19%, with no difference between extension grades and not higher than patients with cervical thyroid nodules [Citation3,Citation11,Citation36]. For those patients with lTG who refuse surgery or cannot undergo surgery, a gross pathology would also remain elusive, as would a complete assessment of the risk of malignancy. The RFA procedure may be effective at improving symptomatic and cosmetic issues, while it should be considered as a viable treatment option, or applied as a down-staging procedure prior to surgery.
In addition to RFA, trans-arterial embolization (TAE) is another minimally invasive procedure for ITG, with several case reports. Ducloux et al. has reported on the outcome of TAE in a case of ITG ineligible for surgery or radioiodine therapy [Citation37]. A 39% total thyroid volume reduction and 52% thyroid nodular volume reduction were achieved, with regression of the intrathoracic extension and tracheal stenosis. Another study reported on the effect of TAE for ITG, the procedure achieved approximately 50% VRR, especially for the intrathoracic section, and regression of tracheal and vessel compression [Citation38]. These reports indicate satisfactory efficacy of TAE for ITG. However, due to limited studies reporting the outcome of TAE, it is difficult to compare the potential benefits and drawbacks of RFA with those of TEA. Further investigation is thus required to compare the effects of TAE and RFA, and to explore the potential for combined therapy.
Although this study reports on the efficacy and safety of the RFA procedure, several limitations nonetheless exist. First, as mentioned above, there is no gross pathology to exclude the possibility of malignancy for those who underwent RFA rather than surgery. The FNA or CNB, performed at least twice, are necessary steps to perform prior to RFA. Second, single-session RFA rarely achieves complete ablation for those patients with large thyroid nodules [Citation23], not to mention ITG. Multiple sessions of RFA may thus be required to achieve the better clinical outcome and to reduce the risk of recurrence. Third, we herein only report on the 6-month outcome for the patients included in the study. A future longitudinal follow-up study could be designed to further evaluate long-term outcomes. Nevertheless, data indicate that RFA remains an effective and minimally invasive treatment for patients with ITG.
In conclusion, US-guided RFA is an effective treatment for ITG in terms of both cervical and intrathoracic reduction with an acceptable complication rate. RFA offers an alternative treatment modality in patients with lTG who refuse or cannot undergo surgery for various reasons, or as a down-staging procedure performed prior to surgery to avoid an extracervical approach.
Acknowledgments
We thank Yu-Hsuan Chang, attending radiologist of National Taiwan University Hospital, for the creation of the medical illustration that greatly improved the manuscript. This research received no specific grant from any funding agency.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hedayati N, McHenry CR. The clinical presentation and operative management of nodular and diffuse substernal thyroid disease. Am Surg. 2002;68(3):245–251.
- Shaha AR. Substernal goiter: what is in a definition? Surgery. 2010;147(2):239–240.
- Erbil Y, Bozbora A, Barbaros U, et al. Surgical management of substernal goiters: clinical experience of 170 cases. Surg Today. 2004;34(9):732–736.
- Moron JC, Singer JA, Sardi A. Retrosternal goiter: a six-year institutional review. Am Surg. 1998;64(9):889–893.
- Khairy GA, Al-Saif AA, Alnassar SA, et al. Surgical management of retrosternal goiter: local experience at a university hospital. Ann Thorac Med. 2012;7(2):57–60.
- Chen AY, Bernet VJ, Carty SE, Surgical Affairs Committee of the American Thyroid Association, et al. American Thyroid Association statement on optimal surgical management of goiter. Thyroid. 2014;24(2):181–189.
- Hinson J, Raven P, and Chew SL. The endocrine system: basic science and clinical conditions. Systems of the body. 2nd ed. Edinburgh; New York: Churchill Livingstone/Elsevier, 2010. p. 185.
- Hegedus L, Bonnema SJ. Approach to management of the patient with primary or secondary intrathoracic goiter. J Clin Endocrinol Metab. 2010;95(12):5155–5162.
- Di Crescenzo V, Vitale M, Valvano L, et al. Surgical management of cervico-mediastinal goiters: our experience and review of the literature. Int J Surg. 2016; 28(Suppl 1):S47–S53.
- Mercante G, Gabrielli E, Pedroni C, et al. CT cross-sectional imaging classification system for substernal goiter based on risk factors for an extracervical surgical approach. Head Neck. 2011;33(6):792–799.
- Tostado KVC, VelÁZquez-Fernandez D, Chapa M, et al. Substernal goiter: correlation between grade and surgical approach. Am Surg. 2018;84(2):262–266.
- Fast S, Bonnema SJ, Hegedus L. The majority of Danish nontoxic goitre patients are ineligible for levothyroxine suppressive therapy. Clin Endocrinol. 2008;69(4):653–658.
- Bonnema SJ, Hegedus L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33(6):920–980.
- Nygaard B, Faber JENS, Veje A, et al. Transition of nodular toxic goiter to autoimmune hyperthyroidism triggered by 131I therapy. Thyroid. 1999;9(5):477–481.
- Kim J-H, Baek JH, Lim HK, Guideline Committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology, et al. 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018;19(4):632–655.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321–1325.
- Baek JH, Lee JH, Sung JY, Korean Society of Thyroid Radiology, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012;262(1):335–342.
- Lin W-C, Kan N-N, Chen H-L, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011; 63(Suppl 11):S240–S52.
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202.
- Pollard DB, Weber CW, Hudgins PA. Preoperative imaging of thyroid goiter: how imaging technique can influence anatomic appearance and create a potential for inaccurate interpretation. AJNR Am J Neuroradiol. 2005;26(5):1215–1217.
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session–prospective randomized study. Radiology. 2012;263(3):909–916.
- Yao Z, Wu T, Zheng B, et al. A novel strategy for single-session ultrasound-guided radiofrequency ablation of large benign thyroid nodules: a pilot cohort study. Front Endocrinol. 2020;11:560508.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a Mayo Clinic case series. Mayo Clin Proc. 2018;93(8):1018–1025.
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(8):920–930.
- Ha EJ, Baek JH, Lee JH, et al. Clinical significance of vagus nerve variation in radiofrequency ablation of thyroid nodules. Eur Radiol. 2011;21(10):2151–2157.
- Ha EJ, Baek JH, Lee JH. Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol. 2015;16(4):749–766.
- Wang J-F, Wu T, Hu K-P, et al. Complications following radiofrequency ablation of benign thyroid nodules: a systematic review. Chin Med J. 2017;130(11):1361–1370.
- Chen X, Xu H, Ni Y, et al. Complete excision of a giant thyroid goiter in posterior mediastinum. J Cardiothorac Surg. 2013;8:207.
- Bin Saeedan M, Aljohani IM, Khushaim AO, et al. Thyroid computed tomography imaging: pictorial review of variable pathologies. Insights Imag. 2016;7(4):601–617.
- Huins CT, Georgalas C, Mehrzad H, et al. A new classification system for retrosternal goitre based on a systematic review of its complications and management. Int J Surg. 2008;6(1):71–76.
- Sephton BM. Extracervical approaches to thyroid surgery: evolution and review. Minim Invasive Surg. 2019;2019:5961690.
- Simo R, Nixon IJ, Poorten VV, et al. Surgical management of intrathoracic goitres. Eur Arch Otorhinolaryngol. 2019;276(2):305–314.
- Sahbaz NA, Tutal F, Aksakal N, et al. Cancer frequency in retrosternal goiter. Am Surg. 2017;83(12):1390–1393.
- Ducloux R, Sapoval M, Russ G. Embolization of thyroid arteries in a patient with compressive intrathoracic goiter ineligible to surgery or radioiodine therapy. Ann Endocrinol. 2016;77(6):670–674.
- Tartaglia F, Salvatori FM, Russo G, et al. Selective embolization of thyroid arteries for preresection or palliative treatment of large cervicomediastinal goiters. Surg Innov. 2011;18(1):70–78.